Abstract
Background
Mesenchymal chondrosarcoma (MSC) is a rare variant of chondrosarcoma. Because of the rarity of the disease, most studies only contain a small number of patients and thus the prognostic variables and role of adjuvant therapies remain controversial.
Questions/purposes
We therefore asked (1) what the overall and disease-free survival were for patients with this diagnosis at 5 and 10 years; (2) whether there were significant prognostic factors associated with survival; and (3) whether use of adjuvant chemotherapy or radiotherapy was associated with survival in patients with MSC.
Methods
We retrospectively reviewed the cases of MSC diagnosed from 1979 to 2010 at one referral center. Forty-three cases were identified. Thirty-seven cases were analyzed for demographics, treatments, and outcomes. Thirty patients with localized disease were analyzed for prognostic factors. The minimum followup was 1 month (mean, 6 years; range, 1 month to 17 years). There were 17 females and 20 males. The mean age at diagnosis was 33 years (range, 11–65 years). Nineteen cases were skeletal and 18 cases were extraskeletal. Seventy-six percent of the tumors were located in the trunk.
Results
Five- and 10-year overall survival was 51% and 37%, respectively. Five- and 10-year disease-free survival was 23% and 5%, respectively. Age (< 30 years) and male sex were associated with poorer overall and disease-free survival in patients presenting with a localized tumor, respectively. Patients who did not receive radiotherapy were more likely to have a local recurrence. Adjuvant chemotherapy failed to show a significant association with overall, disease-free, metastasis-free, or local recurrence-free survival.
Conclusions
The present study reinforced the role of adjuvant radiotherapy for local tumor control.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Mesenchymal chondrosarcoma (MSC) is a rare variant of chondrosarcoma. Histologically, this tumor is characterized by a biphasic pattern consisting of well-differentiated cartilage lobules, which resemble those seen in a low-to-intermediate grade chondrosarcoma, and a proliferation of undifferentiated blue small round cells that simulate the cell type of Ewing’s sarcoma [12, 14]. Reflecting its histological characteristics, MSC shows some clinical features that are similar to Ewing’s sarcoma, including (1) highly malignant biological behavior; (2) occurrence in a young adult population; and (3) a high proportion of extraskeletal tumors [4, 14]. Based on these histological and clinical characteristics, current National Comprehensive Cancer Network guidelines suggest treating MSC in a fashion similar to Ewing’s sarcoma [1], including doxorubicin-based chemotherapy. This therapeutic concept, however, is based on a low level of evidence as a result of the exceeding rarity of MSC. Since the first report of MSC in 1959 [11], only three studies [2, 8, 13] have analyzed clinical course of more than 20 patients with MSC. There remains controversy regarding the prognostic variables and the role of adjuvant chemotherapy and radiotherapy. If one or more of treatment elements such as doxorubicin were to proven to be unhelpful, we would be able to avoid the toxicity associated with them.
The purposes of the present study were to determine (1) what the overall and disease-free survival was for patients with this diagnosis at 5 and 10 years; (2) whether there were significant prognostic factors associated with survival; and (3) whether use of adjuvant chemotherapy or radiotherapy was associated with survival in patients with MSC.
Patients and Methods
After obtaining institutional review board approval, the files of the orthopaedic oncology database and the tumor registry of the University of Texas MD Anderson Cancer Center were interrogated for all cases of MSC diagnosed between 1979 and 2010. Forty-three cases were identified. The diagnosis of MSC was confirmed at MD Anderson by review of relevant pathology slides of all cases. Pathology review was done at the time of diagnosis. One case was excluded because only pathology consultation was performed, and the patient was treated outside MD Anderson. Five cases were also excluded because they were lost to followup within 1 year. The remaining 37 patients represent the subjects of the study. For staging evaluation, all patients underwent CT scans of the chest, abdomen, and pelvis with contrast. Bone scan was performed in all patients with skeletal MSC.
Demographics
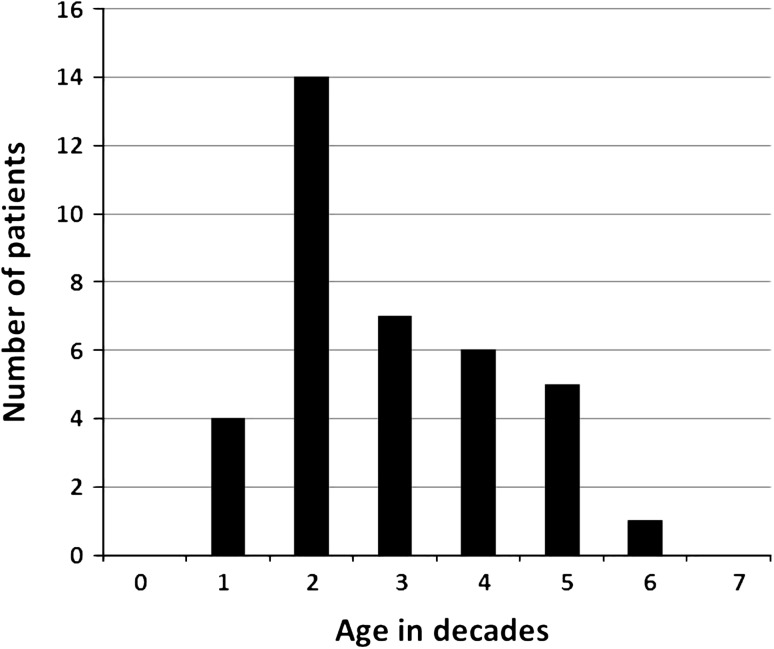
There were 17 females and 20 males. The mean age at diagnosis was 33 years with a range from 11 to 65 years (Fig. 1). Nineteen cases were skeletal (Table 1) and 18 cases were extraskeletal (Table 2). Notably, 76% of the tumors were located in the trunk. The interval between the first documented recognition of the tumor and the initiation of treatment ranged from 1 month to 5 years (mean, 6 months). Seven patients (Cases 16, 17, 18, 19, 35, 36, and 37) presented with metastatic disease.
Fig. 1.
Age distribution of patients with MSC is shown. The mean age at diagnosis was 33 years with a range from 11 to 65 years.
Table 1.
Clinical pictures of patients with skeletal mesenchymal chondrosarcoma
| Case number | Age (years) | Sex | Tumor site at presentation | Treatment for the primary lesion | Metastasis, recurrence (months) | Site of metastasis | Treatment for metastasis or recurrence | Outcome (months) |
|---|---|---|---|---|---|---|---|---|
| Presented without metastasis | ||||||||
| 1 | 44 | M | Scapula | Cx (DI) + Ex | Mets (20) | Bone (multiple), lung | Cx (E) | DOD (51) |
| 2 | 29 | M | Scapula | Cx (VDI)* + Ex* + Rx* | Mets (6) | Lung | Cx (I)* + Ex (lung)* | DOD (26) |
| 3 | 45 | F | Rib | Ex* | None | None | CDF (91) | |
| 4 | 28 | M | Rib | Ex* + Cx* + Rx* | Mets (11) | Bone (spine) | Ex (T9, L1) + Rx | DOD (19) |
| 5 | 65 | F | Rib | Ex* + Rx* | Mets (49) | Bone (femur) | Ex (femoral head)* | DOD (139) |
| 6 | 23 | M | Rib | Ex* | Mets (15) | Bone (multiple) | Cx (MDIDa)* | DOD (41) |
| 7 | 25 | M | Dens | Ex* | Rec (38) | NA | DOD (52) | |
| 8 | 30 | M | Thoracic spine | Ex* + Cx (DI)* + Rx* | Mets (46) | Lung | Cx (DI) + Ex (lung) | AWD (99) |
| 9 | 31 | F | Lumbar spine | Cx (VDI) + Ex | Rec (21), Mets (41) | Bone (skull, spine) | Rx | AWD (41) |
| 10 | 25 | F | Lumbar spine | Ex* | Rec (11), Mets (11) | Bone (multiple) | Rx* | AWD (13) |
| 11 | 21 | M | Sacrum | Ex* + Rx* | Mets (99) | Lung, pancreas | Cx (DI)* + Ex (lung)* | DOD (128) |
| 12 | 38 | F | Sacrum | Ex* + Cx (BCytD)* | Mets (133), Rec (137) | Bone (spine) | Cx (I)* + Ex (L2)* | DOD (207) |
| 13 | 21 | M | Sacrum | Cx (VDI) + Ex | Mets (17) | Subcutaneous tissue | Cx (IrT)* + Rx* | DOD (34) |
| 14 | 27 | M | Femur | Ex* + Cx (CisD)* | Rec (84), Mets (153) | Bone (spine, pelvis) | Rx* + Ex (femur)* + Cx (I)* | DOD (194) |
| 15 | 26 | F | Femur | Cx* + Ex* | Mets (103) | Lung | Cx (I) + Ex (lung) | DOD (187) |
| Presented with metastasis | ||||||||
| 16 | 41 | M | Scapula | Cx (CisTax)* + Rx* | Bone (multiple) | Cx (CisTax)* | DOD (49) | |
| 17 | 14 | F | Thoracic spine | Cx (CisDaMet)* + Ex* | Lung | Ex (lung)* + Cx (IrT) | DOD (57) | |
| 18 | 16 | M | Pelvis | Cx (DI) + Rx + Ex | Lung | Cx (DI) + Ex (lung) | NED (40) | |
| 19 | 22 | F | Femur | Cx (DI) + Ex | Bone (spine) | Cx (DI) + Ex (T1) + Rx | DOD (114) | |
* Treatment outside of MD Anderson; M = male; F = female; Cx = chemotherapy; Ex = excision; Rx = radiotherapy; NA = information not available; B = bleomycin; Cis = cisplatin; Cyt = cytoxan; D = doxorubicin; Da = dacarbazine; E = etoposide; I = ifosphamide; Ir = irinotecan; M = mesna; Met = methotrexate; T = temozoromide; Tax = taxotare; V = vincristine; AWD = alive with disease; CDF = continuously disease-free; DOD = dead of the disease; NED = no evidence of disease. Mesen
Table 2.
Clinical pictures of patients with extraskeletal mesenchymal chondrosarcoma
| Case number | Age (years) | Sex | Tumor site at presentation | Treatment for the primary lesion | Metastasis, recurrence (months) | Site of metastasis | Treatment for metastasis or recurrence | Outcome (months) |
|---|---|---|---|---|---|---|---|---|
| Presented without metastasis | ||||||||
| 20 | 59 | M | Shoulder | Ex + Rx | Mets (21) | Bone (sacrum), lung | Cx (VDI) + Rx | AWD (71) |
| 21 | 54 | F | Chest wall | Cx (VDI) + Ex | Rec (18) | Cx (I) | DOD (20) | |
| 22 | 22 | F | Chest wall | Cx (VDI) | None | None | DOD (20) | |
| 23 | 44 | M | Chest wall | Ex* | Mets (10) | Lung | Cx (VD) +Ex (lung) | DOD (71) |
| 24 | 37 | M | Axillar | Ex* | None | None | CDF (21) | |
| 25 | 32 | M | Axillar | Cx (MDIDa)* + Ex* | Mets (30) | Lung | Cx (MDIDa)* + Ex (lung)* | DOD (46) |
| 26 | 16 | F | Paraspinal | Cx (DCyDa) + Ex | Mets (35), Rec (82) | Bone (orbital) | Cx (I) + Ex (orbital bone) | DOD (98) |
| 27 | 39 | F | Paraspinal | Ex* + Rx* (Brachy) | Mets (80) | Retroperitoneal | Ex (retroperitoneal)* | DOD (183) |
| 28 | 20 | F | Parapelvis | Cx (VDI) + Ex + Rx | None | None | CDF (69) | |
| 29 | 47 | M | Retroperitoneum | None | None | None | DOD (1) | |
| 30 | 45 | F | Vagina | Cx (DI)* + Ex* | Mets (30) | Lung | Cx (VDI) + Ex (lung)* | NED (151) |
| 31 | 24 | M | Thigh | Ex* + Rx* (Brachy) | Mets (5) | Bone (multiple) | Rx* | DOD (29) |
| 32 | 34 | M | Thigh | Ex* | Mets (8) | Lung | Ex (lung)* + Cx (VDI) | AWD (57) |
| 33 | 53 | F | Thigh | Ex | None | None | CDF (56) | |
| 34 | 25 | M | Thigh | Rx + Ex + Cx (VDI) | Mets (37) | Bone (multiple), lung | Cx (IE) | DOD (47) |
| Presented with metastasis | ||||||||
| 35 | 46 | F | Breast | Ex* + Cx (DI) | Lung, inguinal L/N | Cx (DI) + Ex (inguinal L/N) | DOD (71) | |
| 36 | 52 | F | Sigmoid colon | Ex* + Cx (VDI)* + Rx* | Liver | Cx (VDI)* | AWD (14) | |
| 37 | 11 | M | Thigh | Ex* + Rx* + Cx (VDI) | Lung | Cx (VDI) | DOD (49) | |
* Treatment outside of MD Anderson; M = male; F = female; Cx = chemotherapy; Ex = excision; Rx = radiotherapy; Brachy = brachytherapy; L/N = lymphonode; Cis = cisplatin; Cy = cyclophosphamide; D = doxorubicin; Da = dacarbazine; E = etoposide; I = ifosphamide; M = mesna; T = temozoromide; V = vincristine; AWD = alive with disease; CDF = continuously disease-free; DOD = dead of disease; NED = no evidence of disease. Kawaguchi et al. Clinical Orthop
Treatment
Initial treatments for the primary tumor consisted of tumor excision alone in eight patients, excision and chemotherapy in 13 (preoperative in 10 and postoperative in three), excision and radiation therapy in five (postoperative external beam radiation therapy in three and brachytherapy in three), and a combination of excision, chemotherapy, and radiotherapy in eight (preoperative chemotherapy and radiotherapy in one, preoperative chemotherapy and postoperative radiotherapy in two, preoperative radiotherapy and postoperative chemotherapy in one, and postoperative chemotherapy and radiotherapy in four). Three patients (Cases 16, 22, and 29) did not undergo tumor excision and were treated with chemotherapy (Case 22), a combination of chemotherapy and radiation therapy (Case 16), or palliative care (Case 29). Twenty-three patients were treated with chemotherapy; of these, 11 patients presented for consultation. Their chemotherapy treatment protocols were established and monitored by medical oncologists at MD Anderson but administered by the local oncologist because of social or financial restraints. Doxorubicin-based regimens were used for all 23 but two patients (Cases 16 and 17). A total dose of 40 to 70 Gy (mean, 56 Gy) was used for external beam radiation therapy.
Margins From Surgical Resections
The primary tumor was excised in 34 patients. The histological margin of the excised tumor was negative in 21 and positive in four. The information of the surgical margin was not available in nine patients.
Statistical Analyses
Survival was estimated using the Kaplan-Meier plots. Impact of adjuvant chemotherapy and adjuvant radiation therapy on overall survival, disease-free survival, metastasis-free survival, and local-recurrence-free survival was determined using the log-rank test. Also, prognostic significance of age (> 30 or < 30 years), sex, tumor origin (skeletal or extraskeletal), tumor site (trunk or extremities), and surgical margin (positive or negative) on those survival curves was assessed by univariate analysis using the log-rank test. Age 30 years was used as the cutoff point based on the mean age of the patients. The analysis was performed for the 30 patients who had presented without metastatic disease. In addition, association of chemotherapy with disease-free survival was analyzed in the patients who had presented without metastatic disease and were treated with margin-negative resection of the primary tumor. The date that the histological diagnosis was made was used as “time 0.” In metastasis-free survival, patients who did not have metastasis were censored at the last followup date. In local recurrence-free survival, patients who did not have local recurrence were censored at the last followup date. A probability of < 0.05 was accepted as statistically significant. IBM SPSS statistics Version 19 (SPSS Inc, Chicago, IL, USA) was used to perform the Kaplan-Meier method and the log-rank test.
Results
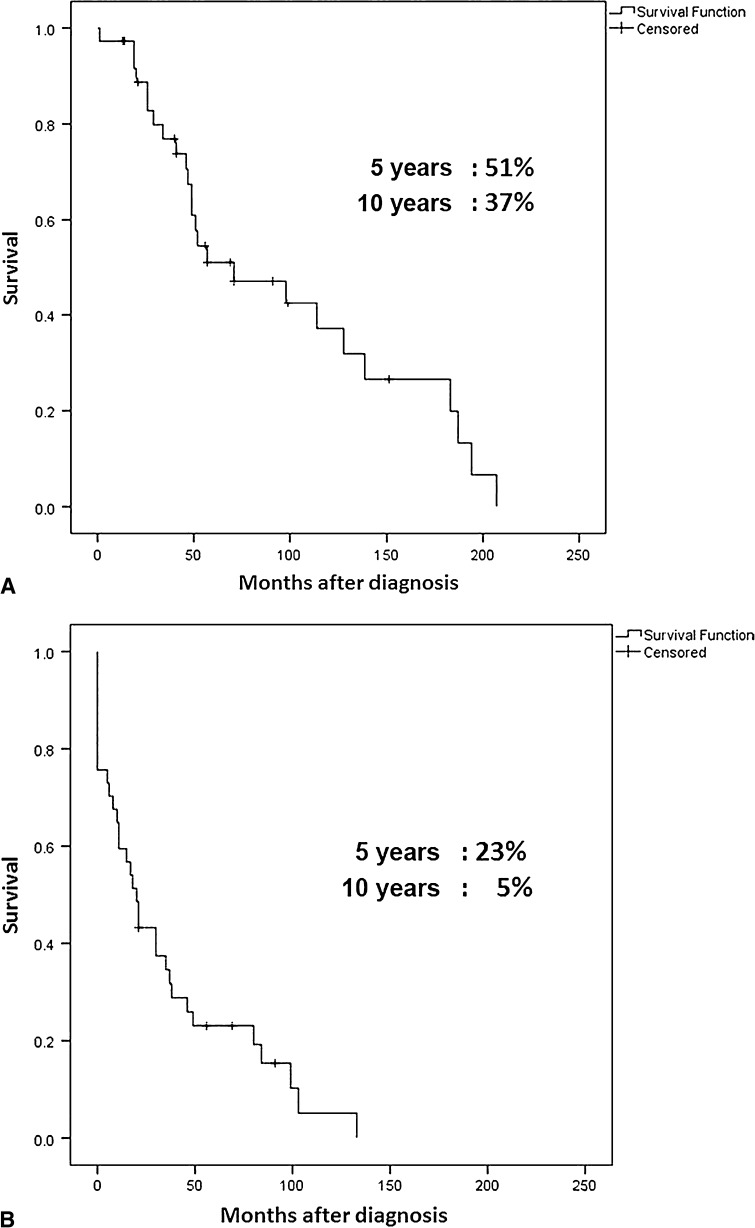
Overall survival was 51% at 5 years and 37% at 10 years (Fig. 2A). Disease-free survival was 23% at 5 years and 5% at 10 years (Fig. 2B). Metastasis-free survival was 37% at 5 years and 15% at 10 years. Local recurrence-free survival was 85% at 5 years and 68% at 10 years. A total of 29 patients had metastatic disease (78%), including 22 patients who developed distant metastasis during the course of treatment and followup and the seven already mentioned who presented with metastases. The most frequent location of the metastatic disease in skeletal MSC was the bone (10 cases) followed by the lung (seven cases) (Table 1). In extraskeletal MSC (Table 2), the most frequent metastasis site was the lung (seven cases) followed by bone (four cases).
Fig. 2A–B.
Overall and disease-free survival rates of 30 patients who presented without metastatic disease are shown. Overall (A) and disease-free (B) survival curves were estimated using the Kaplan-Meier plots. The date that the histological diagnosis was made was used as “time 0”.
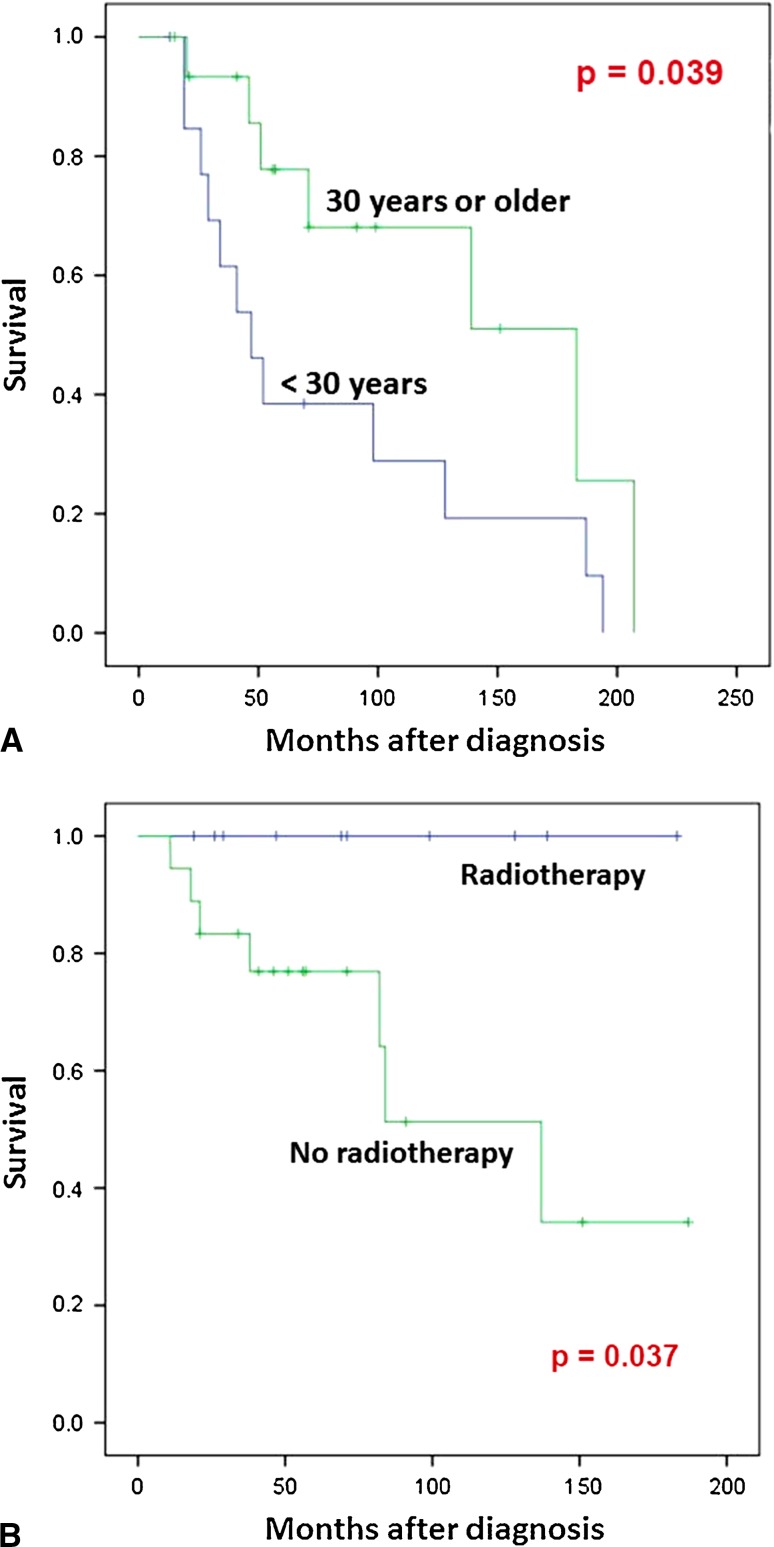
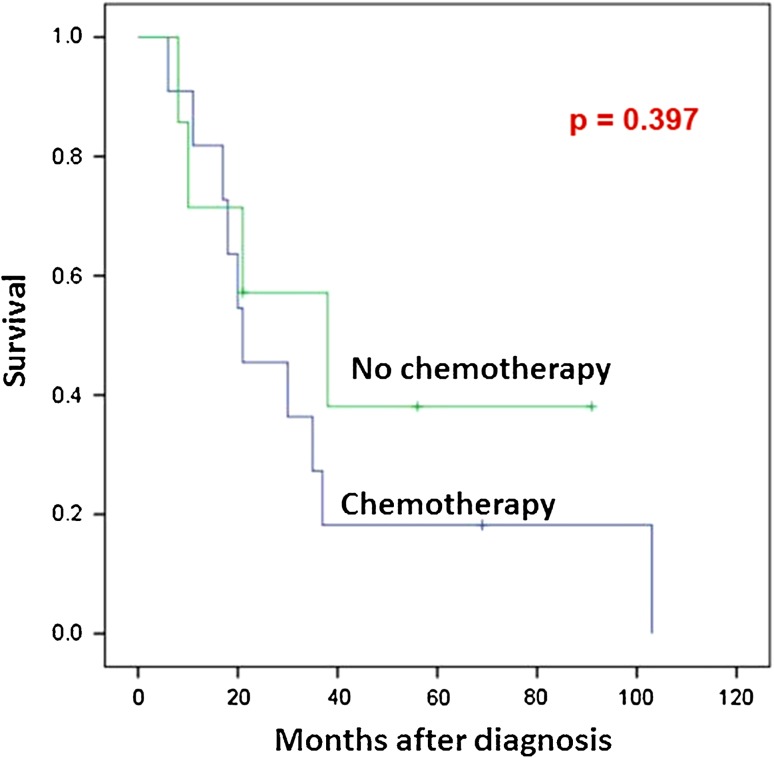
Among patients who presented with localized disease (Table 3), age younger than 30 years and male sex were associated with decreased overall survival (p = 0.039) (Fig. 3A) and disease-free survival (p = 0.024), respectively. Treatment with radiation therapy was significantly associated with improved local-recurrence-free survival (p = 0.037) (Fig. 3B). In contrast, none of origin and site of the tumor, surgical margin, and chemotherapy was significantly associated with overall survival, disease-free survival, metastasis-free survival, or local recurrence-free survival. Subgroup analysis was performed for 19 patients who presented with localized disease and were treated with margin-negative resection of the primary tumor. No significant association was noted between chemotherapy and disease-free survival in the analysis of these 19 cases (p = 0.397) (Fig. 4).
Table 3.
Statistical significance of variables on survivorships of 30 patients who presented without metastasis
| Variable | Number of patients | Overall survival | Disease-free survival | Metastasis-free survival | Recurrence-free survival† |
|---|---|---|---|---|---|
| Age (< 30 years) | 14 | 0.039 | 0.548 | 0.971 | 0.298 |
| Male sex | 17 | 0.06 | 0.024 | 0.088 | 0.424 |
| Bone origin | 15 | 0.602 | 0.224 | 0.43 | 0.505 |
| Trunk | 23 | 0.317 | 0.662 | 0.583 | 0.53 |
| Inadequate margin*,† | 12 | 0.924 | 0.875 | 0.635 | 0.753 |
| No chemotherapy | 14 | 0.961 | 0.908 | 0.748 | 0.388 |
| No Radiotherapy | 20 | 0.527 | 0.95 | 0.257 | 0.037 |
* Includes positive, marginal and unknown margins; †two cases with the primary tumor unresected are excluded.
Fig. 3A–B.
Prognostic significance of age of the patients and radiation therapy is shown. (A) Comparison of patients younger than 30 years and those older than 30 years. Of 30 patients who presented without metastatic disease, 14 patients were younger than 30 years old. Overall survival of these 14 and the remaining 16 patients older than 30 years was estimated using the Kaplan-Meier plots. A statistically significant difference was noted between the groups by the log-rank test (p = 0.039). (B) Comparison of patients treated with radiation and those without. Of 28 patients who had presented without metastatic disease and had undergone excision of the primary tumor, 10 patients received radiotherapy. Local recurrence-free survival of these 10 and the remaining 17 patients was estimated using Kaplan-Meier plots. The date that the histological diagnosis was made was used as “time 0.” A statistically significant difference was noted between the groups by the log-rank test (p = 0.037).
Fig. 4.
No significant association was seen between treatment with chemotherapy and disease-free survival in the 19 patients with MSC who had presented without metastatic disease and had undergone excision of the primary tumor with negative margins.
Discussion
MSC is a rare variant of chondrosarcoma. Because of the rarity of the disease, most studies only contain a small number of patients and thus the prognostic variables and role of adjuvant therapies remain controversial. We retrospectively analyzed 37 patients with MSC to determine (1) survival of patients with this diagnosis at 5 and 10 years; (2) whether there are important prognostic factors associated with longer survival; and (3) whether adjuvant chemotherapy or radiotherapy is associated with longer survival.
The limitations of this study are its retrospective design and inclusion of cases that received surgery outside but chemotherapy on the MD Anderson-monitored protocol. It remains to be determined whether lack of a significant impact of chemotherapy over the survival rates is the result of limited efficacy of the chemotherapy or the selection bias of the patients. A wide array of treatment approaches was used; this limits one’s ability to attribute differences to any one particular intervention (such as chemotherapy) because of the possible influence of confounding variables. Collection of the cases over 30 years also represents a limitation because diagnostic imaging and chemotherapy protocols have advanced and changed during these periods of time.
Overall survival of the patients in this series was 51% at 5 years and 37% at 10 years. The 5-year survival was similar to what has been published in previous studies [8, 13] (Table 4); however, the 10-year overall survival rate in the present series was higher than that published elsewhere (range, 20%–28% in other reports) [2–4, 8, 13] with the exception of a study by Dantonello et al. (in which the 10-year survivorship was 67%) [4]. This may be the result of the relatively short followup of the present study because the disease-free survival rates were as low as 23% at 5 years and 5% at 10 years. Together with the finding that 78% of the patients eventually had metastasis develop, the results of the present study support the highly malignant biological nature of MSC.
Table 4.
Studies of mesenchymal chondrosarcoma with survivorship analysis
| Authors | Number of patients | Average age (years) | Extraskeletal origin (%) | Trunk (%) | Overall survival (%) | |
|---|---|---|---|---|---|---|
| 5 years | 10 years | |||||
| Huvos et al. [8] | 32 | 25 | 14 | 35 | 42 | 28 |
| Nakashima et al. [13] | 23 | ND | ND | ND | 55 | 27 |
| Cesari et al. [2] | 26 | 31 | 35 | 38 | ND | 21 |
| This series | 37 | 33 | 49 | 76 | 51 | 37 |
| Subgroup analysis | ||||||
| Dabska and Huvos [3]* | 19 | 16 | 5 | 32 | 35 | 20 |
| Dantonello et al. [4]† | 15 | 17 | 73 | 80 | ND | 67 |
* Patients 21 years of age or younger; †patients 25 years of age or younger; ND = not described.
The demographics of our study population (mainly young) and the presentation of tumors in this study (almost half were extraskeletal) were consistent with those documented in previous studies [2, 4, 8, 13, 14] (Table 4). In contrast, more MSC was found in the trunk (76%) in the present study than in previous studies, suggesting the presence of possible referral bias.
We found that younger age (< 30 years) was associated with poor overall survival of patients with localized disease. This has not been consistently shown in other reports. Dabska and Huvos [3] analyzed 19 patients, 21 years or younger who were part of the original study group reported by Huvos et al. [8] (Table 4). The subgroup of 19 young patients exhibited poorer overall survival rates than the original group, although the differences were not analyzed. Dantonello et al. [4], on the other hand, reported a 10-year survival rate of 67% in a group of 15 patients who were 25 years of age or younger. The difference may be a function of anatomic location; the study population in the study by Danotello et al. included six cases of MSC of the head/neck, which has a more indolent course than MSC of other anatomical locations [9, 10, 16–18]. In our study, male sex was associated with poor disease-free survival of patients with a localized tumor. Sex-related differences in the survival rates have not been previously analyzed on MSC in the literature. In a study by Giuffrida et al. [6], a significant survival disadvantage was noted for male patients by a univariate analysis of 2890 cases with chondrosarcoma. However, although their study included 126 cases of MSC, subset analysis of MSC was not performed. Comparison of skeletal cases and extraskeletal cases showed no significant difference in survival in the present study. These two entities receive the same treatment at our institution. The numbers of each subgroup are 19 (skeletal) or 18 (extraskeletal), which are too small to proceed with comprehensive prognostic analysis. Therefore, we did not divide the analysis by skeletal and extraskeletal cases.
The present study demonstrates a significant association between adjuvant radiotherapy and local recurrence-free survival. In the literature, Harwood et al. [7] reported three cases with MSC showing some response to radiotherapy (complete remission in one and partial response in two). Gelderblom et al. [5] also described in their current review that MSC is more radiosensitive than other types of chondrosarcoma. In regard to survival analysis, Rushing et al. [15] reported 13 cases with MSC in the central nervous system. All patients underwent tumor excision (gross total excision in 10, subtotal excision in three). Of these, 10 patients who had received postoperative radiotherapy showed a trend toward increased overall survival (p = 0.61). To our best knowledge, none of previous studies with MSC showed a significant association between radiotherapy and local recurrence, which can be attributed to the small number of patients and lack of analysis with local recurrence-free survival. Our findings encourage the use of adjuvant radiotherapy in the treatment of MSC.
Current National Comprehensive Cancer Network guidelines suggest treating MSC in a fashion similar to Ewing’s sarcoma [1]. Accordingly, doxorubicin-based chemotherapy regimens were used in 21 patients in the present study. This chemotherapy regimen failed to yield a significant impact on overall survival, disease-free survival, metastasis-free survival, or local recurrence-free survival of patients with MSC in the present study. Our cohort was too small to analyze any difference between neoadjuvant versus adjuvant chemotherapy. Histological response to doxorubicin-based neoadjuvant chemotherapy was evaluated in nine patients in which only one case showed necrosis of more than 90% (data not shown). Cesari et al. [2], in their analysis of 21 patients with MSC who underwent surgical resection with negative margins, demonstrated significantly better disease-free survival in patients treated with chemotherapy than those without who achieved surgical remission. However, the prognostic impact of chemotherapy was not significant in the analysis of all patients with MSC. In our study group, 19 patients with localized disease were excised with negative margins. Analysis of these 19 patients demonstrated no significant association between chemotherapy and disease-free survival (Fig. 4). Our findings raise the question as to the efficacy of doxorubicin-based chemotherapy regimens in the treatment of MSC.
Metastasis developed frequently in the lungs (14 cases) and the bones (14 cases) in the present study. Notably, bone metastasis was noted in 53% (10 of 19) of skeletal MSC, suggesting the importance of bone surveillance, in addition to lungs. In the literature, the most frequent site of metastasis was documented as the lung [8, 13]. However, skeletal MSC and extraskeletal MSC were mixed together in analysis of those studies.
In conclusion, the present study supports the role of adjuvant radiotherapy for MCS in local control of the tumor. Lack of a significant association between chemotherapy and survival rates of the patients with MSC suggests requirement of more efficacious protocols than the current doxorubicin-based ones. Because MSC showed a highly malignant nature with propensity of metastasis, aggressive systemic therapy is still needed. Our current approach to MSC includes (1) neoadjuvant radiotherapy in case an adequate margin is considered difficult to achieve; and (2) neoadjuvant chemotherapy with an attempt to define efficacious reagents.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device prior to clinical use.
This work was performed at the University of Texas MD Anderson Cancer Center, Houston, TX, USA.
References
- 1.Biermann JS, Adkins DR, Benjamin RS, Brigman B, Chow W, Conrad EU, 3rd, Frassica DA, Frassica FJ, George S, Hande KR, Hornicek FJ, Letson GD, Mayerson J, McGarry SV, McGrath B, Morris CD, O’Donnell RJ, Randall RL, Santana VM, Satcher RL, Siegel HJ, Somaiah N, Yasko AW. Bone cancer. J Natl Compr Canc Netw. 2010;8:688–712. doi: 10.6004/jnccn.2010.0051. [DOI] [PubMed] [Google Scholar]
- 2.Cesari M, Bertoni F, Bacchini P, Mercuri M, Palmerini E, Ferrari S. Mesenchymal chondrosarcoma. An analysis of patients treated at a single institution. Tumori. 2007;93:423–427. doi: 10.1177/030089160709300503. [DOI] [PubMed] [Google Scholar]
- 3.Dabska M, Huvos AG. Mesenchymal chondrosarcoma in the young. Virchows Arch A Pathol Anat Histopathol. 1983;399:89–104. doi: 10.1007/BF00666221. [DOI] [PubMed] [Google Scholar]
- 4.Dantonello TM, Int-Veen C, Leuschner I, Schuck A, Furtwaengler R, Claviez A, Schneider DT, Klingebiel T, Bielack SS, Koscielniak E. Mesenchymal chondrosarcoma of soft tissues and bone in children, adolescents, and young adults: experiences of the CWS and COSS study groups. Cancer. 2008;112:2424–2431. doi: 10.1002/cncr.23457. [DOI] [PubMed] [Google Scholar]
- 5.Gelderblom H, Hogendoorn PC, Dijkstra SD, van Rijswijk CS, Krol AD, Taminiau AH, Bovee JV. The clinical approach towards chondrosarcoma. Oncologist. 2008;13:320–329. doi: 10.1634/theoncologist.2007-0237. [DOI] [PubMed] [Google Scholar]
- 6.Giuffrida AY, Burgueno JE, Koniaris LG, Gutierrez JC, Duncan R, Scully SP. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91:1063–1072. doi: 10.2106/JBJS.H.00416. [DOI] [PubMed] [Google Scholar]
- 7.Harwood AR, Krajbich JI, Fornasier VL. Mesenchymal chondrosarcoma: a report of 17 cases. Clin Orthop Relat Res. 1981;158:144–148. [PubMed] [Google Scholar]
- 8.Huvos AG, Rosen G, Dabska M, Marcove RC. Mesenchymal chondrosarcoma. A clinicopathologic analysis of 35 patients with emphasis on treatment. Cancer. 1983;51:1230–1237. doi: 10.1002/1097-0142(19830401)51:7<1230::AID-CNCR2820510710>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs JL, Merriam JC, Chadburn A, Garvin J, Housepian E, Hilal SK. Mesenchymal chondrosarcoma of the orbit. Report of three new cases and review of the literature. Cancer. 1994;73:399–405. doi: 10.1002/1097-0142(19940115)73:2<399::AID-CNCR2820730227>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 10.Knott PD, Gannon FH, Thompson LD. Mesenchymal chondrosarcoma of the sinonasal tract: a clinicopathological study of 13 cases with a review of the literature. Laryngoscope. 2003;113:783–790. doi: 10.1097/00005537-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein L, Bernstein D. Unusual benign and malignant chondroid tumors of bone. Cancer. 1959;12:1142–1153. doi: 10.1002/1097-0142(195911/12)12:6<1142::AID-CNCR2820120610>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima Y, Park YK, Sugano O. Mesenchymal chondrosarcoma. In: Fletcher DM, Unni KK, Mertens F, editors. Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002. pp. 255–256. [Google Scholar]
- 13.Nakashima Y, Unni KK, Shives TC, Swee RG, Dahlin DC. Mesenchymal chondrosarcoma of bone and soft tissue. A review of 111 cases. Cancer. 1986;57:2444–2453. doi: 10.1002/1097-0142(19860615)57:12<2444::AID-CNCR2820571233>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Riedel RF, Larrier N, Dodd L, Kirsch D, Martinez S, Brigman BE. The clinical management of chondrosarcoma. Curr Treat Options Oncol. 2009;10:94–106. doi: 10.1007/s11864-009-0088-2. [DOI] [PubMed] [Google Scholar]
- 15.Rushing EJ, Armonda RA, Ansari Q, Mena H. Mesenchymal chondrosarcoma: a clinicopathologic and flow cytometric study of 13 cases presenting in the central nervous system. Cancer. 1996;77:1884–1891. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1884::AID-CNCR19>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K, Sato K, Kanazawa H, Wang XL, Kimura T. Mesenchymal chondrosarcoma of the jaw—report of a case and review of 41 cases in the literature. Head Neck. 1993;15:459–464. doi: 10.1002/hed.2880150516. [DOI] [PubMed] [Google Scholar]
- 17.Vencio EF, Reeve CM, Unni KK, Nascimento AG. Mesenchymal chondrosarcoma of the jaw bones: clinicopathologic study of 19 cases. Cancer. 1998;82:2350–2355. doi: 10.1002/(SICI)1097-0142(19980615)82:12<2350::AID-CNCR8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 18.Zakkak TB, Flynn TR, Boguslaw B, Adamo AK. Mesenchymal chondrosarcoma of the mandible: case report and review of the literature. J Oral Maxillofac Surg. 1998;56:84–91. doi: 10.1016/S0278-2391(98)90922-3. [DOI] [PubMed] [Google Scholar]