Abstract
Background
Prophylaxis for pulmonary embolism (PE) after total joint arthroplasty (TJA) presents the clinical dilemma of balancing the risk of postoperative thrombotic risk and anticoagulation-related complications such as bleeding, hematoma formation, and infection. Risk stratification of patients undergoing TJA is needed to tailor prophylaxis based on thrombotic and bleeding risk.
Questions/purposes
The purpose of this study was to identify the preoperative comorbidities that were associated with an increased risk of symptomatic PE after joint arthroplasty in a large group of patients who had TJAs and who were treated with either aspirin or warfarin.
Methods
We conducted a retrospective study of 26,391 primary and revision TJAs performed at our institution between January 2000 and April 2011. A total of 24,567 patients received warfarin prophylaxis for 6 weeks (targeted international normalized ratio of 1.5–2.0) and 1824 patients received 325 mg aspirin twice daily. Symptomatic patients with decreased oxygen saturation were evaluated for PE using either a ventilation/perfusion scan or multidetector CT scan. Symptomatic PEs occurring in patients within 90 days postoperatively identified with CT or ventilation/perfusion scans were considered complications related to surgery, and fatal PEs were those that occurred in patients who died during the hospital admission owing to cardiopulmonary failure after PE. Using a logistic regression analysis, a nomogram was created to predict postoperative symptomatic PE risk.
Results
Risk of postoperative symptomatic PE after primary and revision TJAs was 1.1%. Risk of postoperative fatal PE was 0.02%. Elevated BMI (p < 0.035), procedures on the knee (p < 0.006), higher Charlson Comorbidity Index (p < 0.015), chronic obstructive pulmonary disorder (p = 0.006), atrial fibrillation (p < 0.001), anemia (p < 0.001), presence of deep vein thrombosis (p < 0.001), and depression (p = 0.012) were independent risk factors for symptomatic PE. Based on these risk factors and derived scoring criteria, patients can be classified into low- (0.35%), medium- (1.4%), and high- (9.3%) risk categories.
Conclusions
Patients who are obese, undergo knee procedures, have an elevated Charlson Comorbidity Index, chronic obstructive pulmonary disease, atrial fibrillation, anemia, depression, or postoperative deep vein thrombosis are at greater risk of having a postoperative PE develop. These risk factors should be considered when deciding on postoperative anticoagulation prophylaxis.
Level of Evidence
Level IV, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
During the next two decades, the number of total joint arthroplasties (TJAs) in the United States and worldwide is predicted to increase dramatically. Kurtz et al. [19] predicted that by 2030, there will be 174% and 673% increases in the number of primary THAs and primary TKAs, respectively. One of the most serious complications after TJA is pulmonary embolism (PE) [2, 6, 10, 13, 16]. The risk of symptomatic and asymptomatic PEs after TJA in patients receiving thromboprophylaxis has been reported to be 0.4% to 23% [8, 11, 18, 24, 26, 43]. In addition to patient morbidity and mortality, a PE leads to an extended hospital stay and increased treatment costs, creating an economic burden that can be expected to increase in the future [5, 30]. Consequently, patients undergoing TJA often are prescribed a postoperative anticoagulation regimen in an attempt to minimize the occurrence of postoperative venous thromboembolism (VTE), particularly PE. However, VTE prophylaxis presents the clinical dilemma of balancing postoperative thrombotic risk along with anticoagulation-related complications such as bleeding, hematoma formation, and infection [10]. Therefore, it would be helpful if risks for symptomatic PE in patients undergoing TJA could be clarified to stratify patients by their thrombotic risk to allow tailored prophylaxis.
Several studies have identified risk factors involved in the development of PE after general surgery procedures [3, 7, 8, 21]. For example, Caprini et al. [3] developed scoring criteria to determine the risk of PE in the general surgery population. However, risk factors for PE after TJA have not been as well defined. Whereas a TJA may be considered a prothrombotic risk factor, it remains unclear what role other factors play in affecting postoperative PE risk. Studies currently available in the literature evaluating PE risk after TJA have several limitations including small sample size, limited followup, selective patient populations, and/or incomplete data [4, 22, 25, 37, 43]. Furthermore, some PE risk studies with large cohorts of patients [6, 13] use International Classification of Diseases, 9th Revision (ICD-9) or International Classification of Diseases, 10th Revision coding data to identify complications. Although such coding often is helpful for identifying known comorbidities, there are concerns regarding underreporting and/or inaccurate reporting of perioperative complications. Such concerns will continue to hamper studies that rely on large databases in which a significant proportion of the perioperative information is coded by hospital billing staff and not by medical personnel.
The purpose of our study is to identify risk factors for symptomatic PE after primary and revision joint arthroplasties in patients who receive either prophylactic warfarin or aspirin therapy. Our aim is to develop criteria to stratify patients according to their risk of symptomatic PE to provide targeted postoperative PE prophylaxis.
Materials and Methods
Study Design
Study data were obtained from a prospectively maintained joint arthroplasty database that includes comprehensive documentation of all admissions and discharges of patients who have undergone procedures at one institution. The database includes data from primary admissions and readmissions. After institutional review board approval, all primary and revision TJAs performed at our institution from January 2000 to April 2011 were identified. A total of 26,415 patients undergoing primary and revision TJAs were identified from the institutional database. All patient records were reviewed to identify the type of PE prophylaxis administered. A total of 24,567 patients received postoperative pharmacologic VTE prophylaxis with warfarin (targeted international normalized ratio of 1.5–2) and 1824 patients received prophylaxis with our institutional aspirin protocol (single dose of low-dose warfarin the night of surgery followed by 325 mg aspirin twice daily). All patients receiving anticoagulation other than warfarin or aspirin were excluded (enoxaparin, 21 patients; heparin, two patients; fondaparinux, one patient). From 2000 until December 2007, all surgeons at our institution used warfarin for routine postoperative VTE prophylaxis. Beginning in January 2008, one of our surgeons began using aspirin 325 mg twice a day for VTE prophylaxis, and since then, there has been a gradual trend at our institution toward using aspirin for postoperative VTE prophylaxis. Patient demographics were obtained from hospital admission information. Comorbidities were collected using ICD-9 discharge coding.
Based on clinical suspicion for PE, an imaging workup with either chest CT or ventilation/perfusion scans was obtained. Beginning in 2008, an institutional protocol was put into effect for workup of PE to reduce the overdiagnosis of PE [45]. Under the new protocol, symptomatic patients with symptoms of tachypnea greater than 20 breaths per minute, tachycardia greater than 100 beats per minute, new onset arrhythmia, hemoptysis, dyspnea, or chest pain, receive 2 L of oxygen via nasal cannula. Pulse oximetry then is rechecked after 10 minutes, and if the oxygen saturation is less than 90%, a ventilation/perfusion scan or multidetector CT scan is obtained to rule out PE. In asymptomatic patients with oxygen saturation less than 90% with otherwise normal vital signs, 2 L of oxygen is given via nasal cannula, oxygen saturation is checked 10 minutes later, and if less than 90%, a ventilation/perfusion scan or multidetector CT scan is obtained. A positive radiologic result for PE was defined as PE seen on the CT scan, including subsegmental PEs, and high probability on ventilation/perfusion scans.
Mortality data (for purposes of 90-day mortality after hospital discharge) were obtained using the Social Security Death Index. Patients who were identified to have died within 90 days postoperatively were crossmatched to those who were identified to have had a PE develop. A detailed review of the medical record then was performed to confirm that the mortality was related to the PE.
Patients and Events
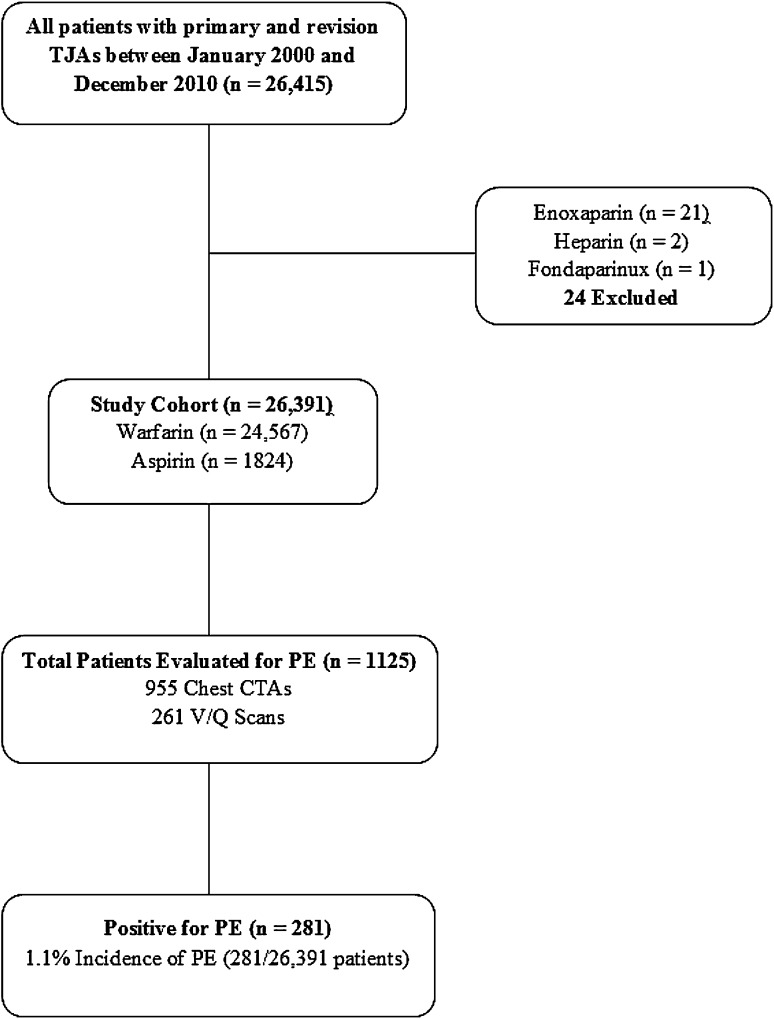
Of the 26,391 patients in the database, 1125 underwent workup for suspected PE. Overall, 1216 radiologic evaluations were performed, including 955 CT scans of the chest and 261 ventilation/perfusion scans. Diagnosis of symptomatic PE was confirmed in 281 of the 1125 patients scanned for suspected PE, yielding a symptomatic PE risk of 1.1% (281 of 26,391 patients) after TJA at our institution (Fig. 1). Two of the 24 patients who were treated with nonwarfarin or nonaspirin anticoagulation had postoperative symptomatic PEs develop. These 24 patients were excluded from the final analysis because of inconsistency in the anticoagulation protocol.
Fig. 1.
The flow diagram for our study is shown. TJA = total joint arthroplasty; PE = pulmonary embolism; CTA = computed tomography angiography; V/Q = ventilation/perfusion scan.
The mean age of the patient population was 69.4 years (range, 11.2–99.6 years) and the average BMI was 32.0 kg/m2 (range, 10.2–77.5 kg/m2) (Table 1). Two hundred forty-six of the 281 (87.5%) patients who had PEs develop were diagnosed during their initial hospital stay; the remaining 35 were diagnosed on readmission. Ninety-day all-cause mortality in our study cohort was 0.33% (86 of 26,391 patients). However, only four deaths were directly attributable to PE for a risk of 0.02%, and there were no deaths documented in our hospital records that were attributable to PE prophylaxis.
Table 1.
Multivariate analysis for pulmonary embolus risk factors
| Multivariate variable | Odds ratio/95% CI | p value |
|---|---|---|
| BMI (kg/m2) | ||
| < 25 | 1.000 (reference value) | |
| ≥ 25 and < 30 | 1.619; 0.93–2.32 | 0.04 |
| ≥ 30 and < 35 | 2.275; 1.21–3.04 | < 0.001 |
| ≥ 35 and < 40 | 2.399; 1.15–3.23 | 0.001 |
| ≥ 40 | 2.320; 0.99–3.12 | 0.003 |
| Procedure | ||
| Primary hip | 1.000 (reference value) | |
| Primary knee | 3.690; 2.27–4.54 | < 0.001 |
| Revision hip | 0.809; 0.76–2.60 | 0.400 |
| Revision knee | 2.152; 1.05–3.60 | 0.006 |
| Charlson Comorbidity Index | ||
| 0 | 1.000 (reference value) | |
| 1 | 1.743; 0.79–4.60 | 0.18 |
| 2 | 2.658; 1.01–5.59 | 0.02 |
| 3 | 3.791; 1.78–9.57 | 0.001 |
| 4 | 3.574; 1.60–9.22 | 0.002 |
| 5 | 4.476; 1.77–14.0 | 0.002 |
| 6 | 6.041; 1.89–32.6 | 0.01 |
| Bilateral procedure | ||
| Unilateral | 1.000 (reference value) | |
| Staged bilateral | 0.525; 0.28–0.70 | 0.001 |
| Simultaneous bilateral | 0.963; 0.91–1.86 | 0.96 |
| Atrial fibrillation | 2.455; 1.70–3.84 | < 0.001 |
| COPD | 1.599; 0.98–2.10 | 0.006 |
| Anemia | 1.697; 1.20–2.26 | < 0.001 |
| Presence of DVT | 19.338; 9.97–54.5 | < 0.001 |
| Connective tissue disease | 0.371; 0.12–1.23 | 0.05 |
| Depression | 1.652; 1.17–2.69 | 0.01 |
COPD = chronic obstructive pulmonary disease; DVT = deep vein thrombosis.
Statistics
All analyses were implemented in the package “rms” for the statistical language R (R Foundation for Statistical Computing, www.r-project.org). Comorbidities were identified by ICD-9 codes and were studied as risk factors for postoperative PE (Table 2). Additional risk factors studied, but not reported in Table 2, included age, BMI, race, sex, procedure, Charlson Comorbidity Index, simultaneous bilateral procedure, and type of anticoagulation (warfarin or aspirin). Chi-square and independent t-tests were used to identify the individual association of each proposed risk factor with the occurrence of PE. Backward stepwise elimination was used in a logistic regression model to analyze the association of risk factors with postoperative PE occurrence accounting for potential confounders. A p value less than 0.05 was considered statistically significant.
Table 2.
International Classification of Diseases, 9th Revision (ICD-9) coding definitions for comorbidities
| Comorbidity | ICD-9 codes |
|---|---|
| Diabetes mellitus | 249-249.79; 250-250.79 |
| Hypothyroidism | 244-244.99 |
| Ischemic heart disease | 412-414.99 |
| Congestive heart failure | 428-428.99 |
| Hypertension | 401-405.99 |
| Peripheral vascular disease | 443-443.99 |
| Atrial fibrillation | 427.3-427.31 |
| Valvulopathy | V42.2; 424-424.3 |
| Chronic obstructive pulmonary disease | 490-496.99 |
| Smoking history | V15.82; 305.1 |
| Sleep apnea | 327.23; 780.51; 780.57 |
| Anemia | 280-285.99 |
| Presence of deep vein thrombosis | 453.4-453.49 |
| History of venous thromboembolism | V12.51; V12.55 |
| History of malignancy | V10-V10.99 |
| Active malignancy | 140-208.99 |
| Leukemia | V10.6-V10.69; 204-208.99 |
| Lymphoma | 200-202.99 |
| HIV | V08-V08.99; V42-V42.99; 42-42.99; 79.53; 795.71 |
| Connective tissue disease | 710-710.99; 714-714.99 |
| Crystalline arthropathy | 274-274.99; 712.1-712.3 |
| Renal disease | V56-V56.99 |
| Dialysis | V45.1-V45.12 |
| Benign prostatic hypertrophy | 600-600.01 |
| Liver disease | 570-573.99 |
| Cerebrovascular disease | V12.54; V17.1; 435-435.99; 437-438.99 |
| Depression | V79.0;296.2-296.39; 311-311.99 |
| Anxiety | 300-300.02 |
| Peptic ulcer disease | 531-533.99 |
| Gastrointestinal reflux disease | 530.11; 530.81 |
| Diverticulosis or gastrointestinal bleeding | 562-562.99; 578-578.99 |
| Inflammatory bowel disease | 555-556.99 |
A nomogram was created using a logistic regression model to predict the risk of PE. Initially, logistic regression models were built using the full set of possible predictive variables. This full model then was pruned using a backward elimination algorithm that removed variables with little impact on the prediction. Finally, penalized maximum likelihood estimation was used to constrain the regression coefficients to improve reliability of the model [41]. These steps are intended to prevent overfitting in the model and improve predictive reliability. Internal validation tools based on bootstrapping were used to confirm that the final logistic regression model was not unduly optimistic. A nomogram was generated using the coefficients of the final model to estimate a linear predictor and map the linear predictor to estimated probabilities of the event. The regression coefficients used to create the nomogram indicated independent effect that each risk factor contributed to the risk of symptomatic PE, relative to one another. We used the relative size of the regression coefficients for each independent risk factor to create a whole number system that was clinically feasible to use.
Results
Pulmonary Embolism Risk by Procedure
The risk of symptomatic PE after primary THA was 0.4% (49 of 12,017 procedures), 1.8% after primary TKA (197 of 10,844 procedures), 0.7% after revision hip arthroplasty (16 of 2188 procedures), and 1.4% after revision knee arthroplasty (19 of 1340 procedures). Furthermore, the risk of symptomatic PE after unilateral procedures was 1.1% (202 of 18,612 procedures), 0.7% after staged bilateral TJAs (31 of 4563 procedures), and 1.5% after simultaneous bilateral TJAs (48 of 3216 procedures).
Risk Factors for Pulmonary Embolism
Elevated BMI, procedures on the knee (especially bilateral procedures), higher Charlson Comorbidity Index, chronic obstructive pulmonary disease, atrial fibrillation, anemia, depression, and presence of postoperative deep vein thrombosis were identified as independent risk factors for symptomatic PE (based on the multivariate analysis; Table 3). A univariate analysis found a longer list of potential risk factors (Table 1); however, because of the likelihood of confounding, the multivariate analysis should be considered more definitive.
Table 3.
Preoperative risk factors for pulmonary embolism
| Variable | No pulmonary embolism (N = 26,110) | Pulmonary embolism (N = 281) | p value |
|---|---|---|---|
| Age (years) | 64.0 ± 12.2 | 68.8 ± 10.3 | < 0.001 |
| BMI (kg/m2) | 30.1 ± 6.7 | 31.6 ± 6.3 | 0.001 |
| Sex | |||
| Male | 11,261 (43.1%) | 97(34.5%) | 0.004 |
| Female | 14,849 (56.9%) | 184 (65.5%) | |
| Race | |||
| White | 19,355 (74.1%) | 216 (76.9%) | 0.17 |
| Black | 2781 (10.7%) | 36 (12.8%) | |
| Hispanic | 153 (0.6%) | 1 (0.4%) | |
| Asian | 109 (0.4%) | 0 (0.0%) | |
| Other | 3712 (14.2%) | 28 (10.0%) | |
| Procedure | |||
| Primary hip | 11,968 (45.8%) | 49 (17.4%) | |
| Primary knee | 10,647 (40.8%) | 197 (70.1%) | |
| Revision hip | 2172 (8.3%) | 16 (5.7%) | < 0.001 |
| Revision knee | 1321 (5.1%) | 19 (6.8%) | |
| ASA class | |||
| 1 | 565 (3.4%) | 1 (0.4%) | |
| 2 | 7521 (44.7%) | 78 (33.6%) | < 0.001 |
| 3 | 8610 (51.1%) | 151 (65.1%) | |
| 138 (0.8%) | 2 (0.9%) | ||
| Charlson Comorbidity Index | |||
| 0 | 2844 (10.9%) | 7 (2.5%) | |
| 1 | 5727 (21.9%) | 35 (12.5%) | |
| 2 | 7085 (27.1%) | 73 (26.0%) | |
| 3 | 6352 (24.3%) | 98 (34.9%) | < 0.001 |
| 4 | 3270 (12.5%) | 52 (18.5%) | |
| 5 | 628 (2.4%) | 13 (4.6%) | |
| 6 | 138 (0.5%) | 3 (1.1%) | |
| ≥ 7 | 66 (0.4%) | 0 (0.0%) | |
| Bilateral procedure | |||
| Unilateral | 18,410 (70.5%) | 202 (71.9%) | 0.002 |
| Staged bilateral (within 1 year) | 4532 (17.4%) | 31 (11.0%) | |
| Simultaneous bilateral | 3168 (12.1%) | 48 (17.1%) | |
| Anticoagulation | |||
| Warfarin | 24,290 (93.0%) | 277 (98.6%) | < 0.01 |
| Aspirin | 1820 (7.0%) | 4 (1.4%) | |
| Diabetes mellitus | |||
| Yes | 2997 (11.5%) | 48 (17.1%) | 0.003 |
| No | 23,113 (88.5%) | 233 (82.9%) | |
| Hypothyroidism | |||
| Yes | 2857 (10.9%) | 35 (12.5%) | 0.42 |
| No | 23,253 (89.1%) | 246 (87.5%) | |
| Ischemic heart disease | |||
| Yes | 3110 (11.9%) | 45 (16.0%) | 0.035 |
| No | 23,000 (88.1%) | 236 (84.0%) | |
| Congestive heart failure | |||
| Yes | 402 (1.5%) | 10 (3.6%) | 0.007 |
| No | 25,708 (98.5%) | 271 (96.4%) | |
| Hypertension | |||
| Yes | 12,555 (48.1%) | 166 (59.1%) | < 0.001 |
| No | 13,555 (51.9%) | 115 (40.9%) | |
| Peripheral vascular disease | |||
| Yes | 229 (0.9%) | 1 (0.4%) | 0.35 |
| No | 25,881 (99.1%) | 280 (99.6%) | |
| Atrial fibrillation | |||
| Yes | 1164 (4.5%) | 39 (13.9%) | < 0.001 |
| No | 24,946 (95.5%) | 242 (86.1%) | |
| Valvulopathy | |||
| Yes | 1123 (4.3%) | 10 (3.6%) | 0.54 |
| No | 24,987 (95.7%) | 271 (96.4%) | |
| Chronic obstructive pulmonary disease | |||
| Yes | 2514 (9.6%) | 44 (15.7%) | 0.001 |
| No | 23,596 (90.4%) | 237 (84.3%) | |
| Smoking history | |||
| Yes | 693 (2.7%) | 9 (3.2%) | 0.57 |
| No | 25,417 (97.3%) | 272 (96.8%) | |
| Sleep apnea | |||
| Yes | 2310 (8.8%) | 35 (12.5%) | 0.03 |
| No | 23,800 (91.2%) | 246 (87.5%) | |
| Anemia | |||
| Yes | 4152 (15.9%) | 68 (24.2%) | < 0.001 |
| No | 25,881 (99.1%) | 280 (99.6%) | |
| Presence of deep vein thrombosis | |||
| Yes | 40 (0.2%) | 11 (3.9%) | < 0.001 |
| No | 26,070 (99.8%) | 270 (96.1%) | |
| History of venous thromboembolism | |||
| Yes | 649 (2.5%) | 8 (2.8%) | 0.70 |
| No | 25,461 (97.5%) | 273 (97.2%) | |
| History of malignancy | |||
| Yes | 2486 (9.5%) | 32 (11.4%) | 0.29 |
| No | 23,624 (90.5%) | 249 (88.6%) | |
| Active malignancy | |||
| Yes | 322 (1.2%) | 2 (0.7%) | 0.43 |
| No | 25,788 (98.8%) | 279 (99.3%) | |
| Leukemia | |||
| Yes | 55 (0.2%) | 0 (0.0%) | 0.44 |
| No | 26,055 (99.8%) | 281 (100.0%) | |
| Lymphoma | |||
| Yes | 54 (0.9%) | 0 (0.0%) | 0.45 |
| No | 26,056 (99.8%) | 281 (100.0%) | |
| HIV | |||
| Yes | 188 (0.7%) | 2 (0.7%) | 0.99 |
| No | 25,922 (99.3%) | 279 (99.3%) | |
| Connective tissue disease | |||
| Yes | 808 (3.1%) | 4 (1.4%) | 0.11 |
| No | 25,302 (96.9%) | 277 (98.6%) | |
| Crystalline arthropathy | |||
| Yes | 547 (2.1%) | 5 (1.8%) | 0.71 |
| No | 25,563 (97.9%) | 276 (98.2%) | |
| Renal disease | |||
| Yes | 43 (0.2%) | 0 (0.0%) | 0.50 |
| No | 26,067 (99.8%) | 281 (100.0%) | |
| Dialysis | |||
| Yes | 14 (0.1%) | 0 (0.0%) | 0.70 |
| No | 26,096 (99.9%) | 281 (100.0%) | |
| Benign prostatic hypertrophy | |||
| Yes | 1186 (4.5%) | 14 (5.0%) | 0.73 |
| No | 24,924 (95.5%) | 267 (95.0%) | |
| Liver disease | |||
| Yes | 103 (0.4%) | 0 (0.0%) | 0.29 |
| No | 26,007 (99.6%) | 281 (100.0%) | |
| Cerebrovascular disease | |||
| Yes | 311 (1.2%) | 5 (1.8%) | 0.37 |
| No | 25,799 (98.8%) | 276 (98.2%) | |
| Depression | |||
| Yes | 1688 (6.5%) | 30 (10.7%) | 0.004 |
| No | 24,422 (93.5%) | 251 (89.3%) | |
| Anxiety | |||
| Yes | 1132 (4.3%) | 15 (5.3%) | 0.41 |
| No | 24,978 (95.7%) | 266 (94.7%) | |
| Peptic ulcer disease | |||
| Yes | 125 (0.5%) | 3 (1.1%) | 0.16 |
| No | 25,985 (99.5%) | 278 (98.9%) | |
| Gastrointestinal reflux disease | |||
| Yes | 6087 (23.3%) | 79 (28.1%) | 0.06 |
| No | 20,023 (76.7%) | 202 (71.9%) | |
| Diverticulosis or gastrointestinal bleeding | |||
| Yes | 230 (0.9%) | 2 (0.7%) | 0.76 |
| No | 25,880 (99.1%) | 279 (99.3%) | |
| Inflammatory bowel disease | |||
| Yes | 119 (0.5%) | 1 (0.4%) | 0.80 |
| No | 25,991 (99.5%) | 280 (99.6%) | |
ASA = American Society of Anesthesiologists.
Patients receiving postoperative 325 mg aspirin twice a day for prophylaxis had a symptomatic PE rate of 0.2% (four of 1824 patients). This was significantly lower (p < 0.01) than the rate of 1.1% (277 of 24,567 patients) for patients receiving warfarin with a target international normalized ratio of 1.5 to 1.8. However, after accounting for confounding variables, anticoagulation was not found to be an independent predictor of the occurrence of symptomatic PE.
Nomogram of Risk for Pulmonary Embolism
A nomogram was created using a logistic regression model to predict the risk of symptomatic PE (Table 4). Low-risk patients (0.35% cumulative risk; range, 0%–0.7%) are classified as those with a score of 0 to 6 points, medium-risk patients (1.4% cumulative risk; range, 1.1%–2.8%) as those with a score between 7 and 15 points, and high-risk patients (9.3% cumulative risk; range, 4.3%–66.7%) as those with a score of 16 points or greater (Table 5).
Table 4.
Pulmonary embolus risk stratification criteria
| Risk factor | Points for each risk factor |
|---|---|
| Knee surgery | 5 |
| Charlson Comorbidity Index (CCI) | 1 × CCI |
| Atrial fibrillation | 4 |
| Postoperative deep vein thrombosis | 14 |
| Chronic obstructive pulmonary disease | 2 |
| Anemia | 2 |
| Depression | 2 |
| BMI (kg/m2) | |
| ≤ 25 | 0 |
| 25–30 | 1 |
| > 30 | 2 |
Table 5.
Risk of PE based on risk group
| Risk group | Total score | Number of patients | Number of PEs | Risk of PE | Patients in each risk group (% of total patients) | PEs in each risk group (% of total PEs) | Cumulative risk in each group |
|---|---|---|---|---|---|---|---|
| Low | 0 | 743 | 0 | 0% | |||
| 1 | 1423 | 0 | 0% | ||||
| 2 | 2382 | 7 | 0.3% | ||||
| 3 | 2866 | 10 | 0.3% | 13,324 (50.4) | 47 (16.7) | 0.35% | |
| 4 | 2534 | 7 | 0.3% | ||||
| 5 | 1709 | 11 | 0.6% | ||||
| 6 | 1667 | 12 | 0.7% | ||||
| Medium | 7 | 2094 | 22 | 1.1% | |||
| 8 | 2847 | 34 | 1.2% | ||||
| 9 | 2934 | 37 | 1.3% | ||||
| 10 | 2062 | 34 | 1.6% | ||||
| 11 | 1048 | 17 | 1.6% | ||||
| 12 | 545 | 14 | 2.6% | 12,474 (47.3) | 179 (63.7) | 1.4% | |
| 13 | 373 | 9 | 2.4% | ||||
| 14 | 318 | 5 | 1.6% | ||||
| 15 | 253 | 7 | 2.8% | ||||
| High | 16 | 187 | 8 | 4.3% | |||
| 17 | 138 | 8 | 5.8% | ||||
| 18 | 56 | 1 | 1.8% | ||||
| 19 | 20 | 1 | 5.0% | ||||
| 20 | 22 | 1 | 4.5% | ||||
| 21 | 27 | 6 | 22.2% | ||||
| 22 | 30 | 7 | 23.3% | ||||
| 23 | 33 | 4 | 12.1% | 593 (2.3) | 55 (19.6) | 9.3% | |
| 24 | 37 | 8 | 21.6% | ||||
| 25 | 21 | 5 | 23.8% | ||||
| 26 | 7 | 2 | 28.6% | ||||
| 27 | 4 | 1 | 25.0% | ||||
| 28 | 3 | 2 | 66.7% | ||||
| 29 | 1 | 0 | 0% | ||||
| 30 | 5 | 1 | 20.0% | ||||
| 32 | 2 | 0 | 0% |
PE = pulmonary embolism.
Discussion
The risk of VTE, mainly PEs and fatal PEs, is the impetus for administration of anticoagulation agents after TJA [2, 5–8, 10, 13, 16, 18–21, 24, 26, 30, 44]. Unfortunately, the administration of chemical prophylaxis is associated with many complications such as increased hematoma formation, wound-related problems, and infection [31, 32, 36]. Sharrock et al. [39] reported that all-cause mortality is greater in patients who received aggressive anticoagulation after TJA. It therefore would be appealing to have a strategy that allows for individualization of postoperative anticoagulation for patients undergoing TJAs based on their risk for the development of PE. To develop such a strategy, we must identify the factors that place patients at the greatest risk for PE. We have provided identification of such risk factors in patients who received routine warfarin or aspirin prophylaxis.
Our study has some limitations. First, this study was limited to the evaluation of symptomatic PEs. We may have missed subclinical PEs in asymptomatic patients. However, we felt that it would have not been possible or medically appropriate to screen all patients for PE. Second, comorbidities were identified using ICD-9 coding data from discharge records at one institution. These records are assumed to be complete and accurate because they generally report known preoperative diagnoses, but underreporting of diagnoses can occur. Third, another shortcoming is that the study design only identifies patients who had their PE diagnosed at this institution and, therefore, misses those who had a PE identified elsewhere. Nonetheless it is reasonable that nearly all of the PEs that occurred were captured, especially given that the majority of PEs occur during the immediate postoperative period [1, 12]. Fourth, 90-day mortality was determined using the Social Security Death Index. Of the 86 patients who died during the postoperative period, we were able to confirm only who four died owing to PE, and no death was directly attributable to PE prophylaxis. However, it was not possible to identify cause of death if the patient died after discharge. Although our rate of documented fatal postoperative PEs is low at 0.02%, a recent meta-analysis by Poultsides et al. showed that non-PE cardiac causes were the leading cause of death after TJA, with PE as the second leading cause at a 0.1% rate of fatal postoperative PEs with pharmacologic prophylaxis [34]. Fifth, in 2008, a new PE screening protocol was instituted at our institution to avoid overdiagnosis of subclinical PEs, and we gradually began to transition from warfarin to aspirin for VTE prophylaxis. It is unclear how these changes affected the risk of PE, but we did not see any increase in the risk of symptomatic PE with use of aspirin for VTE prophylaxis.
A major strength of this study was that all PEs were identified and verified by reviewing radiologic reports and not simply by relying on ICD-9 coding data. When only ICD-9 coding data (ICD-9 codes 415, 415.11, 415.19, 451, 451.0, 451.11, 451.19, 451.2, 451.81, 453, and 453.9) were reviewed, only 205 of the total 283 postoperative PE events were identified. This highlights the potential significant underreporting of adverse events when only ICD-9 coding from databases is used.
Patients with greater BMI, multiple medical comorbidities (as reflected by an elevated Charlson Comorbidity Index), chronic obstructive pulmonary disease, hypertension, and history of atrial fibrillation were more likely to have a PE develop after TJA. There are theoretical bases for the associations between some of these conditions and VTE. Patients with chronic obstructive pulmonary disease, for example, are more likely to have inflammation of the pulmonary vessels and/or experience pulmonary hypertension that leads to stagnation of blood in the inflamed or damaged vessels and subsequent thrombus formation [33]. Patients with arrhythmia such as atrial fibrillation have a high risk of thrombi developing in cardiac atria leading to elevated rates of PE and stroke [29]. Patients with postoperative deep vein thrombosis formation are, as expected, at higher risk for PEs. This is likely the result of the contribution of deep vein thrombosis to embolic phenomena such as PE. Obesity and elevated comorbidity index have been shown to be associated with an increased risk of VTE [9, 14, 17, 40]. This may be secondary to an inflammatory state in these patients that may contribute to increased thrombus formation and subsequent embolization [9, 40]. Simultaneous bilateral TJAs also have been suggested as a higher risk for the development of PE when compared with unilateral TJA [35]. This may be the result of multiple factors, including the difficulty of early mobilization and a higher embolic load from intramedullary debris from bilateral surgery. Other risk factors for PE development such as preoperative anemia and depression are not as easily explained. Subsets of anemia including sickle cell, hemolytic, and even iron deficiency anemia have been associated with PEs [27, 28, 42]. Despite this, it is unclear whether anemia has a direct mechanism to lead to increased risk of PE. Depression has been suggested to be associated with PE. Schulte et al. found depression to be an independent risk factor of PE after spinal surgery [38]. Furthermore, in the psychiatric literature, studies have suggested an association between the lack of physical activity in patients in catatonic and depressive states to development of VTE [15, 23]. Patients with depression may experience self-neglect, which may have resulted in noncompliance with anticoagulation prophylaxis or lack of mobility, both of which may have increased the risk of PE.
Although a goal of our study was to identify risk factors associated with postoperative symptomatic PE development in patients who have undergone TJA, the ultimate purpose of this study is to use this information in making clinical choices regarding patients undergoing TJAs. Therefore, using a logistic regression model, the risk factors identified in this study have been ranked according to their relative risk (Table 4). Using these scoring criteria, three clinically significant risk groups (low, medium, and high) have been created. The application of these relative risk criteria to individual patients may help to determine the type of postoperative anticoagulation that best balances the overall risk of the development of PE with that of postsurgical bleeding.
We found that patients who have an elevated BMI, Charlson Comorbidity Index, chronic obstructive pulmonary disease, atrial fibrillation, postoperative deep vein thrombosis, or anemia, and patients who undergo knee surgery (particularly bilateral knee surgery), are at greater risk of having a postoperative PE develop. These risks should be considered when deciding on postoperative anticoagulation prophylaxis. Further studies must be done to validate the PE risk stratification criteria presented here. These criteria, together with similar risk stratification for postoperative bleeding risk, ultimately can help to determine the appropriate postoperative PE prophylaxis on an individualized basis for patients undergoing TJAs.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bjornara BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88:386–391. doi: 10.1302/0301-620X.88B3.17207. [DOI] [PubMed] [Google Scholar]
- 2.Blom A, Pattison G, Whitehouse S, Taylor A, Bannister G. Early death following primary total hip arthroplasty: 1,727 procedures with mechanical thrombo-prophylaxis. Acta Orthop. 2006;77:347–350. doi: 10.1080/17453670610046244. [DOI] [PubMed] [Google Scholar]
- 3.Caprini JA, Arcelus JI, Hasty JH, Tamhane AC, Fabrega F. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. 1991;17(suppl 3):304–312. [PubMed] [Google Scholar]
- 4.Comp PC, Spiro TE, Friedman RJ, Whitsett TL, Johnson GJ, Gardiner GA, Jr, Landon GC, Jove M, Enoxaparin Clinical Trial Group Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement. Enoxaparin Clinical Trial Group. J Bone Joint Surg Am. 2001;83:336–345. doi: 10.2106/00004623-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Edelsberg J, Ollendorf D, Oster G. Venous thromboembolism following major orthopedic surgery: review of epidemiology and economics. Am J Health Syst Pharm. 2001;58(suppl 2):S4–13. [DOI] [PubMed]
- 6.Fender D, Harper WM, Thompson JR, Gregg PJ. Mortality and fatal pulmonary embolism after primary total hip replacement: results from a regional hip register. J Bone Joint Surg Br. 1997;79:896–899. doi: 10.1302/0301-620X.79B6.7677. [DOI] [PubMed] [Google Scholar]
- 7.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):381S–453S. [DOI] [PubMed]
- 8.Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA, Jr, Wheeler HB. Prevention of venous thromboembolism. Chest. 2001;119(1 suppl):132S–175S. doi: 10.1378/chest.119.1_suppl.132S. [DOI] [PubMed] [Google Scholar]
- 9.Goldhaber SZ, Savage DD, Garrison RJ, Castelli WP, Kannel WB, McNamara PM, Gherardi G, Feinleib M. Risk factors for pulmonary embolism: The Framingham Study. Am J Med. 1983;74:1023–1028. doi: 10.1016/0002-9343(83)90805-7. [DOI] [PubMed] [Google Scholar]
- 10.Guijarro R, Montes J, San Roman C, Arcelus JI, Barillari G, Granero X, Monreal M. Venous thromboembolism and bleeding after total knee and hip arthroplasty: findings from the Spanish National Discharge Database. Thromb Haemost. 2011;105:610–615. [DOI] [PubMed]
- 11.Harris WH, McKusick K, Athanasoulis CA, Waltman AC, Strauss HW. Detection of pulmonary emboli after total hip replacement using serial C15O2 pulmonary scans. J Bone Joint Surg Am. 1984;66:1388–1393. [PubMed] [Google Scholar]
- 12.Hope WW, Demeter BL, Newcomb WL, Schmelzer TM, Schiffern LM, Heniford BT, Sing RF. Postoperative pulmonary embolism: timing, diagnosis, treatment, and outcomes. Am J Surg. 2007;194:814–818; discussion 818–819. [DOI] [PubMed]
- 13.Howie C, Hughes H, Watts AC. Venous thromboembolism associated with hip and knee replacement over a ten-year period: a population-based study. J Bone Joint Surg Br. 2005;87:1675–1680. doi: 10.1302/0301-620X.87B12.16298. [DOI] [PubMed] [Google Scholar]
- 14.Huber O, Bounameaux H, Borst F, Rohner A. Postoperative pulmonary embolism after hospital discharge: an underestimated risk. Arch Surg. 1992;127:310–313. doi: 10.1001/archsurg.1992.01420030076014. [DOI] [PubMed] [Google Scholar]
- 15.Ignatowski M, Sidhu S, Rueve M. Pulmonary embolism as a complication of major depressive disorder with catatonic features: a case report. Psychiatry (Edgmont). 2007;4:51–56. [PMC free article] [PubMed] [Google Scholar]
- 16.Johanson NA, Lachiewicz PF, Lieberman JR, Lotke PA, Parvizi J, Pellegrini V, Stringer TA, Tornetta P, 3rd, Haralson RH, 3rd, Watters WC., 3rd American Academy of Orthopaedic Surgeons clinical practice guideline on prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. J Bone Joint Surg Am. 2009;91:1756–1757. doi: 10.2106/JBJS.I.00511. [DOI] [PubMed] [Google Scholar]
- 17.Josa M, Siouffi SY, Silverman AB, Barsamian EM, Khuri SF, Sharma GV. Pulmonary embolism after cardiac surgery. J Am Coll Cardiol. 1993;21:990–996. doi: 10.1016/0735-1097(93)90358-8. [DOI] [PubMed] [Google Scholar]
- 18.Katz JN, Losina E, Barrett J, Phillips CB, Mahomed NN, Lew RA, Guadagnoli E, Harris WH, Poss R, Baron JA. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83:1622–1629. doi: 10.1302/0301-620X.83B3.10487. [DOI] [PubMed] [Google Scholar]
- 19.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 20.Lachiewicz PF. Prevention of symptomatic pulmonary embolism in patients undergoing total hip and knee arthroplasty: clinical guideline of the American Academy of Orthopaedic Surgeons. Instr Course Lect. 2009;58:795–804. [PubMed] [Google Scholar]
- 21.MacDougall DA, Feliu AL, Boccuzzi SJ, Lin J. Economic burden of deep-vein thrombosis, pulmonary embolism, and post-thrombotic syndrome. Am J Health Syst Pharm. 2006;63(20 suppl 6):S5–S15. doi: 10.2146/ajhp060388. [DOI] [PubMed] [Google Scholar]
- 22.Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96:1140–1146. doi: 10.1097/00000542-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 23.McCall WV, Mann SC, Shelp FE, Caroff SN. Fatal pulmonary embolism in the catatonic syndrome: two case reports and a literature review. J Clin Psychiatry. 1995;56:21–25. [PubMed] [Google Scholar]
- 24.Mohr DN, Silverstein MD, Ilstrup DM, Heit JA, Morrey BF. Venous thromboembolism associated with hip and knee arthroplasty: current prophylactic practices and outcomes. Mayo Clin Proc. 1992;67:861–870. doi: 10.1016/S0025-6196(12)60825-8. [DOI] [PubMed] [Google Scholar]
- 25.Mont MA, Jones LC, Rajadhyaksha AD, Shuler MS, Hungerford DS, Sieve-Smith L, Wang P, Cordista AG, Glueck CJ. Risk factors for pulmonary emboli after total hip or knee arthroplasty. Clin Orthop Relat Res. 2004;422:154–163. doi: 10.1097/01.blo.0000128971.35014.31. [DOI] [PubMed] [Google Scholar]
- 26.Neviaser AS, Chang C, Lyman S, Della Valle AG, Haas SB. High incidence of complications from enoxaparin treatment after arthroplasty. Clin Orthop Relat Res. 2010;468:115–119. doi: 10.1007/s11999-009-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicastro N, Schnider A, Leemann B. Iron-deficiency anemia as a rare cause of cerebral venous thrombosis and pulmonary embolism. Case Rep Med. 2012;2012:497814. doi: 10.1155/2012/497814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novelli EM, Huynh C, Gladwin MT, Moore CG, Ragni MV. Pulmonary embolism in sickle cell disease: a case-control study. J Thromb Haemost. 2012;10:760–766. doi: 10.1111/j.1538-7836.2012.04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogren M, Bergqvist D, Eriksson H, Lindblad B, Sternby NH. Prevalence and risk of pulmonary embolism in patients with intracardiac thrombosis: a population-based study of 23 796 consecutive autopsies. Eur Heart J. 2005;26:1108–1114. doi: 10.1093/eurheartj/ehi130. [DOI] [PubMed] [Google Scholar]
- 30.Ollendorf DA, Vera-Llonch M, Oster G. Cost of venous thromboembolism following major orthopedic surgery in hospitalized patients. Am J Health Syst Pharm. 2002;59:1750–1754. doi: 10.1093/ajhp/59.18.1750. [DOI] [PubMed] [Google Scholar]
- 31.Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24–28. doi: 10.1016/j.arth.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89:33–38. doi: 10.2106/JBJS.F.00163. [DOI] [PubMed] [Google Scholar]
- 33.Peinado VI, Pizarro S, Barbera JA. Pulmonary vascular involvement in COPD. Chest. 2008;134:808–814. doi: 10.1378/chest.08-0820. [DOI] [PubMed] [Google Scholar]
- 34.Poultsides LA, Gonzalez Della Valle A, Memtsoudis SG, Ma Y, Roberts T, Sharrock N, Salvati E. Meta-analysis of cause of death following total joint replacement using different thromboprophylaxis regimens. J Bone Joint Surg Br. 2012;94:113–121. [DOI] [PubMed]
- 35.Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2007;89:1220–1226. doi: 10.2106/JBJS.F.01353. [DOI] [PubMed] [Google Scholar]
- 36.Sachs RA, Smith JH, Kuney M, Paxton L. Does anticoagulation do more harm than good?: A comparison of patients treated without prophylaxis and patients treated with low-dose warfarin after total knee arthroplasty. J Arthroplasty. 2003;18:389–395. doi: 10.1016/S0883-5403(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 37.Schiff RL, Kahn SR, Shrier I, Strulovitch C, Hammouda W, Cohen E, Zukor D. Identifying orthopedic patients at high risk for venous thromboembolism despite thromboprophylaxis. Chest. 2005;128:3364–3371. doi: 10.1378/chest.128.5.3364. [DOI] [PubMed] [Google Scholar]
- 38.Schulte LM, O’Brien JR, Bean MC, Pierce TP, Yu WD, Meals C. Deep vein thrombosis and pulmonary embolism after spine surgery: incidence and patient risk factors. Am J Orthop (Belle Mead NJ). 2013;42:267–270. [PubMed] [Google Scholar]
- 39.Sharrock NE, Gonzalez Della Valle A, Go G, Lyman S, Salvati EA. Potent anticoagulants are associated with a higher all-cause mortality rate after hip and knee arthroplasty. Clin Orthop Relat Res. 2008;466:714–721. [DOI] [PMC free article] [PubMed]
- 40.Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118:978–980. doi: 10.1016/j.amjmed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Steyerberg EW, Eijkemans MJ, Harrell FE, Jr, Habbema JD. Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Med Decis Making. 2001;21:45–56. doi: 10.1177/0272989X0102100106. [DOI] [PubMed] [Google Scholar]
- 42.Thachil J. Autoimmune haemolytic anaemia: an under-recognized risk factor for venous thromboembolism. Transfus Med. 2008;18:377–378. doi: 10.1111/j.1365-3148.2008.00890.x. [DOI] [PubMed] [Google Scholar]
- 43.White RH, Gettner S, Newman JM, Trauner KB, Romano PS. Predictors of rehospitalization for symptomatic venous thromboembolism after total hip arthroplasty. N Engl J Med. 2000;343:1758–1764. doi: 10.1056/NEJM200012143432403. [DOI] [PubMed] [Google Scholar]
- 44.White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158:1525–1531. doi: 10.1001/archinte.158.14.1525. [DOI] [PubMed] [Google Scholar]
- 45.Winters BS, Solarz M, Jacovides CL, Purtill JJ, Rothman RH, Parvizi J. Overdiagnosis of pulmonary embolism: evaluation of a hypoxia algorithm designed to avoid this catastrophic problem. Clin Orthop Relat Res. 2012;470:497–502. doi: 10.1007/s11999-011-2109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]