Abstract
Background/Aims
The major compounds of Cochinchina momordica seed extract (SK-MS10) include momordica saponins. We report that the gastroprotective effect of SK-MS10 in an ethanol-induced gastric damage rat model is mediated by suppressing proinflammatory cytokines and downregulating cytosolic phospholipase A2 (cPLA2), 5-lipoxygenase (5-LOX), and the activation of calcitonin gene-related peptide. In this study, we evaluated the gastroprotective effects of SK-MS10 in the nonsteroidal anti-inflammatory drug (NSAID)-induced gastric damage rat model.
Methods
The pretreatment effect of SK-MS10 was evaluated in the NSAID-induced gastric damage rat model using aspirin, indomethacin, and diclofenac in 7-week-old rats. Gastric damage was evaluated based on the gross ulcer index by gastroenterologists, and the damage area (%) was measured using the MetaMorph 7.0 video image analysis system. Myeloperoxidase (MPO) was measured by enzyme-linked immunosorbent assay, and Western blotting was used to analyze the levels of cyclooxygenase (COX)-1, COX-2, cPLA2, and 5-LOX.
Results
All NSAIDs induced gastric damage based on the gross ulcer index and damage area (p<0.05). Gastric damage was significantly attenuated by SK-MS10 pretreatment compared with NSAID treatment alone (p<0.05). The SK-MS10 pretreatment group exhibited lower MPO levels than the diclofenac group. The expression of cPLA2 and 5-LOX was decreased by SK-MS10 pretreatment in each of the three NSAID treatment groups.
Conclusions
SK-MS10 exhibited a gastroprotective effect against NSAID-induced acute gastric damage in rats. However, its protective mechanism may be different across the three types of NSAID-induced gastric damage models in rats.
Keywords: Cochinchina momordica; Gastroprotection; Anti-inflammatory agents, non-steroidal; Arachidonate 5-lipoxygenase; Phospholipases A2, cytosolic
INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin are among the most commonly prescribed drugs in the world, used as anti-inflammatory and analgesic effect. Furthermore, aspirin is used for its antithrombotic effect. However, this agents have problems of gastrointestinal toxicity including gastric erosion, ulceration, bleeding, and perforation. The development of gastroduodenal ulcers can be explained by an imbalance between aggressive and defensive factors.1,2 Inhibition of gastric acid secretion by proton pump inhibitors (PPIs) or H2 receptor blockers as well as eradication of Helicobacter pylori have been the focus of peptic ulcer therapy. However, the frequency of NSAID-associated or H. pylori-negative peptic ulcer has increased, in which cases the ulcers are mainly caused by damage to the defensive mechanisms rather than an increase of acid secretion.3 PPIs are the only drugs shown to be useful for the prevention of peptic ulcer bleeding. However, the long-term safety of PPIs is unclear.4 Furthermore, as corpus atrophy has been reported to be more prevalent in Japanese than British patients, similar to Koreans, PPI could cause unwanted effects in the NSAID associated gastroduodenal damage in the aged Asian people. Thus, augmentation of the endogenous defensive mechanisms could be more useful than PPI for the prevention of peptic ulcer and/or associated bleeding as well as for the treatment of chronic ulcers due to NSAID.
Most studies examining gastric mucosal injury have investigated the mechanisms of NSAID-induced gastric mucosal lesions.5,6 The depletion of endogenous prostaglandins by inhibiting cyclooxygenase (COX) pathway is known to be a major pathogenic element in the development of these lesions. However, there are prostaglandin independent mechanisms including neutrophil activation,7 hypermotility,8 oxygen free radicals,9 and mucosal proinflammatory cytokines.10 In part due to increasing concern about gastrodefensive factors, gastroprotective agent such as rebamipide and eupatilin have been developed.3,11,12
Cochinchina momordica is the dried ripe seed of Momordica cochinchinensis, known as gac fruit, a perennial vine that is indigenous to Southeast Asia; it has traditionally been used for its anti-inflammatory activity and for suppurative skin infections. Chemical analysis shows that the C. momordica seeds are composed of compounds including fatty acids, saponins, proteins, α-spinasterol, oleanolic acid, and momordica acid. Among these compounds, momordica saponin I, glycoside, a triterpenoid saponin containing disaccharide chain, has been found to be a major active ingredient. The bioactive saponins were found to inhibit gastric mucosal lesions induced by ethanol or indomethacin in rats.13
Previously, we reported that the gastroprotective effect of C. momordica seed extract (SK-MS10) in an ethanol-induced gastric damage rat model is mediated by suppressing proinflammatory cytokines, downregulating cytosolic phospholipase A2 (cPLA2), 5-lipoxygenase (5-LOX), and activation of calcitonin gene-related peptide (CGRP).14 In the next experiment, we found that gross NSAID-induced gastric damage occurred in different patterns according to the type of NSAID such as aspirin, indomethacin, and diclofenac.15 Now we evaluated the effect of an extract from C. momordica seeds (SK-MS10) on the gastroprotective effects in a NSAID-induced acute gastric mucosal damage rat model.
MATERIALS AND METHODS
1. Preparation and composition of SK-MS10
SK-MS10 was supplied by Life Science R&D Center of the SK Chemicals Co., Ltd. (Seongnam, Korea). SK-MS10 was prepared as follows. Five milliliters of aqueous ethanol solution was added to 1 kg (dry weight) of C. momordica, which was purchased at the herb market in Korea. Extraction was performed for 4 hours at 80℃, and this process was performed twice. The extract was filtered and concentrated under reduced pressure at 60℃ using a rotary evaporator. After complete removal of the solvent in a vacuum oven, 21 g of ethanol extract in powder form (SK-MS10) was obtained. SK-MS10 was dissolved in the carboxymethylcellulose (CMC) during the experiment.
2. Animals
Six-week-old male Sprague-Dawley rats (Orient Co., Ltd., Seoul, Korea) were housed in a cage maintained at 23℃, 12/12-hour light/dark cycle under specific pathogen-free conditions. After 1 week of adaptation, 7-week-old rats weighing 250 to 300 g were used for the experiments. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University Bundang Hospital (IACUC number: BA1004-060/018-01).
3. Experimental design
Three groups including control (n=3), pretreatment of SK-MS10 (n=18), and NSAIDs only (n=18) were used for this study. Gastric damage in pretreatment of SK-MS10 and NSAIDs only groups was induced by aspirin (200 mg/kg, n=6), indomethacin (40 mg/kg, n=6), and diclofenac (80 mg/kg, n=6), respectively. The detail process was indicated as below.
The rats were starved but given water for 24 hours prior to the experiments. Before 1 hour of administration of NSAIDs, SK-MS 10 (200 mg/kg) were administered orally by gavage. NSAIDs (aspirin [Sigma-Aldrich Co., St. Louis, MO, USA; 200 mg/kg body weight], indomethacin [Sigma-Aldrich Co.; 40 mg/kg body weight], or diclofenac [Sigma-Aldrich Co.; 80 mg/kg body weight]) or 0.5% CMC (5 mL/kg body weight) as a control were administered orally by gavage through a metal tube attached to a 5- or 10-mL syringe. Each group consisted of six rats. The indomethacin solution was dissolved in 0.5% (wt/vol) CMC with 10% ethanol, pH ranging from 1.3 to 1.5. The aspirin solution was prepared in the same way but with 20% ethanol which maintained aspirin in its readily-absorbed nonionized form. The diclofenac solution was dissolved in 0.5% (wt/vol) CMC. As we used 10% and 20% ethanol to dissolve indomethacin and aspirin, respectively, we tested these percentages on control rat to confirm that it was not a potential confounder. There was no specific lesion occurred with 20% ethanol similar to 0.5% CMC, and 0.5% CMC was administered into control group as a vehicle. Twenty-four hours after NSAID or vehicle administration, the animals were humanely sacrificed and the gastric lesions were scored.
4. Gross ulcer index and damage area
After sacrifice, the isolated stomachs were cut open along the greater curvature and washed in ice-cold saline. To investigate the degree of gross mucosal damage, the mucosal sides of the stomachs were photographed using a digital camera, and part of mucosa was immediately fixed with 10% formalin solution. The gross damage of the gastric mucosa was assessed by an experienced gastroenterologist, who was blind to the treatments, using a gross ulcer index, defined as (number of type I lesions)+(number of type II lesions)×2+(number of type III lesions)×3. The lesion type was classified as follows: type I, presence of edema, hyperemia, or a single submucosal punctiform hemorrhage; type II, presence of submucosal hemorrhagic lesions with small erosions; and type III, presence of a deep ulcer with erosions and invasive lesions.
A total injury score for each stomach was calculated by summing the gross ulcer index of all lesions in that stomach. In addition, the damage area was also measured by the image program. The areas of gross damage (erosion or ulceration) were measured by using a computerized video analysis system (MetaMorph 7.0; Molecular Devices, Downington, PA, USA). The area of mucosal damage was expressed as a percentage of the total mucosal area.
5. Measurement of mucosal myeloperoxidase
An assay of gastric mucosal myeloperoxidase (MPO) concentration was used to quantify the degree of neutrophil infiltration. Three hundred milligrams of scraped mucosa was homogenized for 30 seconds with a polytron homogenizer in 1.0 mL of ice-cold 0.5% hexadecyltrimethylammonium bromide in 50 mM of phosphate buffer (pH 6.0). Hexadecyltrimethylammonium bromide was used to negate the pseudoperoxidase activity of hemoglobin and to solubalize the membrane-bound MPO. The homogenate was sonicated for 10 seconds, freeze-thawed three times and centrifuged for 20 minutes at 18,000 g. The supernatant was isolated and evaluated for the determination of the enzyme concentration, utilizing an enzyme-linked immunosorbent assay kit (Immundiagnostik AG, Bensheim, Germany).
6. Western blotting for cPLA2, 5-LOX, COX-1, and COX-2
The gastric mucosa was homogenized with lysis buffer containing 25 mM Tris-HCL (pH 7.4), ethylene glycol tetraacetic acid (1 mM), dithiothreitol (1 mM), leupeptin (10 µg/mL), aprotinin (10 µg/mL), phenylmethylsulfonyl fluoride (1 mM), and Triton X-100 (0.1%). Briefly, the proteins (each sample, 30 µg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (7.5% wt/wt gel) and transferred to nitrocellulose membranes. All procedures were done in Tris buffer (40 mM, pH 7.55) containing 0.3 M of NaCl and 0.3% Tween 20. The membranes were then blocked with dried milk (6% wt/vol), and subsequently incubated with cPLA2 antibody (mouse monoclonal IgG2b antibody, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), 5-LOX polyclonal antibody (rabbit antibody 1:500; Cayman Chemical, Ann Arbor, MI, USA), COX-1, and COX-2 antibody (goat polyclonal IgG antibody, 1:1,000; Santa Cruz Biotechnology) at 4℃ overnight. The blots were incubated with secondary antibody (goat polyclonal antibody, 1:5,000; Santa Cruz Biotechnology) and an imaging analyzer was used to measure the band densities.
7. Real time polymerase chain reaction for CGRP, COX-1, and COX-2
RNA was extracted from the gastric mucosa using the RNeasy Plus Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. RNA samples were diluted to a final concentration of 0.5 mg/mL in RNase-free water and stored at -80℃ until use. Synthesis of the cDNA was performed with 1 mg of total RNA with M-MLV Reverse Transcription Reagents (Invitrogen, Carlsbad, CA, USA). The 20-µL reverse transcription reaction consisted of 4 µL of first-strand buffer, 500 mM deoxynucleoside triphosphate mixture, 2.5 mM oligo (dT) primer, 0.4 U/mL ribonuclease inhibitor, and 1.25 U/mL moloney murine leukemia virus reverse transcriptase (Invitrogen). The thermal cycling parameters for the reverse transcription were 5 minutes at 65℃, 50 minutes at 37℃, and 15 minutes at 70℃. Real time polymerase chain reaction (PCR) amplification and determination were performed using the ABI PRISM 7000 Sequence Detection System, TaqMan universal PCR master mix, commercially available predesigned, gene specific primers, and FAM-labelled probe sets for quantitative gene expression (TaqMan Gene Expression Assays, rodent CGRP, mouse β-actin; Applied Biosystems, Foster City, CA, USA). All of the probes used in these experiments spanned an exon-intron boundary. The CGRP, COX-1, COX-2, and β-actin mRNA were quantified by parallel estimation. The thermal cycler conditions were 2-minute hold at 50℃ and 10-minute hold at 95℃, followed by 40 cycles of 15 seconds at 95℃ and 1 minute at 60℃.
8. Statistical analysis
All statistical calculations were performed using SPSS software version 18.0 (IBM Co., Armonk, NY, USA). The results were compared using the Mann-Whitney U test and the Wilcoxon rank sum test. All values are reported as means±standard errors. Null hypotheses of no difference were rejected if p-values were less than 0.05.
RESULTS
1. Effect of SK-MS10 on acute gastric lesions induced by NSAIDs
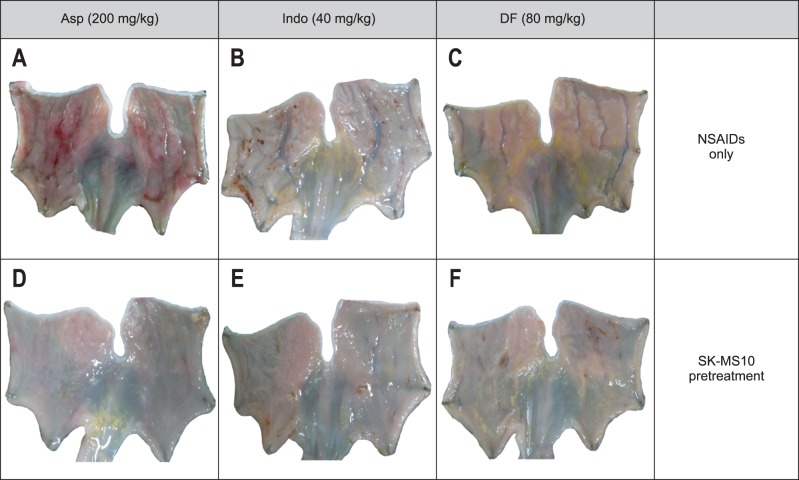
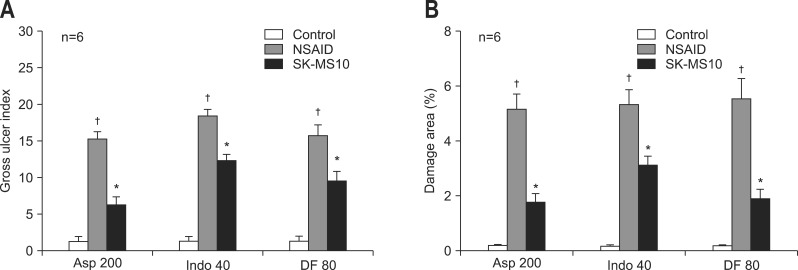
The gross appearance of gastric damage patterns was different depending on the type of NSAIDs (Fig. 1A-C). Aspirin usually caused shallow and linear shape ulcerations, which were associated with hemorrhage more often than those caused by indomethacin or diclofenac. Indomethacin caused a punctuate type clean ulcer. The gastric damage induced by dicolfenac was similar to that caused by indomethacin, but the depth of ulcers was shallow and the damage extent was less severe than that caused by indomethacin. Even though the gross damage looked to be greater in the aspirin group by Fig. 1 but the gross ulcer index or damage area (%) was similar in three groups. All gastric ulcers caused by NSAIDs were significantly attenuated in the SK-MS10 pretreatment group (Figs 1D-F and 2). The significant decrease of NSAID-induced gastric ulcer index by pretreatment of SK-MS10 (Fig. 2A) was very similar to the decrease of the damage area measured by the image program (Fig. 2B).
Fig. 1.
Gross findings of the gastric damage caused by aspirin (200 mg/kg), indomethacin (40 mg/kg), and diclofenac (80 mg/kg), and the gastroprotective effect of SK-MS10 pretreatment in rats. (A) Rat stomach after exposure to aspirin. (B) Rat stomach after exposure to indomethacin. (C) Rat stomach after exposure to diclofenac. (D) Rat stomach after exposure to aspirin with SK-MS10 pretreatment. (E) Rat stomach after exposure to indomethacin with SK-MS10 pretreatment. (F) Rat stomach after exposure to diclofenac with SK-MS10 pretreatment.
Asp, aspirin; Indo, indomethacin; DF, diclofenac; NSAIDs, nonsteroidal anti-inflammatory drugs.
Fig. 2.
(A) Gross ulcer index and (B) damage area as assessed by the image program in the stomachs of 7-week-old rats. The areas of gross damage (erosion or ulceration) were measured using a computerized video analysis system (MetaMorph 7.0; Molecular Devices). The area of mucosal damage was expressed as a percentage of the total mucosal area. The mean±SE with six rats per group.
Asp 200, aspirin 200 mg/kg; Indo 40, indomethacin 40 mg/kg; DF 80, diclofenac 80 mg/kg.
*p<0.05 compared with the nonsteroidal anti-inflammatory drug (NSAID) group; †p<0.05 compared with the control group.
2. Measurement of MPO and real-time PCR of CGRP in the mucosa
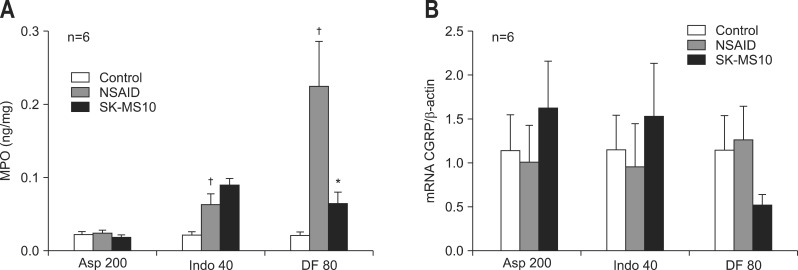
The mucosal levels of MPO significantly increased in indomethacin and diclofenac treatment groups than the control group. After SK-MS10 administration, the mucosal MPO concentration significantly decreased in the diclofenac treated rats (Fig. 3A). The mRNA expression of CGRP in the gastric mucosa increases with SK-MS10 pretreatment in aspirin and indomethacin groups (Fig. 3B). However, in diclofenac group, the CGRP expression by SK-MS10 decreased, contrary to other NSAID groups.
Fig. 3.
(A) Mucosal concentrations of myeloperoxidase (MPO) and (B) real-time polymerase chain reaction of calcitonin gene-related peptide (CGRP) in the stomachs of 7-week-old rats. The mean±SE with six rats per group.
Asp 200, aspirin 200 mg/kg; Indo 40, indomethacin 40 mg/kg; DF 80, diclofenac 80 mg/kg.
*p<0.05 compared with the nonsteroidal anti-inflammatory drug (NSAID) group; †p<0.05 compared with the control group.
3. Western blotting of cPLA2 and 5-LOX in the mucosa
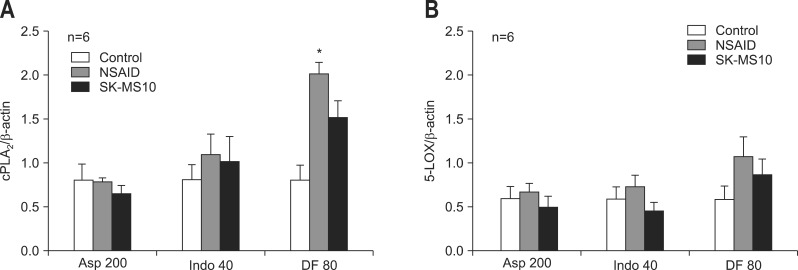
The expression of cPLA2 significantly increased over the control group in diclofenac group after NSAIDs administration. The expression of cPLA2 and 5-LOX increased with NSAID administration except in the aspirin group. This increased expression of cPLA2 and 5-LOX was reduced by SK-MS10 pretreatment in all three NSAID groups (Fig. 4).
Fig. 4.
(A) Western blotting of cytosolic phospholipase A2 (cPLA2) and (B) 5-lipoxygenase (5-LOX) in the stomachs of 7-week-old rats. The mean±SE with six rats per group.
Asp 200, aspirin 200 mg/kg; Indo 40, indomethacin 40 mg/kg; DF 80, diclofenac 80 mg/kg.
*p<0.05 compared with the control group.
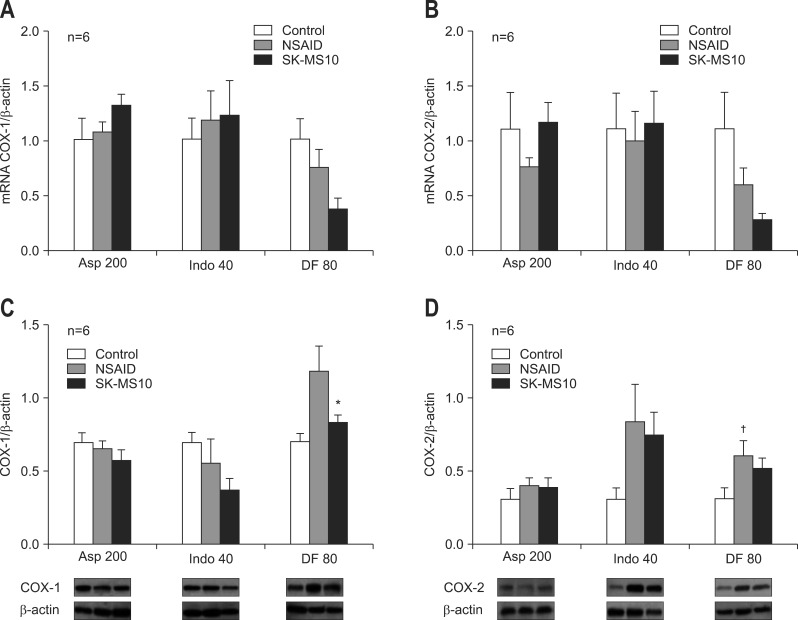
4. Real-time PCR and Western blotting of COX-1 and COX-2 in the mucosa
Real-time PCR of COX-1 (Fig. 5A) and COX-2 (Fig. 5B) did not show significant change by NSAID or by pretreatment of SK-MS10. However, diclofenac decreased the COX-1 and COX-2 and pretreatment of SK-MS10 further decreased expression of COX-1 and COX-2. Compared with control group and SK-MS10 pretreatment group in diclofenac, the expression of mRNA COX-1 (p<0.05) and COX-2 (p<0.05) was significantly decreased.
Fig. 5.
(A) Real-time polymerase chain reaction of cyclooxygenase (COX)-1, (B) COX-2, (C) Western blotting of COX-1, and (D) COX-2 in the stomachs of 7-week-old rats. The mean±SE with six rats per group.
Asp 200, aspirin 200 mg/kg; Indo 40, indomethacin 40 mg/kg; DF 80, diclofenac 80 mg/kg.
*p<0.05 compared with the nonsteroidal anti-inflammatory drug (NSAID) group; †p<0.05 compared with the control group.
The Western blotting showed that diclofenac increased the expression of COX-1 and pretreatment of SK-MS10 significantly decreased it (Fig. 5C). In case of Western blotting of COX-2 diclofenac significantly increased its expression and pretreatment of SK-MS10 decreased it but without statistical change (Fig. 5D). Otherwise, there was no significant change in case of aspirin and indomethacin.
DISCUSSION
In our previous study, increased expression of cPLA2 and 5-LOX after ethanol administration was attenuated by SK-MS10 pretreatment.14 In addition, SK-MS10 pretreatment reduced increase of mucosal tumor necrosis factor-α, interleukin-1β, and MPO concentrations after ethanol administration.14 And another recent study about SK-MS10 showed that its protective effect against cysteamine-induced duodenal ulcer by either cPLA2/5-LOX or glutathione preservation.16 These results suggested that the gastroprotective mechanisms of SK-MS10 in the ethanol model are anti-inflammatory activity by suppressing proinflammatory cytokines and down-regulating cPLA2/5-LOX pathway. In the present study, SK-MS10 significantly attenuated gastric lesions caused by three different kinds of NSAIDs. However, the changes of inflammatory mediators such as MPO, cPLA2, and 5-LOX were rather weak in the NSAID models. The trend was similar in case of MPO. That is, in the ethanol model the level of MPO was 2.42 ng/mg,14 which is quite higher than those of MPO levels of 0.03, 0.06, and 0.23 ng/mg induced by aspirin, indomethacin, and diclofenac administration, respectively. This discrepancy of MPO level was thought to originate from the different type of damage. In case of 100% ethanol-induced gastropathy, it could act partly as a directly corrosive tissue irritant because of the chemical agent itself. Interestingly, the expression of cPLA2 was relatively higher in the diclofenac group than in other NSAIDs (Fig. 4). MPO is most abundantly expressed in neutrophil granulocytes and leukotrien B4, by-product of cPLA2 and 5-LOX, is the major chemotactic factor for leukocytes. Relatively increased expression of cPLA2 in the diclofenac group could be a clue why MPO level of diclofenac was higher comparison to that of other NSAID groups. However, regarding mechanisms of NSAID-induced gastropathy, there are various factors including depletion of prostaglandin by inhibition of COX, neutrophil infiltration, decrease of gastric mucus production, hypermotility, and oxygen free radicals.5,6,8 So there could be some differences of gastroprotective effects by SK-MS10 pretreatment between ethanol-induced and NSAIDs-induced models. That is, the anti-inflammatory effect might be more important in ethanol model, but in NSAID induced gastric damage model we could not find a definite mediator.
CGRP has been reported to stimulate mucin synthesis through an NO-dependent mode of action in the rat gastric corpus and induces vasodilation partly.17,18 As impaired gastric mucosal circulation is one of the key factors in ethanol induced gastric mucosal damage,19,20 activation of CGRP-NO pathway by pretreatment with SK-MS10 was thought to be an important role especially in our previous ethanol model.14 In the aspirin and indomethacin group of the present study, the expression of CGRP was increased by SK-MS10 pretreatment, as expected, although there was no statistical significance. However, in diclofenac group, the CGRP expression by SK-MS10 was decreased contrary to other NSAID groups again without statistical significant. There are various mechanisms concerning NSAID-induced gastropathy, and there could be some differences of mechanisms according to kind of NSAIDs, which could cause different gross finding of NSAID-induced gastric damage in the present study.
Prostaglandin E2 (PGE2) is an important mediator for gastric mucosal protection by decreasing stomach acid secretion, increasing the thickness of mucus layer, and improving the blood flow of mucosa.21,22 Inhibition of COX-2 is thought to be responsible for NSAID's anti-inflammatory effect, while inhibition of COX-1 is responsible for its gastrointestinal side effect as a result of reduced prostaglandin synthesis.23 So, it was expected that NSAIDs might inhibit expression of COX-1 and SK-MS10 pretreatment would reverse it. However, there was no significant decrease of COX-1 by three kinds of NSAIDs either by real time PCR of Western blotting in the present study. However, diclofenac provoked the expression of COX-1 by Western blotting, which was significantly decreased by SK-MS10. In case of COX-2 we also expected that its expression would be decreased by NSAIDs. Similar to COX-1 there was no statistical change in three kinds of NSAIDs. However, diclofenac significantly provoked the expression of COX-2 by Western blotting. It is known that the inhibitory potencies on COX-1 and COX-2 enzymes are different according to the type of NSAIDs.24,25 These different potencies on COX isoforms might be related with different expression of mRNA and Western blotting of COX-1 and COX-2 in the present study. The expressions of COX-1 and COX-2 were similar in the aspirin and indomethacin group but opposite in the diclofenac group. Previous studies showed that the direction of inhibitory potency of diclofenac on COX-1 and COX-2 was similar while the inhibitory potency on COX-1 was stronger than COX-2 in aspirin and indomethacin.26-29 And expression of mRNA and Western blotting of COX-2 in aspirin only group was relatively less than the other NSAIDs only group. By acetylation of COX-2, aspirin triggers formation of lipoxins, a lipid mediator showing anti-inflammatory effects. In addition, it has been suggested that acetylation of COX-2 by aspirin could be related with decreased expression of COX-2.30 Another study suggested that gastric ulcerogenic properties of NSAIDs are not accounted for solely by COX-1 inhibition, but require the inhibition of both COX-1 and COX-2.31 The inhibition of COX-1 up-regulates COX-2 expression, and COX-2/prostaglandin may, in turn, counteract the deleterious effects of gastric hypermotility due to COX-1 inhibition.31
In the present study, the trend of mucosal level of cPLA2, 5-LOX agree among the three NSAIDs. However, the trends of CGRP, COX-1, and COX-2 were consistent in aspirin and indomethacin group while being different in diclofenac group. Actually, ulcerogenic mechanisms of each NSAID are a little different according to their own properties. An indomethacin induced ulcer model was widely used for higher ulcerogenic potential than other NSAIDs. In this indomethacin ulcer model, various antiulcer agents showed some different mechanisms.23 That is, a previous study reported that presynaptic α 2 receptors play a role in the inhibition of indomethacin-, aspirin-, ethanol-, stress-, and pyloric-ligastion-induced ulcers.32 Another study hypothesized that stimulation of α 2 adrenergic receptors may be responsible for the increase of protective factors induced by antiulcer drug.23 In addition, recently lipoxins have been reported to exert important anti-inflammatory.33 The inhibition of cPLA2/5-LOX pathway was thought to be common with both the ethanol and NSAID ulcer model. The pathogenesis of NSAID-induced gastrointestinal damage may also depend on prostaglandin-independent mechanisms, such as uncoupling of oxidative phosphorylation, alterations of mucosal cell turnover as well as neutrophil activation followed by enhanced endothelial adhesion. As the protective effect of SK-MS10 in the NSAID-induced gastric damage model in rat could not be explained by cPLA2/5-LOX pathway, CGRP or COX-1 and COX-2 in the present study the antioxidative property by SK-MS10 might play a role in the NSAID-induced gastric damage model in rat.
There have been many studies about gastroprotective effects of flavonoids in plant extracts showing various mechanisms.33,34 Other studies about gastroprotective agent such as rebamipide, eupatilin have proposed that prostaglandin-independent mechanisms.3,11,12 Taiwan study showed that sesamol potently reduced diclofenac-induced mucosal damage, but neither increased nor maintained diclofenac-induced decreases of PGE2, mucus, or COX activity.35 Aspirin, the classic NSAID, does not produce gastric damage, despite its inhibiting PG production as effectively as the other NSAIDs. Moreover, ulceration does not develop spontaneously in mice with a disrupted COX-1 gene. A mechanism other than the COX pathway might be important in NSAID-induced gastric mucosal injury.35 And there was also a study which suggested that PPIs could activate gastric protective mechanisms independent of acid reduction.36
Our study has several limitations. First, there was no data about prostaglandin and leukotriene which is by-product of COX, 5-LOX, and cPLA2. Second, we did not measure other antioxidative mediators such as lipoxins. Recently lipoxins, a lipid mediator, have been reported to exert to important anti-inflammatory effects in addition to the mucosal protective actions. Third, we focused reproducibility of inhibition mechanism of cPLA2/5-LOX and activation of CGRP by SK-MS10 in the NSAID model similar to the ethanol model. However, it is necessary to explore PLA2 expression, LOX expression, and COX more in detail not by mechanistic manner. In the future, further experiments are necessary. Fourth, as much as the protective effect of SK-MS10 matter, the core results were related to the difference in NSAID-induced gastric damages according to kinds of NSAID. However, we could not provide appropriate explanations or speculations as well as plausible mechanisms in this matter in the present study. In the future we would like to provide accurate answer for this question by further research.
In conclusion, SK-MS10 has a gastroprotective effect against NSAID-induced gastric damage rat model. However, its protective mechanism might be different in three kinds of NSAID-induced gastric damage model in rat, requiring further research.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant for the Global Core Research Center (GCRC) funded by the Korea government (MSIP) (No. 2011-0030001).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Allen A, Flemström G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev. 1993;73:823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- 2.Flemstrom G, Garner A. Gastroduodenal HCO3(-) transport: characteristics and proposed role in acidity regulation and mucosal protection. Am J Physiol. 1982;242:G183–G193. doi: 10.1152/ajpgi.1982.242.3.G183. [DOI] [PubMed] [Google Scholar]
- 3.Arakawa T, Higuchi K, Fujiwara Y, et al. 15th anniversary of rebamipide: looking ahead to the new mechanisms and new applications. Dig Dis Sci. 2005;50(Suppl 1):S3–S11. doi: 10.1007/s10620-005-2800-9. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy DM. Adverse effects of proton pump inhibitor drugs: clues and conclusions. Curr Opin Gastroenterol. 2010;26:624–631. doi: 10.1097/MOG.0b013e32833ea9d9. [DOI] [PubMed] [Google Scholar]
- 5.Beck PL, Xavier R, Lu N, et al. Mechanisms of NSAID-induced gastrointestinal injury defined using mutant mice. Gastroenterology. 2000;119:699–705. doi: 10.1053/gast.2000.16497. [DOI] [PubMed] [Google Scholar]
- 6.Yoshikawa T, Naito Y, Kishi A, et al. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut. 1993;34:732–737. doi: 10.1136/gut.34.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida N, Yoshikawa T, Nakamura Y, et al. Role of neutrophil-mediated inflammation in aspirin-induced gastric mucosal injury. Dig Dis Sci. 1995;40:2300–2304. doi: 10.1007/BF02063228. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi K, Tanaka A, Hayashi Y, Kubo Y. Functional mechanism underlying COX-2 expression following administration of indomethacin in rat stomachs: importance of gastric hypermotility. Dig Dis Sci. 2004;49:180–187. doi: 10.1023/b:ddas.0000017436.05273.fd. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka J, Yuda Y, Yamakawa T. Mechanism of superoxide generation system in indomethacin-induced gastric mucosal injury in rats. Biol Pharm Bull. 2001;24:155–158. doi: 10.1248/bpb.24.155. [DOI] [PubMed] [Google Scholar]
- 10.Okada A, Kinoshita Y, Waki S, et al. Rat gastric mucosal cells express ICAM-1 and proinflammatory cytokines during indomethacin-induced mucosal injury. J Lab Clin Med. 1998;131:538–547. doi: 10.1016/s0022-2143(98)90062-2. [DOI] [PubMed] [Google Scholar]
- 11.Arakawa T, Kobayashi K, Yoshikawa T, Tarnawski A. Rebamipide: overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing. Dig Dis Sci. 1998;43(9 Suppl):5S–13S. [PubMed] [Google Scholar]
- 12.Choi SM, Shin JH, Kang KK, Ahn BO, Yoo M. Gastroprotective effects of DA-6034, a new flavonoid derivative, in various gastric mucosal damage models. Dig Dis Sci. 2007;52:3075–3080. doi: 10.1007/s10620-006-9657-4. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda H, Li Y, Murakami T, Yamahara J, Yoshikawa M. Protective effects of oleanolic acid oligoglycosides on ethanol- or indomethacin-induced gastric mucosal lesions in rats. Life Sci. 1998;63:PL245–PL250. doi: 10.1016/s0024-3205(98)00426-3. [DOI] [PubMed] [Google Scholar]
- 14.Kang JM, Kim N, Kim B, et al. Gastroprotective action of Cochinchina momordica seed extract is mediated by activation of CGRP and inhibition of cPLA(2)/5-LOX pathway. Dig Dis Sci. 2009;54:2549–2560. doi: 10.1007/s10620-008-0671-6. [DOI] [PubMed] [Google Scholar]
- 15.Seo PJ, Kim N, Kim JH, et al. Comparison of indomethacin, diclofenac and aspirin-induced gastric damage according to age in rats. Gut Liver. 2012;6:210–217. doi: 10.5009/gnl.2012.6.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi KS, Kim EH, Hong H, et al. Attenuation of cysteamine-induced duodenal ulcer with Cochinchina momordica seed extract through inhibiting cytoplasmic phospholipase A2/5-lipoxygenase and activating gamma-glutamylcysteine synthetase. J Gastroenterol Hepatol. 2012;27(Suppl 3):13–22. doi: 10.1111/j.1440-1746.2012.07065.x. [DOI] [PubMed] [Google Scholar]
- 17.Ichikawa T, Ishihara K, Kusakabe T, Hiruma H, Kawakami T, Hotta K. CGRP modulates mucin synthesis in surface mucus cells of rat gastric oxyntic mucosa. Am J Physiol Gastrointest Liver Physiol. 2000;279:G82–G89. doi: 10.1152/ajpgi.2000.279.1.G82. [DOI] [PubMed] [Google Scholar]
- 18.Lambrecht N, Burchert M, Respondek M, Müller KM, Peskar BM. Role of calcitonin gene-related peptide and nitric oxide in the gastroprotective effect of capsaicin in the rat. Gastroenterology. 1993;104:1371–1380. doi: 10.1016/0016-5085(93)90345-d. [DOI] [PubMed] [Google Scholar]
- 19.Gyires K, Hermecz I, Knoll J. The effect of some anti-ulcer agents on the early vascular injury of gastric mucosa induced by ethanol in rats. Acta Physiol Hung. 1989;73:149–154. [PubMed] [Google Scholar]
- 20.Szabo S, Trier JS, Brown A, Schnoor J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology. 1985;88(1 Pt 2):228–236. doi: 10.1016/s0016-5085(85)80176-1. [DOI] [PubMed] [Google Scholar]
- 21.Peskar BM, Maricic N. Role of prostaglandins in gastroprotection. Dig Dis Sci. 1998;43(9 Suppl):23S–29S. [PubMed] [Google Scholar]
- 22.Buttgereit F, Burmester GR, Simon LS. Gastrointestinal toxic side effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2-specific inhibitors. Am J Med. 2001;110(Suppl 3A):13S–19S. doi: 10.1016/s0002-9343(00)00728-2. [DOI] [PubMed] [Google Scholar]
- 23.Suleyman H, Albayrak A, Bilici M, Cadirci E, Halici Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation. 2010;33:224–234. doi: 10.1007/s10753-009-9176-5. [DOI] [PubMed] [Google Scholar]
- 24.Simon LS. Role and regulation of cyclooxygenase-2 during inflammation. Am J Med. 1999;106(5B):37S–42S. doi: 10.1016/s0002-9343(99)00115-1. [DOI] [PubMed] [Google Scholar]
- 25.Brzozowski T, Konturek PC, Konturek SJ, et al. Classic NSAID and selective cyclooxygenase (COX)-1 and COX-2 inhibitors in healing of chronic gastric ulcers. Microsc Res Tech. 2001;53:343–353. doi: 10.1002/jemt.1102. [DOI] [PubMed] [Google Scholar]
- 26.Gambero A, Maróstica M, Becker TL, Pedrazzoli J., Jr Effect of different cyclooxygenase inhibitors on gastric adaptive cytoprotection induced by 20% ethanol. Dig Dis Sci. 2007;52:425–433. doi: 10.1007/s10620-006-9487-4. [DOI] [PubMed] [Google Scholar]
- 27.Jang SW, Lee JW, Park SH, et al. Gastroretentive drug delivery system of DA-6034, a new flavonoid derivative, for the treatment of gastritis. Int J Pharm. 2008;356:88–94. doi: 10.1016/j.ijpharm.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 28.Graumlich JF. Preventing gastrointestinal complications of NSAIDs. Risk factors, recent advances, and latest strategies. Postgrad Med. 2001;109:117–128. doi: 10.3810/pgm.2001.05.931. [DOI] [PubMed] [Google Scholar]
- 29.DeWitt DL, Meade EA, Smith WL. PGH synthase isoenzyme selectivity: the potential for safer nonsteroidal antiinflammatory drugs. Am J Med. 1993;95(2A):40S–44S. doi: 10.1016/0002-9343(93)90396-7. [DOI] [PubMed] [Google Scholar]
- 30.Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka A, Araki H, Hase S, Komoike Y, Takeuchi K. Up-regulation of COX-2 by inhibition of COX-1 in the rat: a key to NSAID-induced gastric injury. Aliment Pharmacol Ther. 2002;16(Suppl 2):90–101. doi: 10.1046/j.1365-2036.16.s2.22.x. [DOI] [PubMed] [Google Scholar]
- 32.DiJoseph JF, Eash JR, Mir GN. Gastric antisecretory and antiulcer effects of WHR1582A, a compound exerting alpha-2 adrenoceptor agonist activity. J Pharmacol Exp Ther. 1987;241:97–102. [PubMed] [Google Scholar]
- 33.Zayachkivska OS, Konturek SJ, Drozdowicz D, Konturek PC, Brzozowski T, Ghegotsky MR. Gastroprotective effects of flavonoids in plant extracts. J Physiol Pharmacol. 2005;56(Suppl 1):219–231. [PubMed] [Google Scholar]
- 34.Chatterjee A, Chattopadhyay S, Bandyopadhyay SK. Biphasic effect of phyllanthus emblica L. extract on NSAID-induced ulcer: an antioxidative trail weaved with immunomodulatory effect. Evid Based Complement Alternat Med. 2011;2011:146808. doi: 10.1155/2011/146808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu DZ, Chu PY, Li YH, Liu MY. Sesamol attenuates diclofenac-induced acute gastric mucosal injury via its cyclooxygenase-independent antioxidative effect in rats. Shock. 2008;30:456–462. doi: 10.1097/SHK.0b013e3181672185. [DOI] [PubMed] [Google Scholar]
- 36.Blandizzi C, Fornai M, Colucci R, et al. Lansoprazole prevents experimental gastric injury induced by non-steroidal anti-inflammatory drugs through a reduction of mucosal oxidative damage. World J Gastroenterol. 2005;11:4052–4060. doi: 10.3748/wjg.v11.i26.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]