Abstract
Background
Dedifferentiated chondrosarcoma remains a significant therapeutic challenge. Studies performed to date have not identified efficacious chemotherapy regimens for this disease.
Questions/purposes
We sought to (1) evaluate the disease-specific survival at 2 and 5 years of patients with dedifferentiated chondrosarcoma; (2) assess the prognostic variables (both patient- and treatment-related), including the use of chemotherapy with ifosfamide, that relate to survivorship; and (3) assess specific toxicities associated with ifosfamide use.
Methods
Data from 41 patients with dedifferentiated chondrosarcoma diagnosed and treated at the University of Texas MD Anderson Cancer Center from 1986 to 2010 were analyzed for demographics, treatments, oncologic outcomes, and prognostic variables. There were 14 women and 27 men. The mean age at diagnosis was 58 years (range, 26–86 years). Seven patients presented with metastasis. Surgical resection alone was performed in 11 patients; resection and chemotherapy in 26 patients; resection and radiotherapy in two patients; and resection, chemotherapy, and radiotherapy in two patients. Ifosfamide-based regimens were used for 16 patients. In general, ifosfamide was used when the tumor was located in the trunk or if cisplatin was discontinued as a result of toxicity. Minimum followup was 8 months (median, 68 months; range, 8–281 months). Survival was estimated using Kaplan-Meier plots and analyzed by using the Cox proportional hazards model.
Results
Disease-specific survival rates at 2 and 5 years were 33% and 15%, respectively. Multivariate analysis revealed that treatment without ifosfamide-based chemotherapy was the only independent negative prognostic factor for disease-specific survival (hazard ratio, 0.4; 95% confidence interval, 0.17–0.92; p = 0.03). Ifosfamide was discontinued in a patient as a result of renal dysfunction and was decreased in dose in another patient who developed encephalopathy.
Conclusions
In this small retrospective study, it appeared that ifosfamide-based adjuvant chemotherapy combined with surgical resection offered a treatment advantage compared with patients who did not receive the drug in patients with dedifferentiated chondrosarcoma, although disease-specific survival for patients who have this rare tumor remains dismal.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Dedifferentiated chondrosarcoma is defined as a cartilaginous neoplasm characterized histologically by islands of benign or low-grade chondroid tumors adjacent to components of nonchondroid high-grade sarcoma [4, 16, 20]. Treatment of dedifferentiated chondrosarcoma remains a major challenge. As a result of the rarity of the disease, only two institutes [3, 5, 9, 18, 22] have reported the outcomes of more than 30 cases.
Whereas the National Comprehensive Cancer Network guidelines suggest treating dedifferentiated chondrosarcoma in a fashion similar to the recommended treatment for osteosarcoma [1], studies performed to date have not identified efficacious chemotherapy agents. Effectiveness of ifosfamide remains inconclusive [5].
We therefore sought to (1) evaluate the disease-specific survival at 2 and 5 years of patients with dedifferentiated chondrosarcoma; (2) assess the prognostic variables (both patient- and treatment-related), including the use of chemotherapy with ifosfamide, that relate to survivorship; and (3) assess specific toxicities associated with ifosfamide use.
Patients and Methods
With the approval of the institutional review board, we searched the orthopaedic oncology database and tumor registry of the University of Texas MD Anderson Cancer Center to identify all patients treated for dedifferentiated chondrosarcoma diagnosed between 1986 and 2010. Forty-three cases were identified. The diagnosis of dedifferentiated chondrosarcoma was confirmed at MD Anderson by a musculoskeletal pathologist (MD) by review of all relevant case pathology slides. Of the 43 patients identified, two were excluded because the sizes of the primary tumors, which had been excised at an outside facility, were not available.
For our retrospective analysis of the 41 study-eligible cases (14 women and 27 men), we obtained and evaluated data on demographics, treatments, toxicities associated with chemotherapy, oncologic outcomes, and prognostic variables by chart review. Of 41 patients, 34 patients have died. The remaining seven patients were followed up from 8 months to 281 months (median, 68 months).
Demographics
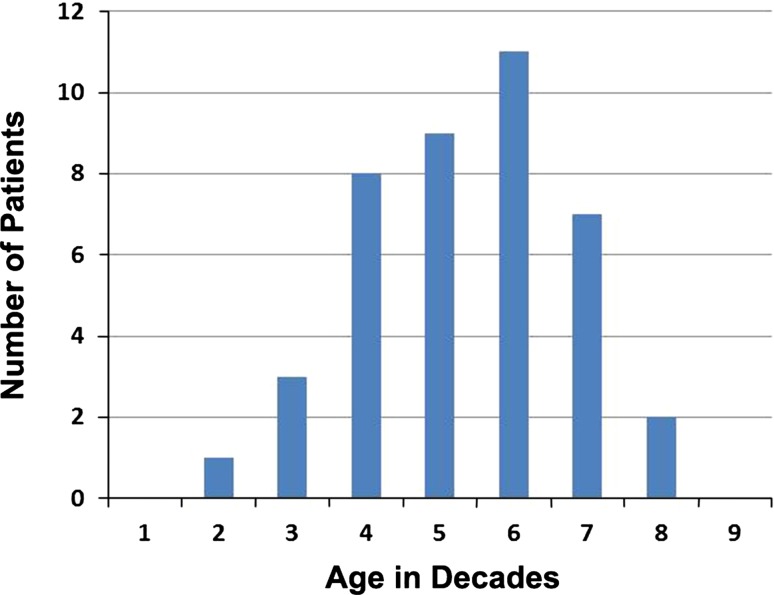
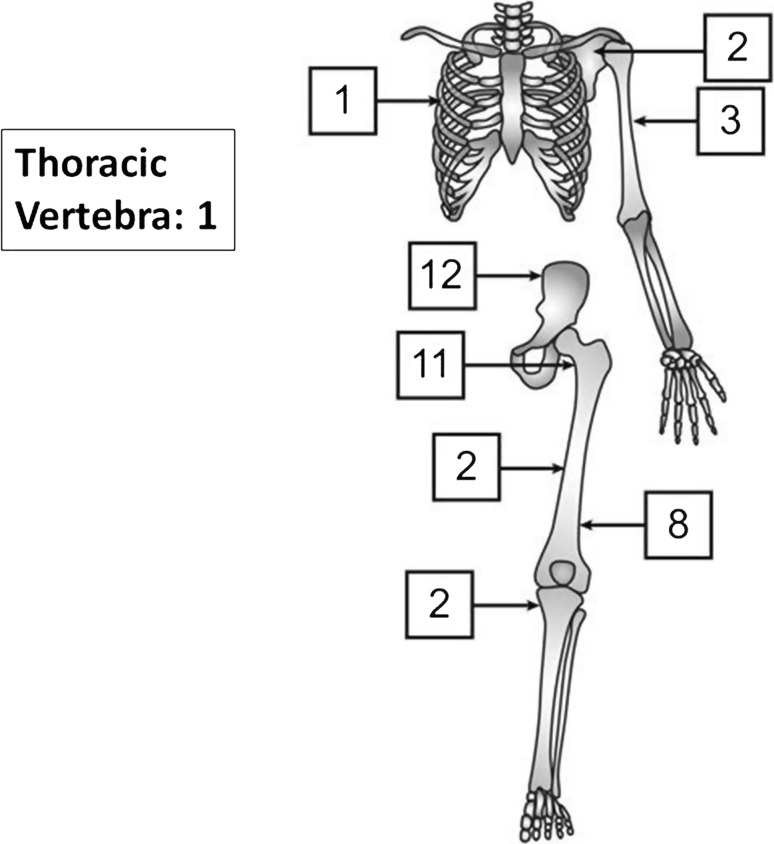
Mean age at diagnosis was 58 years (range, 26–86 years; Fig. 1). More than half of tumors were located in the pelvis or the proximal femur (Fig. 2). The tumor diameter was 9.6 cm on average (range, 2–23 cm). Fourteen patients had pathological fractures. Preoperative diagnosis was correct (dedifferentiated chondrosarcoma) in 21 patients. Lesions in the other 20 patients were initially categorized as conventional chondrosarcoma (13 patients; Grade 1 in three patients, Grade 2 in 10 patients), osteosarcoma (two patients), unclassified high-grade spindle cell sarcoma (two patients), mesenchymal chondrosarcoma (one patient), malignant fibrous histiocytoma (one patient), and low-grade sarcoma (one patient). Seven patients presented with metastasis (in the lung in six cases and ribs and pleura in the seventh).
Fig. 1.
Age distribution of patients is shown.
Fig. 2.
Anatomic locations of tumors is shown. Reprinted with permission from artist Kim-Anh T. Vu.
Treatment
Treatment for the primary tumor was tumor excision alone in 11 patients, excision and chemotherapy in 26 patients, excision and radiotherapy in two patients, and a combination of excision, chemotherapy, and radiotherapy in two patients. Chemotherapy was used preoperatively alone in 11 patients, postoperatively alone in 11 patients, and both preoperatively and postoperatively in six patients. Regimens used were a combination of doxorubicin, cisplatin, and ifosfamide in 14 patients; doxorubicin and cisplatin in 10 patients; doxorubicin and ifosfamide in two patients; and doxorubicin alone in two. Dose of reagents used was 60 to 75 mg/m2 doxorubicin, 100 to 120 mg/m2 cisplatin, and 10 g/m2 ifosfamide, respectively. Preoperative chemotherapy was applied for four cycles in 10 patients and three cycles in five patients. In the remaining two patients, preoperative chemotherapy was applied for only one cycle and two cycles, respectively. This is the result of development of pathologic fracture with progressive disease and they underwent surgical treatment. Number of cycles of postoperative chemotherapy varies from one to nine. Eleven patients received three cycles or more of chemotherapy. During the period in question, ifosfamide was used when the tumor was located in the trunk (Table 1) or when cisplatin was discontinued as a result of toxicity. Surgical margins were negative in 34 patients and positive in seven.
Table 1.
Association between chemotherapy and patient characteristics
| Characteristic | Number of patients | p value | Number of patients | p value | ||
|---|---|---|---|---|---|---|
| Any chemotherapy | No chemotherapy | Ifosfamide | No ifosfamide | |||
| Age (years) | ||||||
| ≥ 60 | 10 (50%) | 10 (50%) | 0.020 | 5 (25%) | 15 (75%) | 0.111 |
| < 60 | 18 (85.7%) | 3 (14.3%) | 11 (52.4%) | 10 (47.6%) | ||
| Sex | ||||||
| Male | 18 (66.7%) | 9 (33.3%) | 0.266 | 10 (37%) | 17 (63%) | 0.717 |
| Female | 10 (71.4%) | 4 (28.6%) | 6 (42.9%) | 8 (57.1%) | ||
| Tumor location | ||||||
| Trunk | 11 (73.3%) | 4 (26.7%) | 0.734 | 9 (60%) | 6 (40%) | 0.037 |
| Extremity | 17 (65.4%) | 9 (34.6%) | 7 (26.9%) | 19 (73.1%) | ||
| Tumor size (cm) | ||||||
| > 8 | 16 (72.7%) | 6 (27.3%) | 0.511 | 9 (40.9%) | 13 (59.1%) | 0.790 |
| ≤ 8 | 12 (63.2%) | 7 (36.8%) | 7 (36.8%) | 12 (63.2%) | ||
| Initial diagnosis | ||||||
| Incorrect | 13 (65%) | 7 (35%) | 0.628 | 9 (45%) | 11 (55%) | 0.444 |
| Correct | 15 (71.4%) | 6 (28.6%) | 7 (33.3%) | 14 (66.7%) | ||
| Pathologic fracture | ||||||
| Yes | 8 (57.1%) | 6 (42.9%) | 0.269 | 2 (14.3%) | 12 (85.7%) | 0.041 |
| No | 20 (74.1%) | 7 (25.9%) | 14 (51.9%) | 13 (48.1%) | ||
| Surgical margin | ||||||
| Positive | 2 (28.6%) | 5 (71.4%) | 0.024 | 0 (0%) | 7 (100%) | 0.031 |
| Negative | 26 (76.5%) | 8 (23.5%) | 16 (47.1%) | 18 (52.9%) | ||
| Metastasis at diagnosis | ||||||
| Yes | 6 (85.7%) | 1 (14.3%) | 0.399 | 2 (28.6%) | 5 (71.4%) | 0.685 |
| No | 22 (64.7%) | 12 (35.3%) | 14 (41.2%) | 20 (58.8%) | ||
For seven patients with metastatic lesions on presentation, treatment included chemotherapy in combination with thoracotomy for two patients and chemotherapy alone for five.
Statistical Methods
Disease-specific survival was defined as the time from diagnosis to death resulting from the disease. The distributions of time to events were estimated by the Kaplan-Meier method.
The Cox proportional hazards regression model was used to assess the difference in disease-specific survival rates and event-free survival rates among the following patient groups: (1) age ≥ 60 versus < 60 years; (2) female versus male; (3) tumors located on the trunk of the body versus in extremities; (4) tumor size > 8 cm versus ≤ 8 cm; (5) correct versus incorrect initial diagnosis; (6) occurrence versus absence of pathologic fracture; (7) negative versus positive surgical margin; (8) chemotherapy (regardless of the reagents used) versus no chemotherapy; (9) treatment with versus without ifosfamide-based chemotherapy; and (10) presence versus absence of metastasis at the time of diagnosis. Age of 60 years was used as the cutoff point to stratify age groups for outcomes and prognostic implications like in the study by Grimer et al. [10]. A tumor size of 8 cm was used as the cutoff point to stratify size groups according to the TNM classification of bone tumors [7].
Fisher’s exact test was used to assess whether an association existed between chemotherapy and patients’ characteristics. A probability value of < 0.05 was considered statistically significant. SAS Version 9.3 (SAS Institute Inc, Cary, NC, USA) and S-Plus Version 8.2 (TIBCO Software Inc, Palo Alto, CA, USA) were used for all statistical analyses.
Results
Disease-specific Survivorship, Local Recurrence, and Metastases
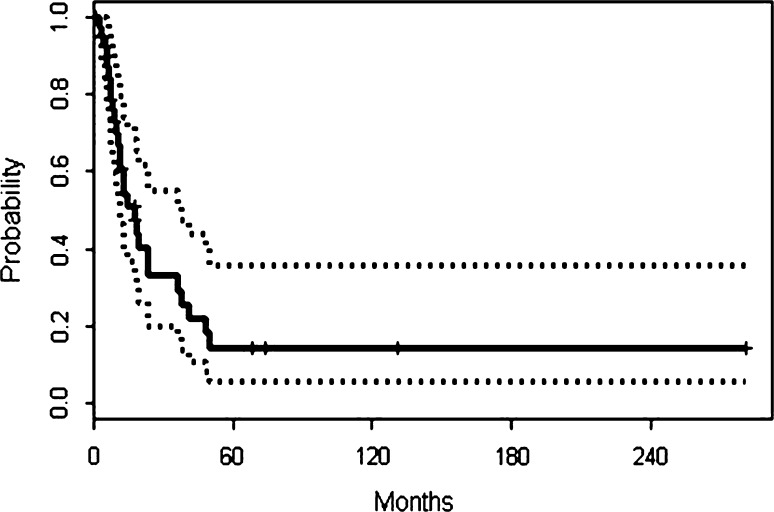
Disease-specific survival rates at 2 and 5 years were 33% (95% confidence interval [CI], 0.1988–0.550) and 15% (95% CI, 0.0603–0.358), respectively (Fig. 3). The event-free survival rate was 18% at 2 years and 15% at 5 years. Median disease-specific survival time was 18 months. The 5-year disease-specific survival rate for the 14 patients with localized dedifferentiated chondrosarcoma who received ifosfamide-based chemotherapy was 32%.
Fig. 3.
Disease-specific survival curve based on Kaplan-Meier plots is shown. The date of histological diagnosis was “time 0.”
Local recurrence of dedifferentiated chondrosarcoma developed in 14 patients including three of the seven patients with positive surgical margins and 11 of the 34 who had negative margins at the initial excision. A total of 27 patients developed distant metastasis, including 20 patients during the course of treatment and followup, in addition to the seven patients who presented with metastasis. At the latest followup, 27 patients had died of dedifferentiated chondrosarcoma. Seven patients had died of other causes including acute myocardial infarction, cardiomyopathy, leukemia, syndrome of inappropriate antidiuretic hormone secretion (SIADH), and suicide or an unknown cause. Acute myocardial infarction and cardiomyopathy were developed in patients who had not received chemotherapy. Six patients were disease-free. One patient was alive with disease at the latest followup.
Prognostic Factors
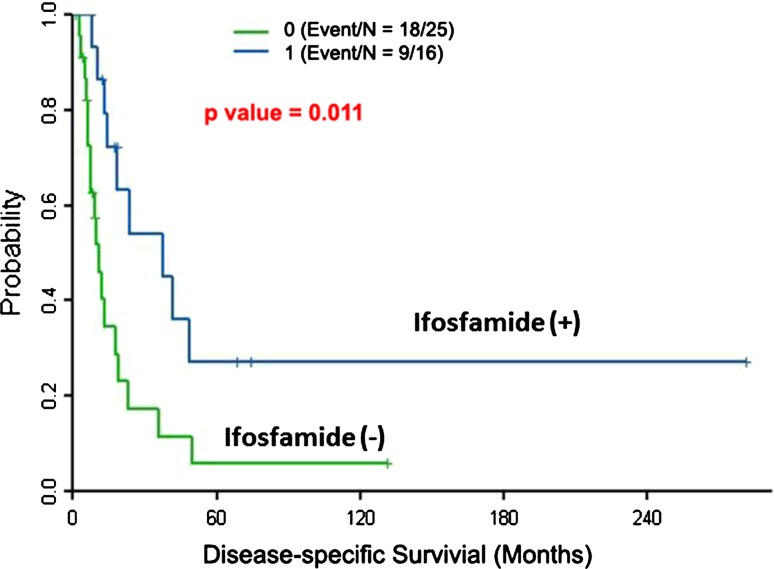
Ifosfamide-based chemotherapy was the only independent prognostic factor for disease-specific survival based on the multivariate analysis using a Cox regression model (hazard ratio, 0.4; 95% CI, 0.17–0.92; p = 0.03) (Table 2). Patients treated with ifosfamide had 2- and 5-year disease-specific survivorship of 54% and 27%, respectively; patients not treated with ifosfamide had 2- and 5-year disease-specific survivorship of 17% and 6%, respectively (Fig. 4).
Table 2.
Multivariate Cox regression analysis of DSS
| Prognostic factor | p value | HR (95% CI) |
|---|---|---|
| Ifosfamide | 0.030 | 0.40 (0.17–0.92) |
| Pathologic fracture | 0.063 | 2.52 (0.95–6.69) |
| Metastasis at diagnosis | 0.179 | 2.00 (0.73–5.47) |
A Cox regression model was fitted to estimate the effect of ifosfamide on DSS with adjustment for pathologic fracture and metastasis at diagnosis; DSS = disease-specific survival; HR = hazard ratio; CI = confidence interval.
Fig. 4.
Prognostic significance of ifosfamide-based chemotherapy is shown. Of the 41 patients, 16 received ifosfamide-based chemotherapy. Disease-specific survival rates of these 16 and the remaining 25 patients were estimated using Kaplan-Meier plots. The difference between the disease-specific survival rates of the two groups was statistically significant (p = 0.011). Patients treated with ifosfamide had 2- and 5-year disease-specific survivorship of 54% and 27%, respectively; patients not treated with ifosfamide had 2- and 5-year disease-specific survivorship of 17% and 6%, respectively.
Although the univariate analysis identified a number of factors potentially associated with poor disease-specific survivorship, including pathologic fracture (p = 0.004), and presence of metastasis at diagnosis (p = 0.014), none was subsequently confirmed on the multivariate analysis, so these should not be considered independent predictors of survivorship in patients in this study. Other variables that were evaluated but found not to be predictive of survivorship included age older than 60 years (p = 0.055) and positive surgical margin (p = 0.070), sex (p = 0.679), location (p = 0.680), size (p = 0.942), initial incorrect diagnosis (p = 0.319), and treatment with any chemotherapy (p = 0.303).
Subsequently event-free survival of 34 patients who had presented without metastasis was analyzed. The univariate analysis identified positive margin (p = 0.01), treatment with any chemotherapy (p = 0.0002), and again ifosfamide-based chemotherapy (p = 0.03) as potential prognostic predictors. Multivariate analysis revealed that treatment with any chemotherapy represented the independent prognostic factor (hazard ratio, 0.2; 95% CI, 0.09–0.6; p = 0.003) when adjusting for positive margin. Because ifosfamide-based chemotherapy is a part of “treatment with any chemotherapy,” these two variables were not analyzable together on a multivariate Cox regression model.
Risks and Toxicities Associated With Ifosfamide Use
Ifosfamide was used in 16 patients. Of these, one patient developed renal dysfunction during adjuvant chemotherapy with a combination of doxorubicin (75 mg/m2) and ifosfamide (10 g/m2). The regimen was switched to combination chemotherapy with doxorubicin, cyclophosphamide, and decarbazine, which the patient tolerated and completed three cycles. At final followup of 3 years after the ifosfamide therapy, the patient’s serum creatinine level remains elevated to 1.35 mg/dL. Another patient developed neurotoxicity (confusion, hallucination, and lethargy) on Day 4 of ifosfamide therapy (2 g/m2 Days 1–5, total 10 mg/m2). Ifosfamide scheduled on Day 5 was not given. The patient’s neurological status improved 24 hours after vigorous hydration. Ifosfamide chemotherapy was resumed with a reduced total dose at 8 g/m2 and the patient completed three cycles of ifosfamide therapy. Before ifosfamide therapy, this patient had received three cycles of doxorubicin (60 mg/m2) and cisplatin (100 mg/m2). Hematologic toxicity was reported in all patients with various degrees of leucopenia and thrombocytopenia, which were manageable with colony-stimulating factor support. No occurrence of hemorrhagic cystitis was reported with concurrent infusion of mesna. Apart from ifosfamide, cisplatin therapy was discontinued in four patients as a result of renal dysfunction (three patients) and healing loss (one patient).
Discussion
Dedifferentiated chondrosarcoma remains a major therapeutic challenge. Studies performed to date have not identified efficacious chemotherapy agents [3, 5, 9, 10, 13, 18, 21, 22]. We therefore sought to determine disease-specific survivorship (as well as local recurrences and metastases), prognostic variables associated with patient survival, and toxicities associated with ifosfamide-based chemotherapy for patients diagnosed with dedifferentiated chondrosarcoma.
This study has several limitations. The retrospective design of our review could result in transfer bias (loss to followup) and selection bias (variable indications for using ifosfamide). In regard to transfer bias, 34 patients were followed up to death. In the remaining seven patients, four patients were followed up for more than 2 years. Therefore, 38 of 41 patients (90%) were followed up to death or more than 2 years. To compensate for the selection bias, we analyzed the association between ifosfamide use and patients clinical characteristics (Table 1) and conducted multivariate Cox regression analysis. Also, only six patients underwent both preoperative and postoperative chemotherapy in our study group. This was the result of (1) inaccurate preoperative diagnosis in 11 patients; (2) the toxicity of the regimens in two older patients; and (3) tumor progression in nine patients who died before starting postoperative chemotherapy. The prognostic impact of doxorubicin and cisplatin was not separately analyzed as a result of an insufficient number of patients so treated.
The present study is the first to report an apparent therapeutic benefit of ifosfamide-based chemotherapy on disease-specific survival in patients with dedifferentiated chondrosarcoma. Despite the apparent prognostic impact of ifosfamide-based chemotherapy, the survival rate of patients treated with this regimen was only 32% at 5 years and was only 15% for all 41 patients in our analysis. Definite proof for the efficacy of ifosfamide awaits prospective analysis of larger numbers of patients. Several preclinical efforts are currently underway to develop targeted therapy for dedifferentiated chondrosarcoma based on molecular approaches [14, 15, 17]. The low survival rates of patients we observed with dedifferentiated chondrosarcoma are comparable to those reported in previous studies (Table 3) [3, 5, 9, 10, 13, 18, 21, 22]. This indicates the need for more systemic options.
Table 3.
Findings of studies of 20 or more patients with dedifferentiated chondrosarcoma
| Authors | Institute | Number of patients | Mean age (years) | Male (%) | Tumor in femur or pelvis (%) | Five-year survival (months) | Year of publication | References |
|---|---|---|---|---|---|---|---|---|
| Capanna et al. [3] | IOR | 46 | 52 | 54 | 61 | 6 | 1988 | 4 |
| Mercuri et al. [18] | IOR | 74 | 56 | 53 | 72 | 13 | 1995 | 18 |
| Staals et al. [22] | IOR | 123 | 59 | 54 | 73 | 24 | 2006 | 22 |
| Frassica et al. [9] | Mayo Clinic | 78 | 55 | 53 | 65 | 11 | 1986 | 10 |
| Dickey et al. [5] | Mayo Clinic | 42 | 60 | 57 | 81 | 7 | 2004 | 6 |
| Mitchell et al. [21] | ROH | 22 | 56 | 68 | 67 | 18 | 2000 | 20 |
| Grimer et al. [10] | ROH | 337* | 59 | 53 | 74 | 24 | 2007 | 11 |
| Johnson et al. [13] | MDACC | 26 | 61 | 62 | 69 | ND | 1986 | 13 |
| This study | MDACC | 41 | 58 | 66 | 80 | 15 |
* Multiinstitutional study; IOR = Instituto Ortopedico Rizzoli, ROH = Royal Orthopaedic Hospital; MDACC = MD Anderson Cancer Center; ND = not described.
In terms of prognostic variables, Grimer et al. [10] in their multiinstitutional study reported that the axial location of the tumor, pathologic fracture, positive surgical margin, and age older than 60 years were independent negative prognostic factors for dedifferentiated chondrosarcoma. In our univariate analysis, pathologic fracture showed a significant association with poor disease-specific survival and positive surgical margin and age older than 60 years approached significance; however, none of them were identified as independent prognostic factors in our multivariate model. This is likely the result of the small number of patients in our study. Studies performed to date have not shown a significant prognostic impact of adjuvant chemotherapy in patients with dedifferentiated chondrosarcoma [3, 5, 9, 10, 13, 18, 21, 22]. Ifosfamide has been identified as one of the active drugs for bone and soft tissue sarcomas and was incorporated into chemotherapeutic regimens for osteosarcoma in the 1980s [6, 11, 19]. Dickey et al. [5], who incorporated ifosfamide into chemotherapy regimens consisting of doxorubicin and cisplatin for dedifferentiated chondrosarcoma, attributed the observed absence of therapeutic benefit to a small number of patients and the retrospective design of the study. In their study, 22 of the 42 patients with dedifferentiated chondrosarcoma had chemotherapy. However, the number of patients who received ifosfamide-based chemotherapy is not documented. In the present study, 28 of the 41 patients with dedifferentiated chondrosarcoma received chemotherapy. Of these 28 patients, 16 patients were treated with ifosfamide-based chemotherapy.
The acute toxicity associated with ifosfamide included renal toxicity and encephalopathy (neurotoxicity) in the present study. In the literature, Ferrari et al. [8] reported ifosfamide-related renal toxicity and neurotoxicity in 2% and 5% of 182 patients, respectively, who had been treated with combination chemotherapy with methotorexate, doxorubicin, cisplatin, and ifosfamide. They also reported 4% incidence of chronic renal failure in survivors and stressed importance of continuous followup [8]. In our study, the patient with renal toxicity remains with borderline elevation of creatinine at final followup. Encephalopathy is also a serious adverse reaction to ifosfamide. Suggested predisposing factors include renal and liver dysfunction, low serum albumin, pelvic disease, prior cisplatin treatment, female sex, and age older than 65 years [12]. Our patient showing encephalopathy was a 75-year-old woman who had undergone prior cisplatin treatment. For elderly patients, careful baseline evaluation of mental status and constant clinical observation during ifosfamide infusion, especially during the first course of therapy, is recommended [2].
We conclude that ifosfamide-based adjuvant chemotherapy combined with surgical resection provided a survival benefit in the small series of dedifferentiated chondrosarcoma; however, the prognosis remains generally poor. Whereas the National Comprehensive Cancer Network guidelines suggest treating dedifferentiated chondrosarcoma in a fashion similar to the recommended treatment for osteosarcoma, we recommend adding ifosfamide to the regimen. The potential benefit of ifosfamide in patients with dedifferentiated chondrosarcoma needs to be verified in a prospective analysis. Close monitoring of neurological status and renal function throughout the ifosfamide infusion is crucial to facilitate early detection and treatment of ifosfamide-related renal and neurotoxicities.
Acknowledgments
We thank Ms Kim-Anh T. Vu for the illustration.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the University of Texas MD Anderson Cancer Center, Houston, TX, USA.
References
- 1.Biermann JS, Adkins DR, Benjamin RS, Brigman B, Chow W, Conrad EU, 3rd, Frassica DA, Frassica FJ, George S, Hande KR, Hornicek FJ, Letson GD, Mayerson J, McGarry SV, McGrath B, Morris CD, O’Donnell RJ, Randall RL, Santana VM, Satcher RL, Siegel HJ, Somaiah N, Yasko AW. Bone cancer. J Natl Compr Canc Netw. 2010;8:688–712. doi: 10.6004/jnccn.2010.0051. [DOI] [PubMed] [Google Scholar]
- 2.Brunello A, Basso U, Rossi E, Stefani M, Ghiotto C, Marino D, Crivellari G, Monfardini S. Ifosfamide-related encephalopathy in elderly patients: report of five cases and review of the literature. Drugs Aging. 2007;24:967–973. doi: 10.2165/00002512-200724110-00008. [DOI] [PubMed] [Google Scholar]
- 3.Capanna R, Bertoni F, Bettelli G, Picci P, Bacchini P, Present D, Giunti A, Campanacci M. Dedifferentiated chondrosarcoma. J Bone Joint Surg Am. 1988;70:60–69. [PubMed] [Google Scholar]
- 4.Dahlin DC, Beabout JW. Dedifferentiation of low-grade chondrosarcomas. Cancer. 1971;28:461–466. doi: 10.1002/1097-0142(197108)28:2<461::AID-CNCR2820280227>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 5.Dickey ID, Rose PS, Fuchs B, Wold LE, Okuno SH, Sim FH, Scully SP. Dedifferentiated chondrosarcoma: the role of chemotherapy with updated outcomes. J Bone Joint Surg Am. 2004;86:2412–2418. [PubMed] [Google Scholar]
- 6.Dirix LY, Van Oosterom AT. The role of ifosfamide in the treatment of adult soft tissue sarcomas, Ewing’s sarcoma, and osteosarcoma: a review. Semin Oncol. 1990;17:50–57. [PubMed] [Google Scholar]
- 7.Dorfman HD, Czerniak B, Kotz R, Vanel D, Park YK, Unni KK. WHO classification of tumours of bone: introduction. In: Fletcher CDM, Unni KK, Mertens F, editors. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002. pp. 227–232. [Google Scholar]
- 8.Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini G, Muller C, Tienghi A, Wiebe T, Comandone A, Bohling T, Del Prever AB, Brosjo O, Bacci G, Saeter G, Italian Scandinavian Sarcoma Group Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–8852. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 9.Frassica FJ, Unni KK, Beabout JW, Sim FH. Dedifferentiated chondrosarcoma. A report of the clinicopathological features and treatment of seventy-eight cases. J Bone Joint Surg Am. 1986;68:1197–1205. [PubMed] [Google Scholar]
- 10.Grimer RJ, Gosheger G, Taminiau A, Biau D, Matejovsky Z, Kollender Y, San-Julian M, Gherlinzoni F, Ferrari C. Dedifferentiated chondrosarcoma: prognostic factors and outcome from a European group. Eur J Cancer. 2007;43:2060–2065. doi: 10.1016/j.ejca.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Harris MB, Cantor AB, Goorin AM, Shochat SJ, Ayala AG, Ferguson WS, Holbrook T, Link MP. Treatment of osteosarcoma with ifosfamide: comparison of response in pediatric patients with recurrent disease versus patients previously untreated: a Pediatric Oncology Group study. Med Pediatr Oncol. 1995;24:87–92. doi: 10.1002/mpo.2950240205. [DOI] [PubMed] [Google Scholar]
- 12.Isofamide: Drug Index, BC Cancer Agency Cancer Drug Manual. Available at: www.bccancer.bc.ca. Accessed September 22, 2013.
- 13.Johnson S, Tetu B, Ayala AG, Chawla SP. Chondrosarcoma with additional mesenchymal component (dedifferentiated chondrosarcoma). I. A clinicopathologic study of 26 cases. Cancer. 1986;58:278–286. doi: 10.1002/1097-0142(19860715)58:2<278::AID-CNCR2820580213>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Kim MJ, Cho KJ, Ayala AG, Ro JY. Chondrosarcoma: with updates on molecular genetics. Sarcoma. 2011;2011:405437. doi: 10.1155/2011/405437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malchenko S, Seftor EA, Nikolsky Y, Hasegawa SL, Kuo S, Stevens JW, Poyarkov S, Nikolskaya T, Kucaba T, Wang M, Abdulkawy H, Casavant T, Morcuende J, Buckwalter J, Hohl R, Deyoung B, Kernstine K, Bonaldo Mde F, Hendrix MJ, Soares MB, Soares VM. Putative multifunctional signature of lung metastases in dedifferentiated chondrosarcoma. Sarcoma. 2012;2012:820254. [DOI] [PMC free article] [PubMed]
- 16.Mavrogenis AF, Ruggieri P, Mercuri M, Papagelopoulos PJ. Dedifferentiated chondrosarcoma revisited. J Surg Orthop Adv. 2011;20:106–111. [PubMed] [Google Scholar]
- 17.Meijer D, de Jong D, Pansuriya TC, van den Akker BE, Picci P, Szuhai K, Bovee JV. Genetic characterization of mesenchymal, clear cell, and dedifferentiated chondrosarcoma. Genes Chromosomes Cancer. 2012;51:899–909. doi: 10.1002/gcc.21974. [DOI] [PubMed] [Google Scholar]
- 18.Mercuri M, Picci P, Campanacci L, Rulli E. Dedifferentiated chondrosarcoma. Skeletal Radiol. 1995;24:409–416. doi: 10.1007/BF00941235. [DOI] [PubMed] [Google Scholar]
- 19.Meyer WH, Pratt CB, Poquette CA, Rao BN, Parham DM, Marina NM, Pappo AS, Mahmoud HH, Jenkins JJ, Harper J, Neel M, Fletcher BD. Carboplatin/ifosfamide window therapy for osteosarcoma: results of the St Jude Children’s Research Hospital OS-91 trial. J Clin Oncol. 2001;19:171–182. doi: 10.1200/JCO.2001.19.1.171. [DOI] [PubMed] [Google Scholar]
- 20.Milchgrub S, Hogendoorn PCW. Dedifferentiated chondrosarcoma. In: Fletcher DM, Unni KK, Mertens F, editors. Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002. pp. 252–254. [Google Scholar]
- 21.Mitchell AD, Ayoub K, Mangham DC, Grimer RJ, Carter SR, Tillman RM. Experience in the treatment of dedifferentiated chondrosarcoma. J Bone Joint Surg Br. 2000;82:55–61. doi: 10.1302/0301-620X.82B1.9020. [DOI] [PubMed] [Google Scholar]
- 22.Staals EL, Bacchini P, Bertoni F. Dedifferentiated central chondrosarcoma. Cancer. 2006;106:2682–2691. doi: 10.1002/cncr.21936. [DOI] [PubMed] [Google Scholar]