Abstract
Background/Aims
The most common cause of chronic periodontitis is poor oral hygiene. Gastroesophageal reflux disease (GERD) enhances the proximal migration of gastric contents and may cause poor oral hygiene. We hypothesized that GERD may increase thse risk of chronic periodontitis and investigated this potential relationship.
Methods
A retrospective cross-sectional study was conducted in outpatients between January 1, 2010, and April 30, 2012. GERD was defined as being present based on at least two of the following criteria: etiologic agent(s), identifiable signs and symptoms, and consistent anatomic alterations. A total of 280 patients with chronic periodontitis and 280 controls were analyzed. Information regarding patient demographics and other potential confounding factors for chronic periodontitis were collected through individual medical records.
Results
GERD was revealed to be independently associated with an increased incidence of chronic periodontitis (odds ratio [OR], 2.883; 95% confidence interval [CI], 1.775 to 4.682). The other three variables of dental caries (OR, 1.531; 95% CI, 1.042 to 2.249), tobacco use (OR, 2.335; 95% CI, 1.461 to 3.730), and history of medication (calcium channel blocker, cyclosporine, or phenytoin) (OR, 2.114; 95% CI, 1.160 to 3.854) were also determined to be independent risk factors.
Conclusions
The present study supported our hypothesis that GERD can be a risk factor for chronic periodontitis.
Keywords: Gastroesophageal reflux, Chronic periodontitis, Oral hygiene
INTRODUCTION
Chronic inflammatory disorders of the structures surrounding the teeth lead to chronic periodontitis, which is characterized by the irreversible loss of supporting connective tissue, alveolar bone, and periodontal ligament.1 Chronic periodontitis is common and severe in the American adult population, with at least 35% of dentate adults having periodontitis and 10% to 15% having the severe forms of periodontitis.2 The presence and progression of untreated chronic periodontitis jeopardizes the intact dentition, leading to tooth loss and compromised patient-based outcomes such as esthetics, impaired oral functioning, and other domains of oral-health-related quality of life.3,4 Patients with chronic periodontitis require management such as supportive periodontal care and surgery, including replacement of their dentition. These patients may perceive such treatments to be costly maintenance requirements over their lifetime. Consequently, the active prevention of chronic periodontitis is important, and the investigation of the potential risk factors of chronic periodontitis is imperative.
Based on the Montreal Definition and Classification agreement, gastroesophageal reflux disease (GERD) is defined as a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications.5 The prevalence of GERD in Western countries is higher than in Eastern countries, and is estimated to be about 10% to 20%.6 Extraesophageal manifestations resulting from GERD include laryngeal (reflux laryngitis, hoarseness, chronic cough, vocal cord ulcers, and granulomas), pharyngeal (mucositis), respiratory (asthma, bronchitis, chronic cough, and aspiration pneumonia), sinus (sinusitis), middle ear (otitis media), and oral conditions (tooth erosion, sour taste, and mucositis).7-9
We speculated that GERD may be one of the causes of chronic periodontitis since it can induce poor oral conditions, which can easily result in chronic periodontitis through poor salivary function or microbial colonization in GERD patients.10-13 Despite the fact that GERD causes many extraesophageal manifestations, few reports have studied the relationship between chronic periodontitis and GERD.14,15 Therefore, in this study, we aimed to investigate whether there is a relationship between chronic periodontitis and GERD.
MATERIALS AND METHODS
1. Patients
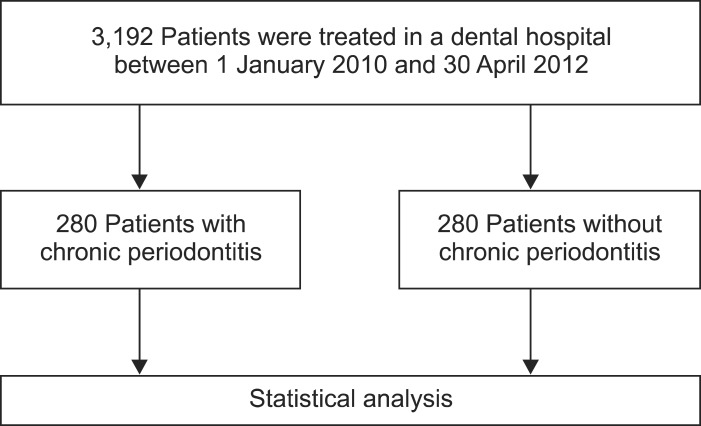
A cross-sectional study was conducted by the Department of Gastroenterology, Kosin University College of Medicine, Busan, Korea. This study consisted of outpatients who visited the Department of Dentistry, Kosin University Gospel Hospital between January 1, 2010 and April 30, 2012 to assess the association between chronic periodontitis and GERD. A diagnosis of chronic periodontitis was based on the International Classification of Disease, 10th revision (ICD-10, K05.3), and 280 patients with chronic periodontitis were enrolled. Among the outpatients who visited the department for the treatment of dental issues in the same period, we enrolled 280 patients without chronic periodontitis who were randomly selected after adjusting for age and gender (Fig. 1). Institutional Review Board approval was obtained prior to start of the study (KUGH IRB 12-063).
Fig. 1.
Patient groups included in this study.
2. Variables
Demographic information (age and gender) and the presence of GERD in each patient were investigated. Functional gastrointestinal diseases were also analyzed. Other potential confounders, including ICD-10 diagnoses of hypertension, diabetes, hepatitis, autoimmune disease, cancer, dental disease, and a history of tobacco use or alcohol consumption, drug use (calcium channel blocker [CCB], cyclosporine, or phenytoin), chemotherapy, and surgical history were collected.
3. Definitions
The diagnosis of chronic periodontitis was based on clinical findings of gingival inflammation, loss of attachment in excess of 1 mm, and probing pocket depths ≥4 mm at three or four sites for more than four teeth per quadrant.16 Plaque index, gingival index, pocket depth, and clinical attachment loss using a sterile William's graduated periodontal probe were collected. Plaque was assessed using the Silness and Loe plaque index, and gingival status was determined using the Loe and Silness gingival index.17,18
The diagnosis of GERD was based on the Montreal Definition and Classification agreement. GERD was defined as a morbid entity characterized by at least two of these criteria: 1) recognized etiologic agent(s), 2) identifiable group of signs and symptoms, and 3) consistent anatomic alterations.5 The factors that contribute to reflux include weight gain, fatty foods, caffeinated or carbonated beverages, alcohol, tobacco use, and drugs.19 Drugs decreasing lower esophageal sphincter pressure include anticholinergics, antihistamines, tricyclic antidepressants, CCB, progesterone, and nitrates. The most prominent symptom of GERD is heartburn with or without the regurgitation of gastric contents into the mouth. Other symptoms include epigastric pain, chest pain, coughing, hoarseness, and throat irritation.20 Consistent anatomic alterations, such as reflux esophagitis and Barrett's esophagitis, were included in the case definition of GERD.5
Functional dyspepsia was diagnosed when one or more symptoms (postprandial fullness, early satiation, epigastric pain, or epigastric burning) were present with no evidence of structural disease. Irritable bowel syndrome was diagnosed by abdominal pain and cramps, changes in bowel movements (diarrhea, constipation, or both), gassiness, bloating, nausea, and other symptoms without organic abnormalities.
4. Statistical analysis
The univariate associations between chronic periodontitis and variables were explored using chi-square or Student t-test. To determine independent risk factors, multivariate analysis using a logistic regression model was planned when multiple factors were identified from the univariate analysis. All analyses were performed using SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL, USA), and p<0.05 were considered statistically significant.
RESULTS
1. Baseline characteristics
The present study was composed of 50% males (n=280) and 50% females (n=280). The age of the patients ranged from 14 to 82 years, with a mean age of 48.7±16.3 years. Of our 560 subjects, 50.0% had chronic periodontitis (n=260) and 19.3% (n=108) had GERD. There was no difference in gender or age between the chronic periodontitis and nonperiodontitis groups (Table 1).
Table 1.
Baseline Characteristics of the Study Population
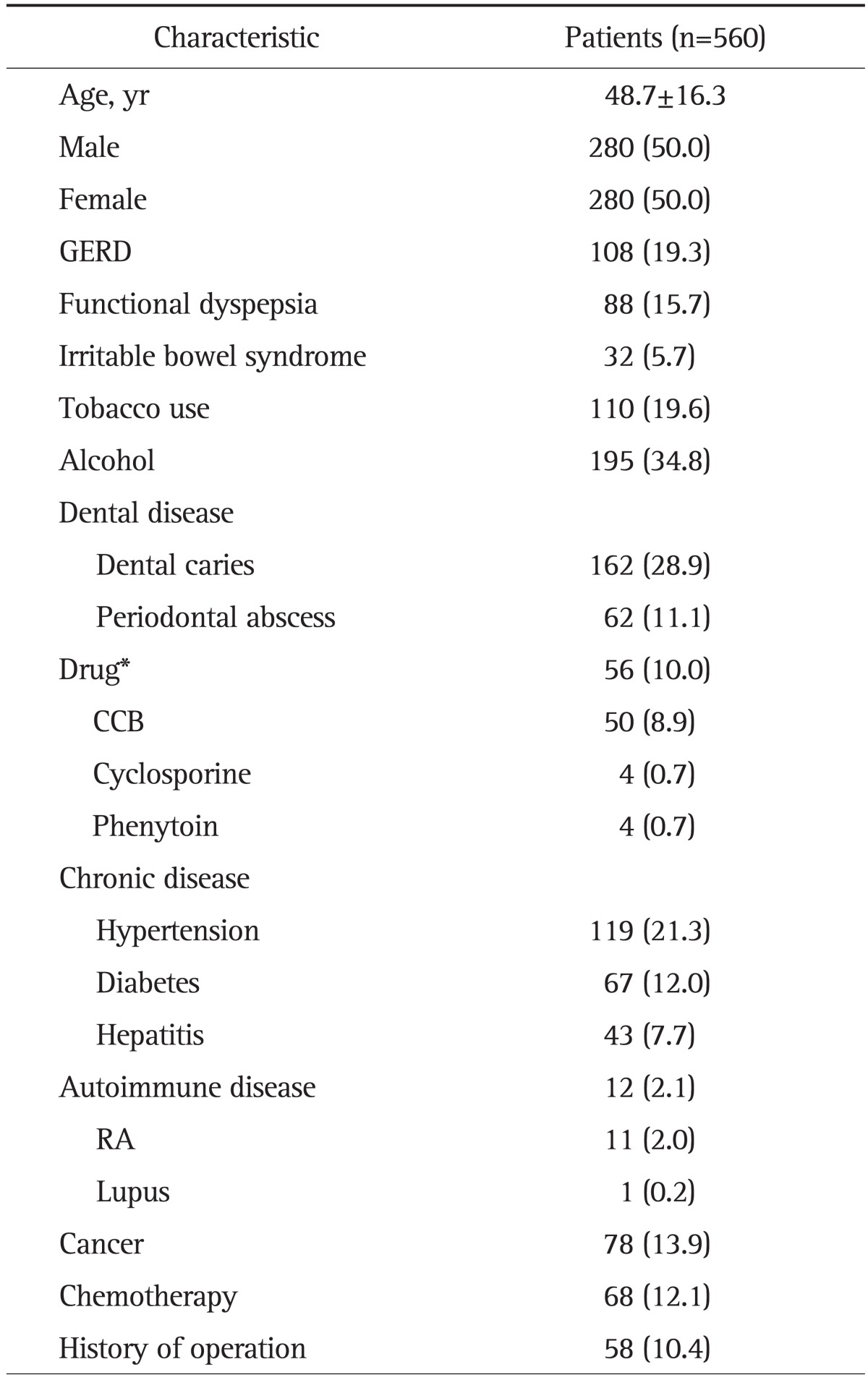
Data are presented as mean±SD or number (%).
GERD, gastroesophageal reflux disease; CCB, calcium channel blocker; RA, rheumatoid arthritis.
†Drug includes only three medications: CCB, cyclosporine, and phenytoin.
2. Chronic periodontitis, functional gastrointestinal disease, and other dental disease
There was a strong correlation between the presence of GERD and patients with chronic periodontitis (p<0.001) (Table 2). However, there were no correlations between chronic periodontitis and dyspepsia (p=0.131) or irritable bowel syndrome (p=0.298). Dental caries exhibited a strong relationship with the presence of chronic periodontitis (p=0.020), but periodontal abscess did not demonstrate a correlation with chronic periodontitis (p=0.346).
Table 2.
Comparison of Functional Gastrointestinal Disease and Dental Disease between Patients with and without Chronic Periodontitis
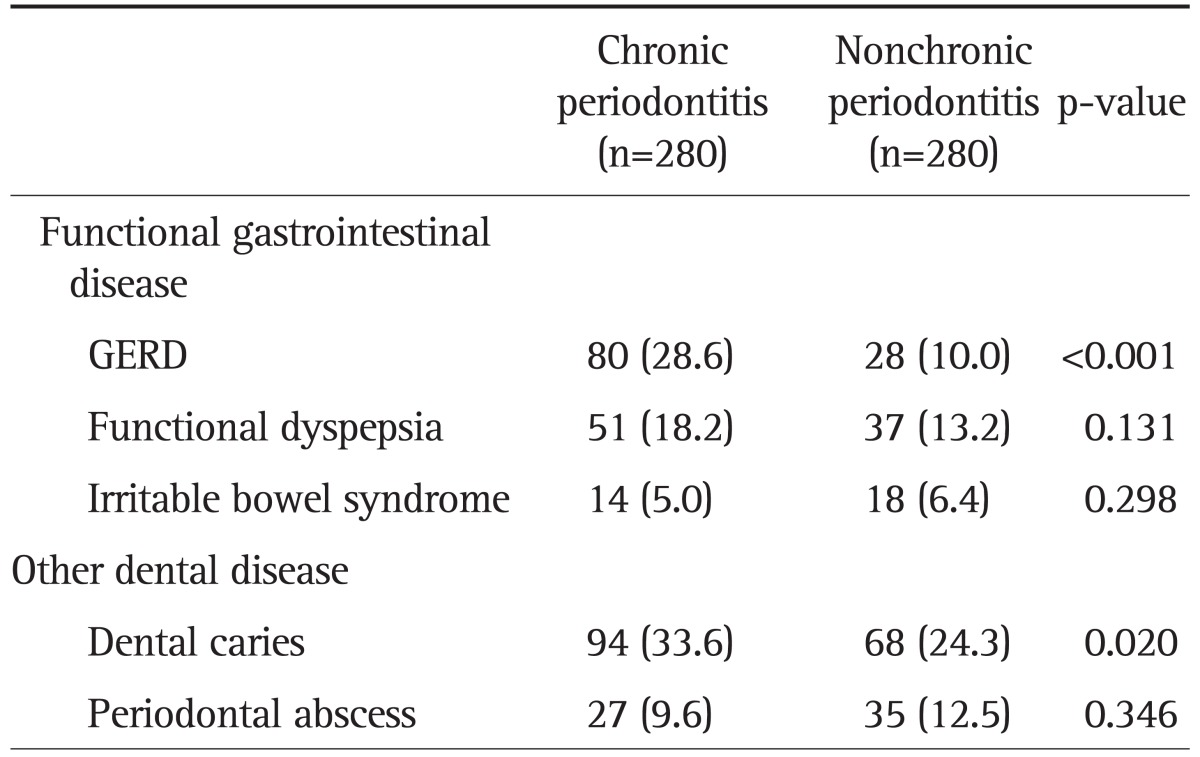
Data are presented as number (%).
GERD, gastroesophageal reflux disease.
3. Chronic periodontitis, chronic diseases, alcohol, and smoking
We did not find any correlations between GERD and three chronic diseases or autoimmune diseases including hypertension (p=0.148), diabetes (p=0.435), hepatitis (p=0.526), rheumatoid arthritis (p=0.545), or lupus (p=1.000). Chronic periodontitis was associated with tobacco use (p<0.001) (Table 3), but not with alcohol use (p=0.595).
Table 3.
Comparison of Chronic Disease, Alcohol, and Smoking between Patients with and without Chronic Periodontitis
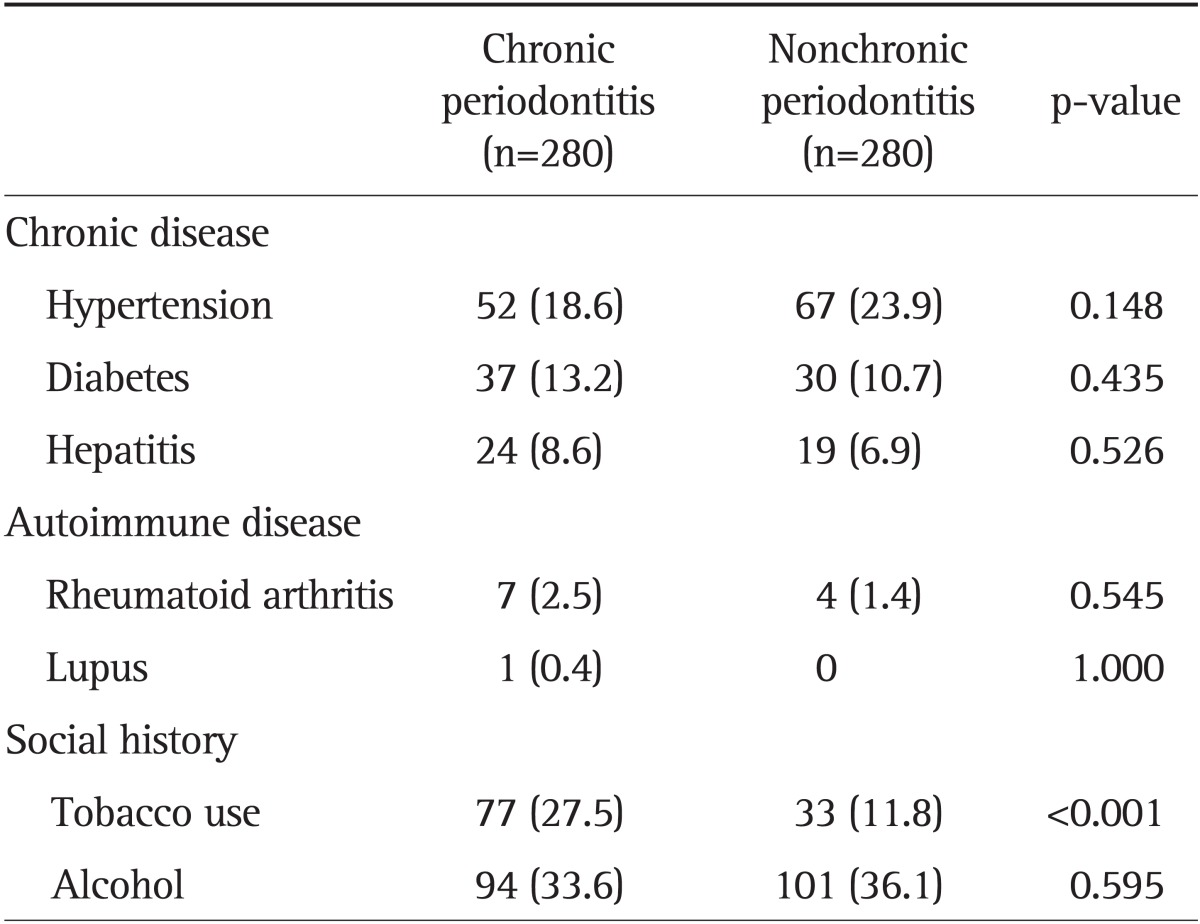
Data are presented as number (%).
4. Chronic periodontitis and medications
Chronic periodontitis was associated with an increased incidence of history of medication use, which is known to increase periodontitis (p=0.016) (Table 4). Nevertheless, there were no statistically significant findings in the relationships between chronic periodontitis and each drug of CCB (p=0.102), cyclosporine (p=0.124), or phenytoin (p=0.624). Chronic periodontitis was not correlated with cancer or anticancer chemotherapy.
Table 4.
Comparison of Cancer, Chemotherapy, and Prescribed Drugs between Patients with and without Chronic Periodontitis
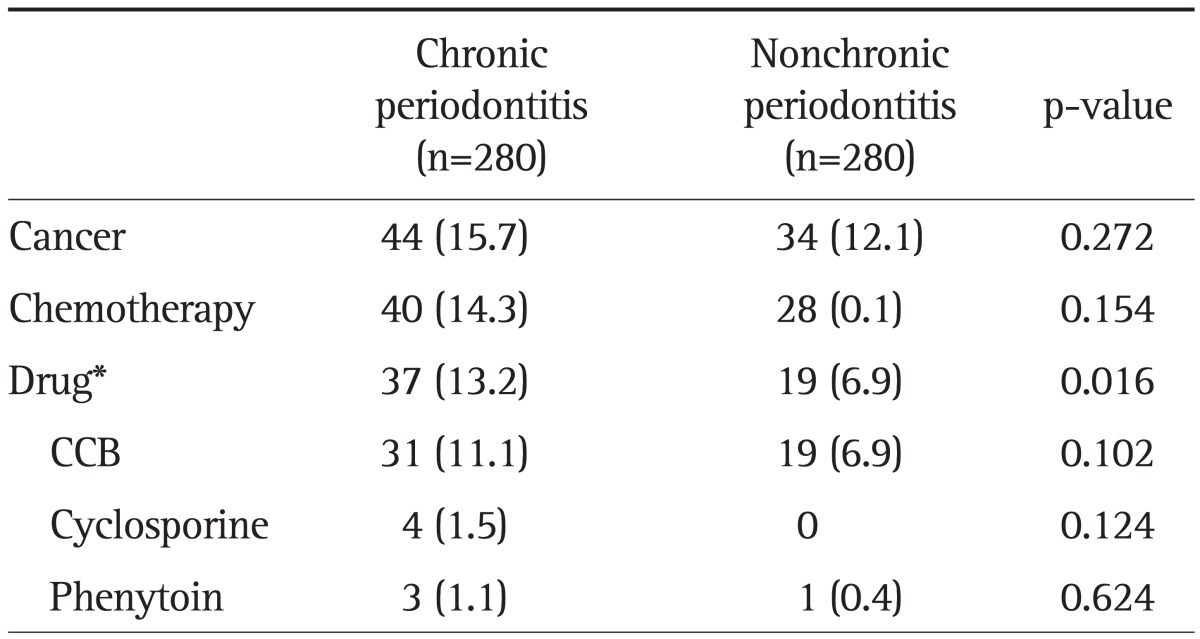
Data are presented as number (%).
CCB, calcium channel blocker.
*Drug includes only three medications: CCB, cyclosporine, and phenytoin.
5. Multivariate analysis
Logistic regression analysis revealed that GERD was independently associated with an increased incidence of chronic periodontitis (p<0.001) (Table 5). Furthermore, three other variables were recognized as independent factors of chronic periodontitis: tobacco use (p<0.001), dental caries (p=0.030), and medication (p=0.015).
Table 5.
Independent Risk Factors for Chronic Periodontitis (Logistic Regression Model)
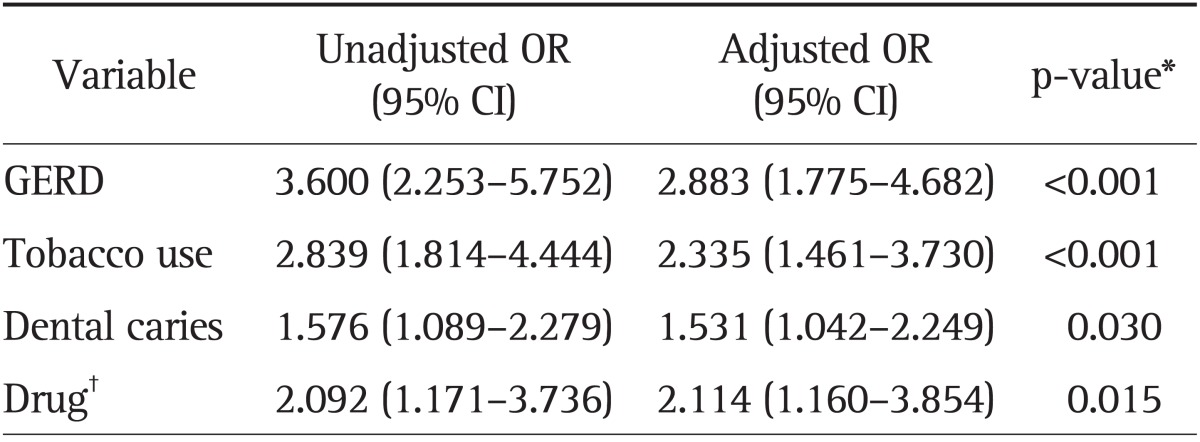
OR, odds ratio; CI, confidence interval; GERD, gastroesophageal reflux disease.
*p-value is the adjusted p-value; †Drug includes only three medicines: calcium channel blocker, cyclosporine, and phenytoin.
DISCUSSION
The present study showed that, apart from the other variables which are well known to be significantly associated with chronic periodontitis, there was a significantly higher prevalence of GERD in patients with chronic periodontitis. In addition, it was shown that GERD was an independent risk factor for chronic periodontitis regardless of the established risk factors of chronic periodontitis such as dental caries, tobacco use, and a history of drug use. Dzhamaldinova14 suggested that there was a pathogenic link between chronic inflammatory periodontal disease and the presence of pathological gastroesophageal reflux. That study showed that patients with inflammatory periodontal diseases coupled with GERD were marked by a more pronounced therapeutic effect of combined treatment in comparison with isolated local treatment for chronic inflammatory periodontal disease.14 The inflammatory response of the periodontal tissues to infection is influenced by environmental factors, including oral hygiene and genetic factors.21-23 Randomized controlled trials have revealed that oral hygiene (plaque) is a risk factor for chronic periodontitis.24-26 These trials showed an improvement in surrogate measures of chronic periodontitis resulting from improved plaque control. Greater tooth loss was found in patients with poorer oral hygiene, and it is assumed that periodontitis was the major cause.24-26
The most reasonable explanation for GERD as a predisposing factor for chronic periodontitis would be poor salivary function. Mixed saliva coats all of the relevant internal anatomical surfaces with mucin-rich secretions, providing a protective diffusion barrier against mechanical, thermal, chemical, and microbial damage.27 Saliva also acts as an endogenous antacid to protect against symptomatic gastroesophageal reflux. Therefore, decreased salivary secretion results in insufficient acid neutralization.28 Hyposalivation in GERD patients has been demonstrated in several papers,29-31 and a few studies have discovered an association between reduced salivary flow and periodontal disease among the elderly.32,33 The esophago-salivary reflex is the major mechanism that governs salivary secretion in response to the presence of gastroesophageal refluxate within the esophageal lumen. Esophageal mucosal exposure to the volume of gastroesophageal refluxate and to its low pH activates esophageal mechano- and chemoreceptors. Such stimulation results in the activation of afferent fibers. Parasympathetic (nerve VII or IX) efferent fibers induce the vigorous secretion of water and electrolytes, including buffers, within the salivary glands.34 In physiologic situations, as the volume of gastroesophageal refluxate increases, more saliva is secreted. However, the salivary volume of patients with GERD was not dependent on the volume of refluxate.34 Patients with GERD may have an impaired esophago-salivary reflex which causes hyposalivation, even in the presence of a large volume of refluxate, although the detailed mechanism has yet to be elucidated. From these findings, we tentatively decided that hyposalivation in GERD patients makes the oral internal anatomical surfaces vulnerable to acidic and proteolytic stomach contents and ultimately result in chronic periodontitis.
Another plausible effect of hyposalivation may be the intraoral bacterial proliferation that occurs in GERD patients. Periodontal disease is characterized by the formation of mixed biofilms on the teeth and gingival tissues.35 Chronic periodontitis is known to be caused mainly by a specific group of gram-negative anaerobic bacteria, including Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis.36 Human saliva and gingival fluid contains at least 45 different antimicrobial proteins (AMPs) necessary to protect the oral epithelia from the large number of possible invading microbes and to maintain the oral homeostasis of commensal and pathogenic bacteria.37 Among AMPs, chemokine ligand 28 (CCL28) was effective at killing two anaerobic periodontal pathogens: P. gingivalis and A. actinomycetemcomitans. A reduction in salivary flow decreases the amount of CCL28 and results in diminished oral self-defense mechanisms.38 Therefore, we can postulate that hyposalivation in GERD may have an influence on the development of chronic periodontitis by allowing for the proliferation of intraoral bacteria.
In our study, diabetes was not significantly associated with chronic periodontitis. Although diabetes has been identified as an important risk factor for chronic periodontal disease,39,40 the interpretation of the results is complicated by a number of factors: small sample sizes, the absence of standard reporting of the type of diabetes, the lack of longitudinal studies and control groups, and the inadequate control of covariates such as age, duration of diabetes, and level of control of diabetes.41
A history of medication use including CCB, cyclosporine, and phenytoin (multiple or each) was significantly more common in the chronic periodontitis group compared with the control group. There are a growing number of medications that may induce gingival overgrowth, resulting in chronic periodontitis.42 However, there were no statistically significant differences in the use of CCB, cyclosporine, or phenytoin with regards to chronic periodontitis. This result might be caused by the small sample size and insufficiency of individual medical records.
Our study has several limitations that should be mentioned. First, the present study is dependent on ICD-10 code accuracy and individual medical records. Some medical records were not clear and were excluded from our study. Second, because of the retrospective cross-sectional nature of our data, we did not follow our patients over time to evaluate a causative mechanism. As a result, the data and findings in this study are, at best, correlative. Third, this is not a large-scale study. The negative findings regarding the relationship between chronic periodontitis and diabetes or each specific medicine may result from the small sample size. In future studies, large epidemiological studies are necessary to confirm the association between chronic periodontitis and GERD.
Although chronic periodontitis is a serious problem causing substantial morbidities and increased medical costs in the elderly, few reports have suggested an association between chronic periodontitis and GERD. In the present study, GERD was more prevalent in patients with chronic periodontitis and was regarded as an independent risk factor. Gastroenterologists should consider the development of chronic periodontitis when they care for patients with GERD. In addition, it may be helpful to consider the presence of GERD as an etiology of unexplained chronic periodontitis. Dentists may need to manage GERD in patients with chronic periodontitis and GERD.
ACKNOWLEDGEMENTS
We quite appreciate Dentist Kyeong Lok Park for analyzing all dental medical records and giving advice to complete this paper.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 2.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heasman PA, Vernazza CR, Gaunt FL, Pennington MW. Cost-effectiveness of adjunctive antimicrobials in the treatment of periodontitis. Periodontol 2000. 2011;55:217–230. doi: 10.1111/j.1600-0757.2010.00341.x. [DOI] [PubMed] [Google Scholar]
- 4.Tomar SL. Public health perspectives on surveillance for periodontal diseases. J Periodontol. 2007;78(7 Suppl):1380–1386. doi: 10.1902/jop.2007.060340. [DOI] [PubMed] [Google Scholar]
- 5.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 6.Richter JE. The many manifestations of gastroesophageal reflux disease: presentation, evaluation, and treatment. Gastroenterol Clin North Am. 2007;36:577–599. doi: 10.1016/j.gtc.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett D. Intrinsic causes of erosion. Monogr Oral Sci. 2006;20:119–139. doi: 10.1159/000093359. [DOI] [PubMed] [Google Scholar]
- 8.Heidelbaugh JJ, Gill AS, Van Harrison R, Nostrant TT. Atypical presentations of gastroesophageal reflux disease. Am Fam Physician. 2008;78:483–488. [PubMed] [Google Scholar]
- 9.Tolia V, Vandenplas Y. Systematic review: the extra-oesophageal symptoms of gastro-oesophageal reflux disease in children. Aliment Pharmacol Ther. 2009;29:258–272. doi: 10.1111/j.1365-2036.2008.03879.x. [DOI] [PubMed] [Google Scholar]
- 10.Crow HC, Ship JA. Are gingival and periodontal conditions related to salivary gland flow rates in healthy individuals? J Am Dent Assoc. 1995;126:1514–1520. doi: 10.14219/jada.archive.1995.0080. [DOI] [PubMed] [Google Scholar]
- 11.Farsi N, Al Amoudi N, Farsi J, Bokhary S, Sonbul H. Periodontal health and its relationship with salivary factors among different age groups in a Saudi population. Oral Health Prev Dent. 2008;6:147–154. [PubMed] [Google Scholar]
- 12.Macfarlane S, Furrie E, Macfarlane GT, Dillon JF. Microbial colonization of the upper gastrointestinal tract in patients with Barrett's esophagus. Clin Infect Dis. 2007;45:29–38. doi: 10.1086/518578. [DOI] [PubMed] [Google Scholar]
- 13.Pei Z, Yang L, Peek RM, Levine SM, Jr, Pride DT, Blaser MJ. Bacterial biota in reflux esophagitis and Barrett's esophagus. World J Gastroenterol. 2005;11:7277–7283. doi: 10.3748/wjg.v11.i46.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dzhamaldinova TD. Dynamics of inflammatory periodontal diseases under the influence of gastroesophageal reflux disease therapy. Eksp Klin Gastroenterol. 2010:46–51. [PubMed] [Google Scholar]
- 15.Dzhamaldinova TD, Maksimovskaia LN, Li ED. Manifestations of gastroesophageal reflux disease in the oral cavity. Eksp Klin Gastroenterol. 2010:23–27. [PubMed] [Google Scholar]
- 16.Karthikeyan BV, Pradeep AR. Gingival crevicular fluid and serum leptin: their relationship to periodontal health and disease. J Clin Periodontol. 2007;34:467–472. doi: 10.1111/j.1600-051X.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- 17.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 18.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 19.Yamamichi N, Mochizuki S, Asada-Hirayama I, et al. Lifestyle factors affecting gastroesophageal reflux disease symptoms: a cross-sectional study of healthy 19864 adults using FSSG scores. BMC Med. 2012;10:45. doi: 10.1186/1741-7015-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaezi MF. Use of symptom indices in the management of GERD. Gastroenterol Hepatol (N Y) 2012;8:185–187. [PMC free article] [PubMed] [Google Scholar]
- 21.Heitz-Mayfield LJ. Disease progression: identification of high-risk groups and individuals for periodontitis. J Clin Periodontol. 2005;32(Suppl 6):196–209. doi: 10.1111/j.1600-051X.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- 22.Kinane DF, Peterson M, Stathopoulou PG. Environmental and other modifying factors of the periodontal diseases. Periodontol 2000. 2006;40:107–119. doi: 10.1111/j.1600-0757.2005.00136.x. [DOI] [PubMed] [Google Scholar]
- 23.Takashiba S, Naruishi K. Gene polymorphisms in periodontal health and disease. Periodontol 2000. 2006;40:94–106. doi: 10.1111/j.1600-0757.2005.00142.x. [DOI] [PubMed] [Google Scholar]
- 24.Axelsson P, Nyström B, Lindhe J. The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. J Clin Periodontol. 2004;31:749–757. doi: 10.1111/j.1600-051X.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 25.Burt BA, Ismail AI, Eklund SA. Periodontal disease, tooth loss, and oral hygiene among older Americans. Community Dent Oral Epidemiol. 1985;13:93–96. doi: 10.1111/j.1600-0528.1985.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 26.Hujoel PP, Cunha-Cruz J, Loesche WJ, Robertson PB. Personal oral hygiene and chronic periodontitis: a systematic review. Periodontol 2000. 2005;37:29–34. doi: 10.1111/j.1600-0757.2004.03795.x. [DOI] [PubMed] [Google Scholar]
- 27.Helm JF, Dodds WJ, Pelc LR, Palmer DW, Hogan WJ, Teeter BC. Effect of esophageal emptying and saliva on clearance of acid from the esophagus. N Engl J Med. 1984;310:284–288. doi: 10.1056/NEJM198402023100503. [DOI] [PubMed] [Google Scholar]
- 28.Helm JF, Dodds WJ, Hogan WJ. Salivary response to esophageal acid in normal subjects and patients with reflux esophagitis. Gastroenterology. 1987;93:1393–1397. doi: 10.1016/0016-5085(87)90270-8. [DOI] [PubMed] [Google Scholar]
- 29.Kao CH, Ho YJ, ChangLai SP, Liao KK. Evidence for decreased salivary function in patients with reflux esophagitis. Digestion. 1999;60:191–195. doi: 10.1159/000007658. [DOI] [PubMed] [Google Scholar]
- 30.Campisi G, Lo Russo L, Di Liberto C, et al. Saliva variations in gastro-oesophageal reflux disease. J Dent. 2008;36:268–271. doi: 10.1016/j.jdent.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Di Fede O, Di Liberto C, Occhipinti G, et al. Oral manifestations in patients with gastro-oesophageal reflux disease: a single-center case-control study. J Oral Pathol Med. 2008;37:336–340. doi: 10.1111/j.1600-0714.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 32.Hirotomi T, Yoshihara A, Ogawa H, Ito K, Igarashi A, Miyazaki H. A preliminary study on the relationship between stimulated saliva and periodontal conditions in community-dwelling elderly people. J Dent. 2006;34:692–698. doi: 10.1016/j.jdent.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Hirotomi T, Yoshihara A, Ogawa H, Ito K, Igarashi A, Miyazaki H. Salivary spinability and periodontal disease progression in an elderly population. Arch Oral Biol. 2008;53:1071–1076. doi: 10.1016/j.archoralbio.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Sarosiek J, McCallum RW. What role do salivary inorganic components play in health and disease of the esophageal mucosa? Digestion. 1995;56(Suppl 1):24–31. doi: 10.1159/000201298. [DOI] [PubMed] [Google Scholar]
- 35.Socransky SS. Relationship of bacteria to the etiology of periodontal disease. J Dent Res. 1970;49:203–222. doi: 10.1177/00220345700490020401. [DOI] [PubMed] [Google Scholar]
- 36.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 37.Gorr SU, Abdolhosseini M. Antimicrobial peptides and periodontal disease. J Clin Periodontol. 2011;38(Suppl 11):126–141. doi: 10.1111/j.1600-051X.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 38.Watkins HR, Lapp CA, Hanes PJ, et al. CCL28 effects on periodontal pathogens. J Periodontol. 2007;78:2356–2363. doi: 10.1902/jop.2007.060504. [DOI] [PubMed] [Google Scholar]
- 39.Bridges RB, Anderson JW, Saxe SR, Gregory K, Bridges SR. Periodontal status of diabetic and non-diabetic men: effects of smoking, glycemic control, and socioeconomic factors. J Periodontol. 1996;67:1185–1192. doi: 10.1902/jop.1996.67.11.1185. [DOI] [PubMed] [Google Scholar]
- 40.Orbak R, Tezel A, Canakçi V, Demir T. The influence of smoking and non-insulin-dependent diabetes mellitus on periodontal disease. J Int Med Res. 2002;30:116–125. doi: 10.1177/147323000203000203. [DOI] [PubMed] [Google Scholar]
- 41.Ryan ME, Carnu O, Kamer A. The influence of diabetes on the periodontal tissues. J Am Dent Assoc. 2003;134 Spec No:34S–40S. doi: 10.14219/jada.archive.2003.0370. [DOI] [PubMed] [Google Scholar]
- 42.Marshall RI, Bartold PM. A clinical review of drug-induced gingival overgrowths. Aust Dent J. 1999;44:219–232. doi: 10.1111/j.1834-7819.1999.tb00224.x. [DOI] [PubMed] [Google Scholar]