Abstract
Background/Aims
The prognosis of pancreatic adenocarcinoma (PAC) is poor. The serum carbohydrate antigen 19-9 (CA 19-9) level has been identified as a prognostic indicator of recurrence and reduced overall survival. The aim of this study was to identify preoperative prognostic factors and to create a prognostic model able to assess the early recurrence risk for patients with resectable PAC.
Methods
A series of 177 patients with PAC treated surgically at the St. Andrea Hospital of Rome between January 2003 and December 2011 were reviewed retrospectively. Univariate and multivariate analyses were utilized to identify preoperative prognostic indicators.
Results
A preoperative CA 19-9 level >228 U/mL, tumor size >3.1 cm, and the presence of pathological preoperative lymph nodes statistically correlated with early recurrence. Together, these three factors predicted the possibility of an early recurrence with 90.4% accuracy. The combination of these three preoperative conditions was identified as an independent parameter for early recurrence based on multivariate analysis (p=0.0314; hazard ratio, 3.9811; 95% confidence interval, 1.1745 to 15.3245).
Conclusions
PAC patient candidates for surgical resection should undergo an assessment of early recurrence risk to avoid unnecessary and ineffective resection and to identify patients for whom palliative or alternative treatment may be the treatment of choice.
Keywords: Pancreatic neoplasms, Early recurrence, Preoperative CA 19-9, Prognosis, Pancreatic adenocarcinoma
INTRODUCTION
Pancreatic adenocarcinoma (PAC) is an extremely aggressive tumor; as such, it is the fourth leading cause of cancer-related death in the United States.1 In 2008, the estimated incidence in the United States was 37,700 cases while an estimated 34,300 patients died of the disease.2 Worldwide, over 200,000 people die annually of pancreatic cancer.3 Most patients with pancreatic cancer present with locally advanced or metastatic disease upon diagnosis. Only 10% to 20% of patients are candidates for curative surgery.4 The only curative treatment is radical surgical resection; yet, unfortunately, one fourth of patients present with unresectable tumors at the time of surgery.5 Surgical resection, with or without adjuvant therapy, is associated with a median survival of 11 to 23 months and with a 5-year survival of about 20%.6 Pancreatic surgical resections are technically demanding procedures, associated with high postoperative morbidity and mortality rates (about 30% to 50% and 5% to 10%, respectively).7 The prognosis of patients with resectable PAC remains poor despite improvements in surgical management and adjuvant therapy, as 60% of patients experience local and systemic relapse during the first 12 months after surgery.8
A preoperative prognostic assessment is crucial in order to estimate tumor resectability, choose appropriate neoadjuvant chemoradiotherapy, and avoid unnecessary laparotomies or delays in systemic chemoradiotherapy. Carbohydrate antigen 19-9 (CA 19-9) is the most accurate serum biomarker for pancreatic cancer available and has shown some utility as diagnostic and prognostic marker.9 Several studies have shown that high levels of preoperative CA 19-9 (>300 U/mL) correlate with advanced disease.10 Additional evidence indicates that serum CA 19-9 is an independent predictor of both recurrence and reduced survival after resection and that, furthermore, its levels correlate with tumor burden and metastatic disease. Patients with resectable tumors have significantly lower CA 19-9 levels than those with unresectable tumors.11
The aim of the study is: 1) to evaluate whether CA 19-9 can serve as prognostic preoperative parameter able to predict early recurrence, 2) to create a prognostic preoperative model (utilizing the CA 19-9 and other preoperative parameters) able to determine the risk of early recurrence in patients undergoing to surgical pancreatic resection.
MATERIALS AND METHODS
1. Patients, preoperative parameters, and surgical procedures
A series of 177 consecutive patients undergoing resection for PAC between January 2003 and December 2011 at the St. Andrea Hospital, University of Rome, were reviewed retrospectively. Demographic characteristics, clinical findings, details of the surgical procedures, and histopathological parameters are outlined in Table 1.
Table 1.
Baseline Characteristics of Patients with Pancreatic Adenocarcinoma

CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen 19-9.
If tumors were located in the pancreatic head or uncus, a pancreaticoduodenectomy (either pylorus-preserving or the classic Whipple procedure) was performed. Distal pancreatectomy or splenopancreatectomy was the procedure of choice for tumors located in the pancreatic body or tail, along with standard lymphadenectomy (level I lymph nodes). Pancreatojejunostomy was routinely performed for reconstruction after pancreaticoduodenectomy and was carried out by anastomosing the pancreatic parenchyma and the jejunum in an end-to-side, single-layer, full-thickness anastomosis. Superior mesenteric-portal vein resection was performed in cases of suspected infiltration of the portal vein, either preoperatively or intraoperatively. The operative specimen underwent standard histopathological evaluation by an experienced pathologist. The residual tumor classification of the Union for International Cancer Control was used to define the radicality of resection; R2 cases were excluded from the analysis.
Several preoperative variables were analyzed: tumor size; preoperative lymph nodes metastases; vascular infiltration; and preoperative CA 19-9, carcinoembryonic antigen (CEA), and total bilirubin. Data were collected, recorded in a database, and analyzed. Patients who underwent to chemoradiotherapy before surgery were not considered. Patients were followed at regular intervals after discharge, either by clinical examination or by contacting their general practitioner and/or oncologist to obtain information about progression of the disease or cancer-related death. Report of local recurrence or distant metastasis was confirmed histologically or radiographically. Early recurrence was defined as the radiographic evidence of tumor tissue around the surgical site or the appearance of distal metastases within 6 months of surgical treatment.
Several studies have shown that high levels of CA 19-9 correlate with advanced disease;10 however, levels of this marker are also elevated in a broad range of benign and malignant conditions including cholecystitis, choledocholithiasis, hepatitis, cirrhosis, pancreatitis, achalasia, inflammatory bowel disease, rheumatoid arthritis, Hashimoto thyroiditis, cholangiocarcinoma, and colorectal, hepatocellular, esophageal, lung, and ovarian cancer.12 CA 19-9 levels may be artificially elevated in a setting of cholestasis and correcting it in relation to bilirubin may improve its applicability.9 We controlled for bilirubin levels, as increased cholestasis falsely elevates CA 19-9 levels, likely a result of the decreased capacity of a cholestatic liver to degrade and excrete CA 19-9. Analyses were carried out using CA 19-9 values in patients with normal bilirubin levels and in those with impaired biliary excretion (bilirubin >2 mg/dL). In patients with total bilirubin levels of 2 mg/dL or more, the corrected CA 19-9 (cCA 19-9) was calculated by dividing the CA 19-9 value by the bilirubin value (mg/dL).
2. Statistics
Statistical analyses were performed using MedCalc version 10.2.0.0 for Windows (MedCalc Software, MariaKerke, Belgium). The significance of differences in distribution was calculated using the t-test and one-way analysis of variance for continuous variables and the chi-square test or Fisher exact test (depending on the number of cases in each subgroup) for categorical variables. Survival was estimated using the Kaplan-Meier method while differences were assessed by means of the log-rank test. Survival was defined as the time from diagnosis to disease-related death and was censored at the last follow-up date if no events had occurred.
A p<0.05 was used as the threshold value for statistical significance for variable selection for multivariate modeling using the Cox proportional hazards model in order not to overlook any potentially important predictors. Only those variables found to be significant in the univariate analysis were included in the model. We then used receiver operating characteristic (ROC) curve analysis to determine the best threshold values for CA 19-9 level and tumor volume. Sensitivity, specificity, and positive and negative predictive values were calculated for each threshold value of CA 19-9, CEA, and tumor volume. We considered values statistically significant when the p<0.05.
RESULTS
1. Patients and diagnoses
Of the 177 patients, 92 were male and 85 female with a median age of 66.8 years. Tumor location was confined to the head of pancreas in 142 patients and to the body or tail in 35 patients. We performed pancreaticoduodenectomy in 140 patients, distal splenopancreatectomy in 35, and a total pancreatectomy in two patients. The mean preoperative size of the tumors was 3.98 cm; preoperative mean cCA 19-9 and cCEA were 525 U/mL and 3.22 ng/mL, respectively. Histological examination showed a grading of 3 to 4 in 93 patients; according to the American Joint Committee on Cancer (AJCC; 6th edition from 2002 to 2009 and 7th edition from 2009 to 2012) pancreatic cancer staging, 89 patients presented with a stage IIb tumor, 29 with a stage III tumor, 28 with a stage IIa, 12 with a stage Ib tumor, and 19 with a stage Ia PAC.
2. Preoperative factors that influenced early recurrence
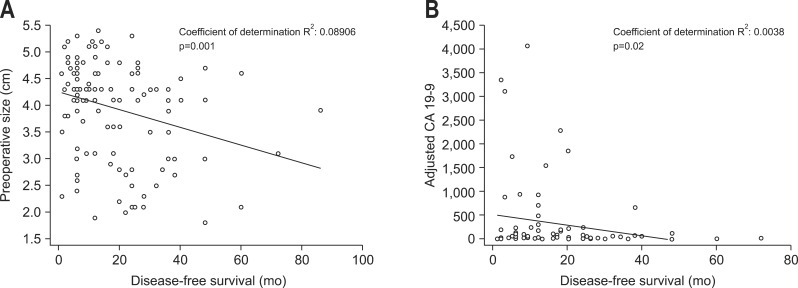
After univariate analysis, preoperative cCA 19-9, tumor size, and the presence of node metastases were statistically correlated with early recurrence (Table 2). The significance of preoperative cCA 19-9 and tumor size in predicting early recurrence is shown by regression lines through the regression coefficient (Fig. 1). ROC curve analyses were performed in order to establish the level of cCA 19-9 and tumor size related to early recurrence. The most predictive value for preoperative cCA 19-9 was 228 U/L or higher (specificity, 66.9%; sensitivity, 62.0%) and for tumor size was 3 cm or higher (specificity, 80.7%; sensitivity, 51.3%).
Table 2.
Univariate Analysis Considering Preoperative Factors Influencing Early Recurrence
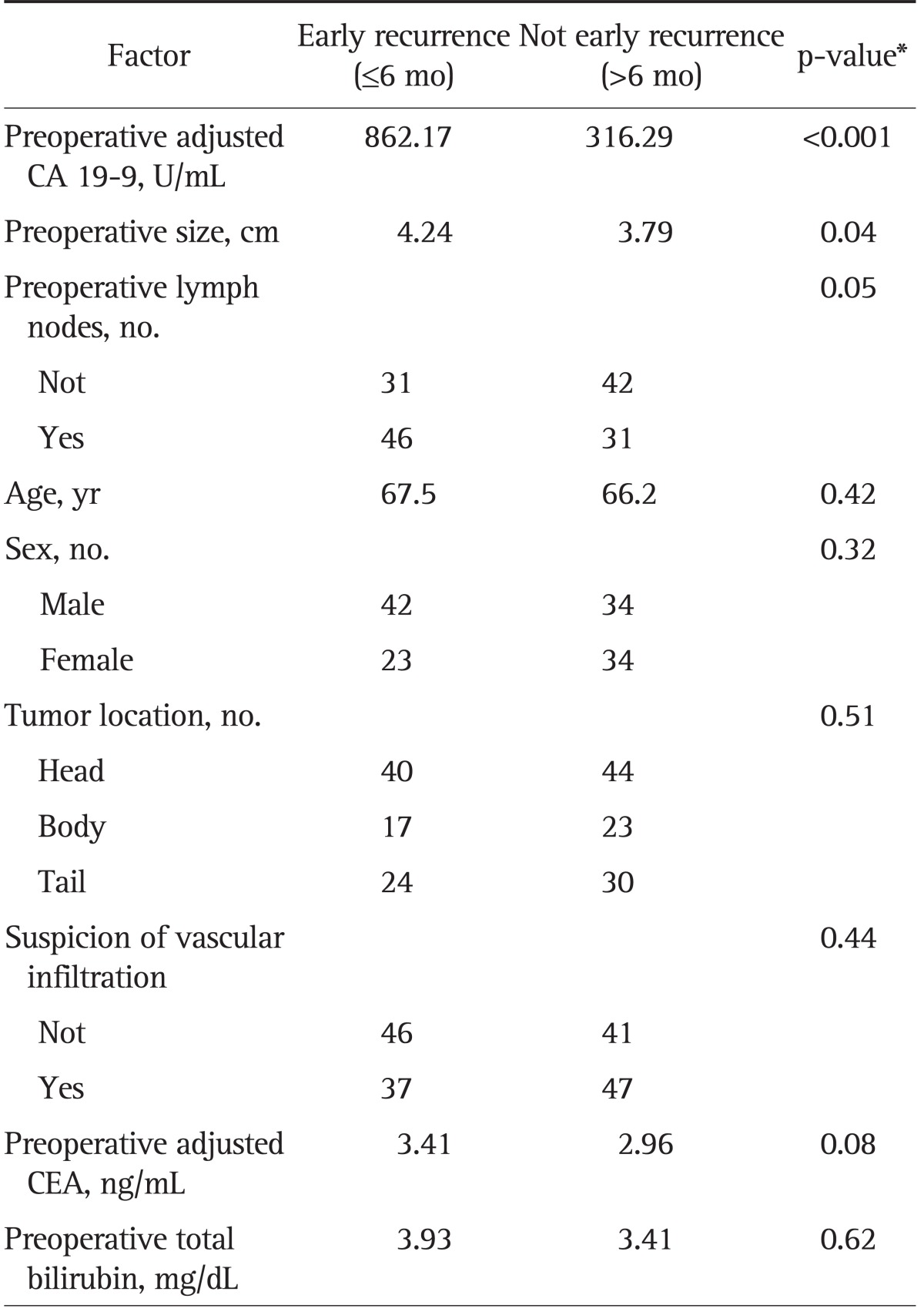
CA 19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen.
*By analysis of variance.
Fig. 1.
Regression lines and coefficients for (A) preoperative tumor size and (B) corrected carbohydrate antigen 19-9 (cCA 19-9).
Using these values, we tested the utility of each marker and the combination of the threshold values for the prediction of early recurrence (Table 3); when all the three preoperative parameters were less than the threshold values the prediction of early recurrence was assessed with 90.4% accuracy (Table 3).
Table 3.
Accuracy of Preoperative Parameters in Predicting Early Recurrence
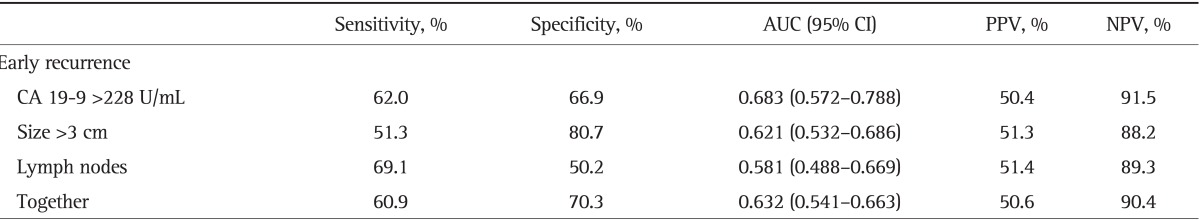
AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; CA 19-9, carbohydrate antigen 19-9.
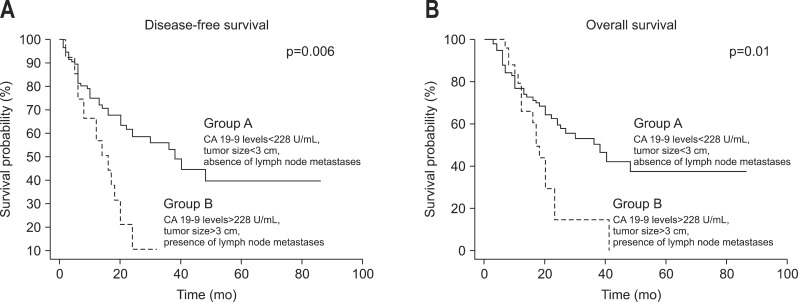
We also found that after univariate analysis, a preoperative cCA 19-9 level >228 U/mL and tumor size ≥3.1 cm significantly predict disease-free survival (DFS) and overall survival (OS) in Table 4. Patients were divided into two subgroups based upon preoperative cCA 19-9 level, tumor size, and the presence of lymph node metastases at the preoperative staging. Group A consisted of 49 patients with CA 19-9 levels <228 U/mL, tumor size <3 cm, and the absence of lymph node metastases while group B consisted of 128 patients with cCA 19-9 levels >228 U/mL, a tumor size ≥3.1 cm, and preoperative metastatic nodes. The two subgroups were equivalent in terms of age, sex, and tumor location (p=n.s.). Table 4 demonstrated that group B patients experienced shorter median OS and DFS compared to group A patients (OS, 14.8 months vs 20.5 months, p<0.001; DFS, 11.4 months vs 20.6 months, p<0.001, respectively) (Fig. 2).
Table 4.
Univariate Analysis Considering Preoperative Prognostic Factors and Survival
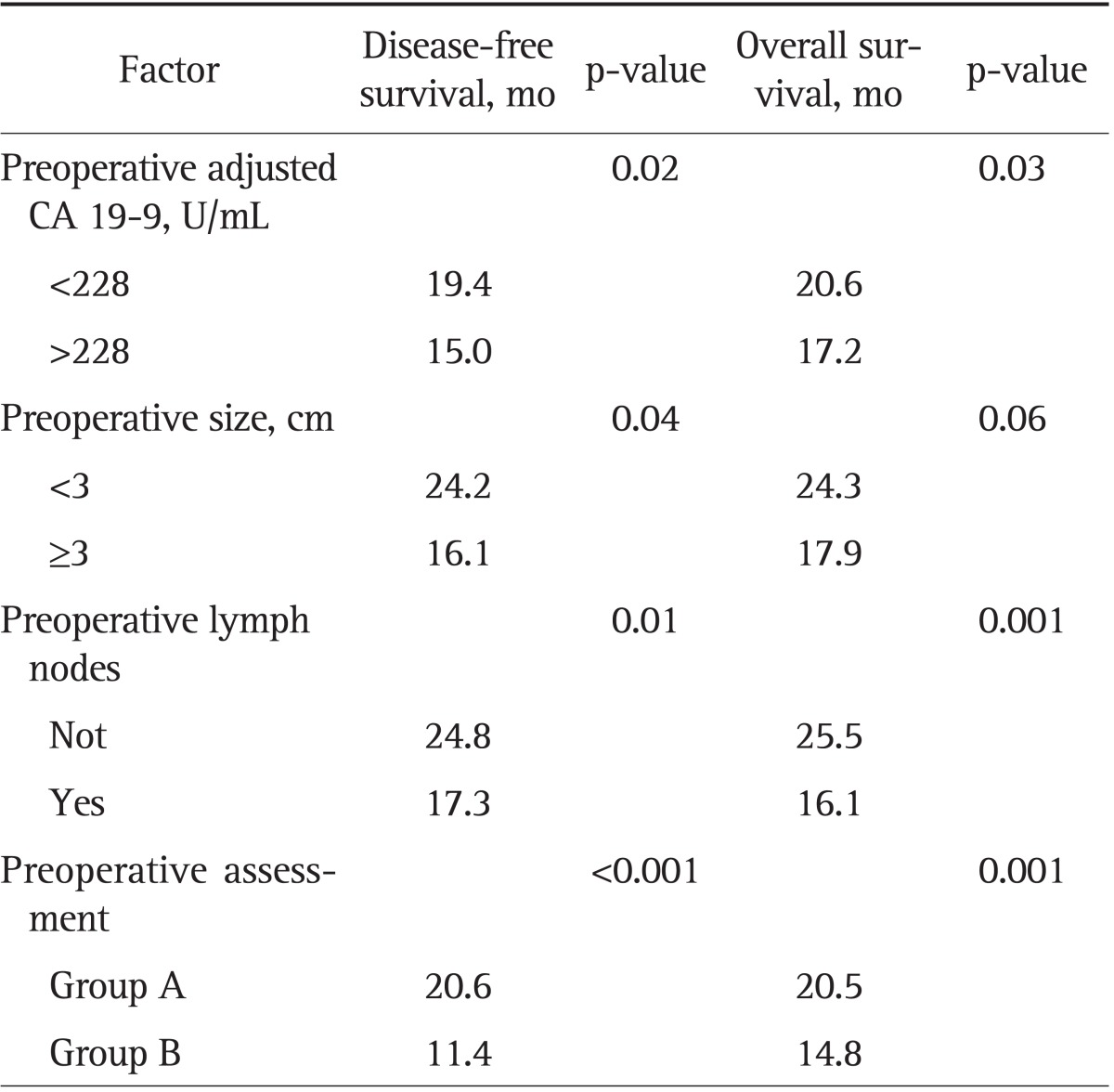
CA 19-9, carbohydrate antigen 19-9.
Fig. 2.
Kaplan-Meier curves for preoperative assessment combining preoperative tumor size, corrected carbohydrate antigen 19-9 (cCA 19-9), and preoperative lymph nodes: (A) disease-free survival and (B) overall survival.
After multivariate analysis considering both preoperative and postoperative parameters, the preoperative assessment combining preoperative cCA 19-9, tumor size, and the presence of nodes metastases (p=0.0314; hazard ratio, 3.9811; 95% confidence interval, 1.1745 to 15.3245), a complete microscopic resection (R0), the ratio of metastatic/resected lymph nodes >0.3 (lymph node ratio), and AJCC stage were each independently correlated with early recurrence (Table 5).
Table 5.
Multivariate Analysis Considering Factors Influencing Early Recurrence
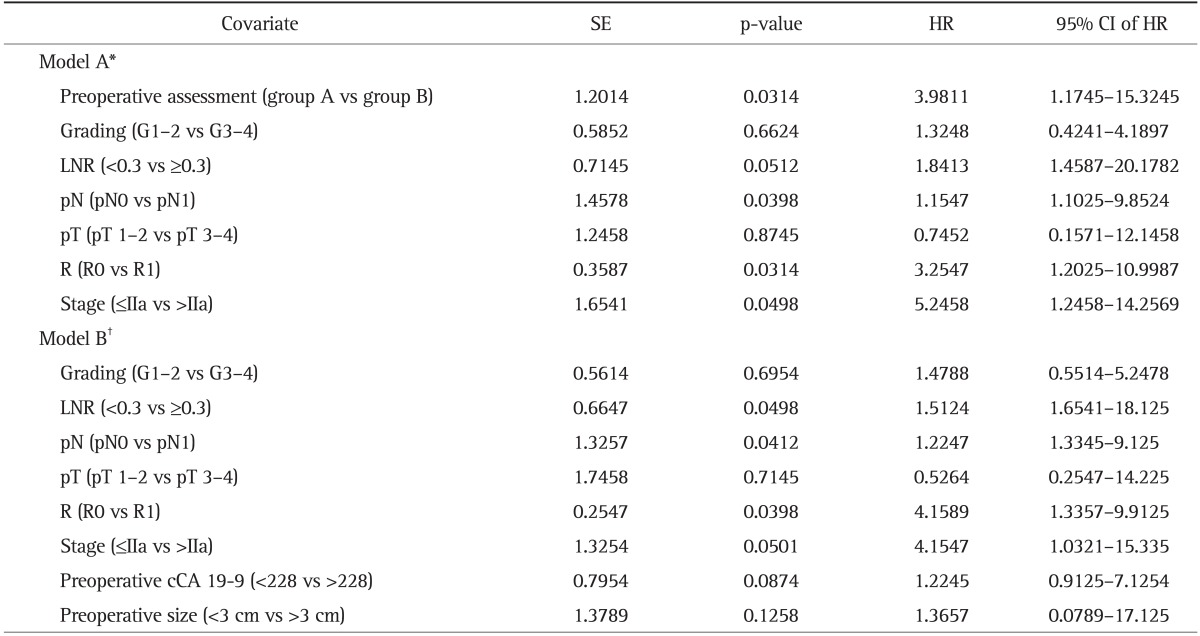
SE, standard error; HR, hazard ratio; CI, confidence interval; LNR, lymph node ratio; cCA 19-9, corrected carbohydrate antigen 19-9.
*Model A, multivariate analysis considering preoperative assessment as subgroups A-B; †Model B, multivariate analysis independently considering the preoperative parameters (preoperative cCA 19-9 and preoperative size).
DISCUSSION
PAC is an aggressive tumor characterized by early dissemination to regional lymph nodes and distant metastases. At the time of presentation, it is essential to evaluate disease status in order to achieve the best management of the disease. Only 20% to 25% of the patients who present with a diagnosis of PAC can potentially benefit from surgical resection,13 yet a great majority of those patients have a locally advanced or metastatic disease at surgery.3 Even when complete surgical resection is performed, prognosis is poor with a high local or metastatic recurrence rate within the first 12 months.14 For this reason, given the systemic nature of PAC and the morbidity and mortality rates involved with surgery, it is essential to clearly determine the relative risk of relapse at the time of initial staging evaluation. It has become crucial, in fact, to propose a risk stratification of disease progression and surgical failure prior to surgery in order to avoid unnecessary laparotomies and the loss of quality of life as a consequence of the surgery. It allows the physician to redirect appropriate patients to palliative or neoadjuvant treatment, which maximize resources and reduces the costs of surgery and hospitalization.
Computed tomography (CT) is the most widely available and best validated modality for imaging patients with PAC; it also permits an accurate evaluation of the local extent of cancer.15 CT, in association with the endoscopic ultrasound (EUS), allows visualization of the primary tumor in relation to the superior mesenteric artery, celiac axis, superior mesenteric vein, and portal vein.2 Although CT techniques have improved, it still misses occult peritoneal or liver metastatic disease in 4% to 15% of patients and occult vascular involvement in 4% to 19% of patients.4,15
Preoperative CT and EUS actually allow selection of patients who are better candidates for trials for neoadjuvant treatment in tertiary cancer centers.16
Cancer associated antigen (CA 19-9) has become the most important tumor marker for pancreatic cancer.11 Many reports have verified its clinical usefulness in diagnosis and monitoring of disease progression.9 CA 19-9 can also be used as a tool for predicting patient response to chemotherapy and chemoradiation. There are many reports in the literature of the utility of preoperative CA 19-9 for the assessment of expected curability and resectability in patients with pancreatic cancer as higher levels of CA 19-9 correlate with a major probability of requiring R1/R2 resection and, consequently, a worse OS and DFS. Several threshold values for the use of CA 19-9 as a prognostic indicator have been reported.5,17-20 Ferrone et al.21 reported that operative treatment and tumor size <3 cm were factors independently associated with longer survival.
In this study, we investigated the outcomes of patients who underwent pancreatic resection for pancreatic cancer and we identified several factors predictive of early recurrence. In particular, we tried to identify a panel of significant preoperative predictors of surgical failure and early disease progression. We demonstrated that preoperative cCA 19-9 levels, tumor size, and preoperative metastatic lymph nodes were statistically significant predictors of early recurrence, DFS, and OS using univariate analysis. After multivariate analysis, however, these parameters alone failed to demonstrate a significant value in terms of survival. For this reason, we rebuilt the multivariate analysis model using a sub-classification system which considered all three parameters together (Table 5). In this analysis, these three preoperative parameters together were able to independently predict the early recurrence of PAC.
Kim et al.,5 in a recent paper demonstrating the value of cCA 19-9 in predicting R0 resectability of PAC, pose the following question: how can we assess and manage the patients in whom preoperative CT demonstrates only a localized and respectable tumor, but whose tumor markers show higher levels than cutoff levels? Kim et al.5 recommended further preoperative evaluations such as positron emission tomography (PET)/CT and/or diagnostic laparoscopy for detecting occult metastases to decrease the number of unnecessary laparotomies.
In conclusion we showed the feasibility of a preoperative prognostic assessment in order to better stratify patients who are candidates for surgical resection. We identified three common preoperative parameters able, in combination, to calculate the risk of early disease progression after surgery.
We propose that these variables could be used to effectively select patients for alternative or palliative treatment, or further diagnostic evaluations (explorative laparoscopy, PET/CT). Neoadjuvant chemoradiotherapy could be an effective alternative choice for patients presenting unfavorable and high risk recurrence parameters, to better select patients responsive to a preoperative treatment (under-sized or under-staged) from patients developing a disease progression and then candidate to palliative care.
ACKNOWLEDGEMENTS
Marco La Torre is supported by the PhD University Grant program "Clinical and Experimental Research Methodologies in Oncology" provided by the Faculty of Medicine and Psychology, University of Rome La Sapienza, Rome, Italy.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Kim CB, Ahmed S, Hsueh EC. Current surgical management of pancreatic cancer. J Gastrointest Oncol. 2011;2:126–135. doi: 10.3978/j.issn.2078-6891.2011.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim YC, Kim HJ, Park JH, et al. Can preoperative CA19-9 and CEA levels predict the resectability of patients with pancreatic adenocarcinoma? J Gastroenterol Hepatol. 2009;24:1869–1875. doi: 10.1111/j.1440-1746.2009.05935.x. [DOI] [PubMed] [Google Scholar]
- 6.Hata S, Sakamoto Y, Yamamoto Y, et al. Prognostic impact of postoperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2012;19:636–641. doi: 10.1245/s10434-011-2020-9. [DOI] [PubMed] [Google Scholar]
- 7.La Torre M, Nigri G, Ferrari L, Cosenza G, Ravaioli M, Ramacciato G. Hospital volume, margin status, and long-term survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2012;78:225–259. [PubMed] [Google Scholar]
- 8.La Torre M, Nigri G, Cavallini M, Mercantini P, Ziparo V, Ramacciato G. The glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19:2917–2923. doi: 10.1245/s10434-012-2348-9. [DOI] [PubMed] [Google Scholar]
- 9.Humphris JL, Chang DK, Johns AL, et al. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012;23:1713–1722. doi: 10.1093/annonc/mdr561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlieman MG, Ho HS, Bold RJ. Utility of tumor markers in determining resectability of pancreatic cancer. Arch Surg. 2003;138:951–955. doi: 10.1001/archsurg.138.9.951. [DOI] [PubMed] [Google Scholar]
- 11.Berger AC, Meszoely IM, Ross EA, Watson JC, Hoffman JP. Undetectable preoperative levels of serum CA 19-9 correlate with improved survival for patients with resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2004;11:644–649. doi: 10.1245/ASO.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Uygur-Bayramicli O, Dabak R, Orbay E, et al. Type 2 diabetes mellitus and CA 19-9 levels. World J Gastroenterol. 2007;13:5357–5359. doi: 10.3748/wjg.v13.i40.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 14.La Torre M, Cavallini M, Ramacciato G, et al. Role of the lymph node ratio in pancreatic ductal adenocarcinoma. Impact on patient stratification and prognosis. J Surg Oncol. 2011;104:629–633. doi: 10.1002/jso.22013. [DOI] [PubMed] [Google Scholar]
- 15.Soriano A, Castells A, Ayuso C, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99:492–501. doi: 10.1111/j.1572-0241.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 16.Varadhachary GR. Preoperative therapies for resectable and borderline resectable pancreatic cancer. J Gastrointest Oncol. 2011;2:136–142. doi: 10.3978/j.issn.2078-6891.2011.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujioka S, Misawa T, Okamoto T, et al. Preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels for the evaluation of curability and resectability in patients with pancreatic adenocarcinoma. J Hepatobiliary Pancreat Surg. 2007;14:539–544. doi: 10.1007/s00534-006-1184-3. [DOI] [PubMed] [Google Scholar]
- 18.La Torre M, Ziparo V, Nigri G, Cavallini M, Balducci G, Ramacciato G. Malnutrition and pancreatic surgery: prevalence and outcomes. J Surg Oncol. 2013;107:702–708. doi: 10.1002/jso.23304. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Wang YM, Sun CD, Lu Y, Wu LQ. Clinical value of serum CA19-9 levels in evaluating resectability of pancreatic carcinoma. World J Gastroenterol. 2008;14:3750–3753. doi: 10.3748/wjg.14.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.La Torre M, Ramacciato G, Nigri G, et al. Post-operative morbidity and mortality in pancreatic surgery. The role of surgical Apgar score. Pancreatology. 2013;13:175–179. doi: 10.1016/j.pan.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24:2897–2902. doi: 10.1200/JCO.2005.05.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]