Abstract
Background/Aims
Inflammatory pseudotumor (IPT) of the liver is a rare disease characterized by chronic infiltration of inflammatory cells. However, the clinical characteristics and outcomes of IPT remain uncertain.
Methods
Clinical features, image findings, and outcomes of 55 patients with histologically proven IPT were evaluated.
Results
They consisted of 26 men and 19 women with median age of 65 years. Serum carcinoembryonal antigen and carbohydrate antigen 19-9 levels were normal in 42 patients (93.3%). Enhanced CT scans indicated poorly defined peripheral enhancement (82.5%) at the arterial phase and poorly defined hyperattenuating lesions with internal hypoattenuating areas at the equilibrium phase (77.0%). Gadolinium-enhancement MRI revealed poorly defined peripheral rim-like enhancement (77.8%). Ten patients underwent surgical resection and 35 were treated conservatively with or without antibiotics. No recurrence was noted after surgical resection during follow-up (1 to 48 months). In all patients who received conservative treatment, complete resolution or size reduction was noted during follow-up (1 to 192 months).
Conclusions
CT and MRI provide clues to the diagnosis of IPT in patients with liver masses and normal tumor markers. However, due to the lack of pathognomonic findings, the clinician's suspicion and histological diagnosis are necessary to make an accurate diagnosis of IPT.
Keywords: Inflammatory pseudotumor, Liver
INTRODUCTION
Inflammatory pseudotumor (IPT) or inflammatory myofibroblastic tumor is a relatively rare disease characterized by chronic infiltration of inflammatory cells and area of fibrosis.1 It is also known as plasma cell granuloma due to the predominant infiltration of plasma cells.2 Etiology of IPT remains uncertain although infectious condition, autoimmune phenomenon, or systemic inflammatory response syndrome has been suggested as possible one.2-5 Nowadays, IPTs are more frequently recognized due to increasing number of abdominal imaging performed in clinical practice. IPT occurs most commonly in the lung, but can be found in other locations including spinal cord, eye, spleen, lymph node, soft tissue, and liver.6-8
IPT of liver is quite infrequent and accounts for 8% of extrapulmonary IPTs. Incidence of IPT of liver was reported to be around 0.7% in recent studies.2,9,10 However, since most previous studies were descriptive ones involving only small numbers of patients, clinical features and image findings of this rare condition remains uncertain. Despite recent progress in diagnostic ability of radiologic study, it is difficult to differentiate IPT of liver from malignant tumor such as atypical hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (CC) and metastatic cancer11,12 or liver abscess showing incomplete liquefaction and granulation.10
Furthermore, optimal treatment and prognosis have not been established.10,13-15 Due to its diagnostic ambiguity, some cases with IPT of the liver were diagnosed and treated through surgical resection. Other cases were reported to be successfully managed with antibiotics, nonsteroidal anti-inflammatory drugs,16 or no medication.2,12,17,18
Aim of this report is to evaluate the clinical features, laboratory and image findings, treatment, and prognosis of IPT of liver in a large number of patients.
MATERIALS AND METHODS
Forty-five patients diagnosed as IPT at three major hospitals in Korea (Samsung Medical Center, Asan Medical Center, and Yonsei University Severance Hospital) between February 1995 and January 2011 were consecutively enrolled in this study. IPT was histologically confirmed with percutaneous needle biopsy (n=35), surgical resection (n=9), or both (n=1) in all patients. Their demographic and clinical features, laboratory and image findings, treatments, and outcomes were evaluated retrospectively.
Multiphase contrast-enhanced computed tomographic (CT) images were obtained with an multidetector computed tomographic scanner (LightSpeed VCT; GE Healthcare, Milwaukee, WI, USA). A total 120 mL of nonionic contrast material (300 mg I/mL iopromide, Ultravist 300; Bayer Schering Pharma, Berlin, Germany) was administered intravenously with an automatic injector at a rate of 3 to 4 mL/sec. Triple-phase CT images from the dome of the diaphragm to the lower pole of the right kidney were obtained during a single breath-hold 25 to 35, 60 to 70, and 180 seconds after the initiation of the contrast injection, representing the hepatic arterial, portal venous, and equilibrium phases of enhancement. The axial images were reconstructed with 5.0-mm slice thickness and reconstruction interval.
Magnetic resonance images were acquired with a 3-T whole body magnetic resonance imaging (MRI) system (InteraAchieva 3 T; Philips Medical Systems, Best, the Netherlands) with a 16 channel phased array coil as the receiver coil. The liver was imaged in the axial plane in all sequences. For gadoxetic acid-enhanced MRI, acquisitions in the unenhanced, arterial (20 to 35 seconds), portal venous (60 seconds), late (3 minutes) and 20 minute hepatobiliary phases were performed with a T1-weighted 3D turbo field echo sequence (T1 high resolution isotropic volume examination, THRIVE; Philips Medical Systems) (repetition time/echo time, 3.1/1.5; flip angle, 10'; matrix size, 256×256; bandwidth, 724.1 Hz/pixel) with a 2-mm section thickness and field of view of 32 to 38 cm. The measured voxel size was 1.5×1.5×4.0 mm, and the reconstructed voxel size was 1.17×1.17×2.0 mm. The contrast agent was administered IV with a power injector at a rate of 1 mL/sec for a dose of 0.025 mmol/kg body weight followed by a 20-mL saline flush.
RESULTS
1. Demography and clinical features
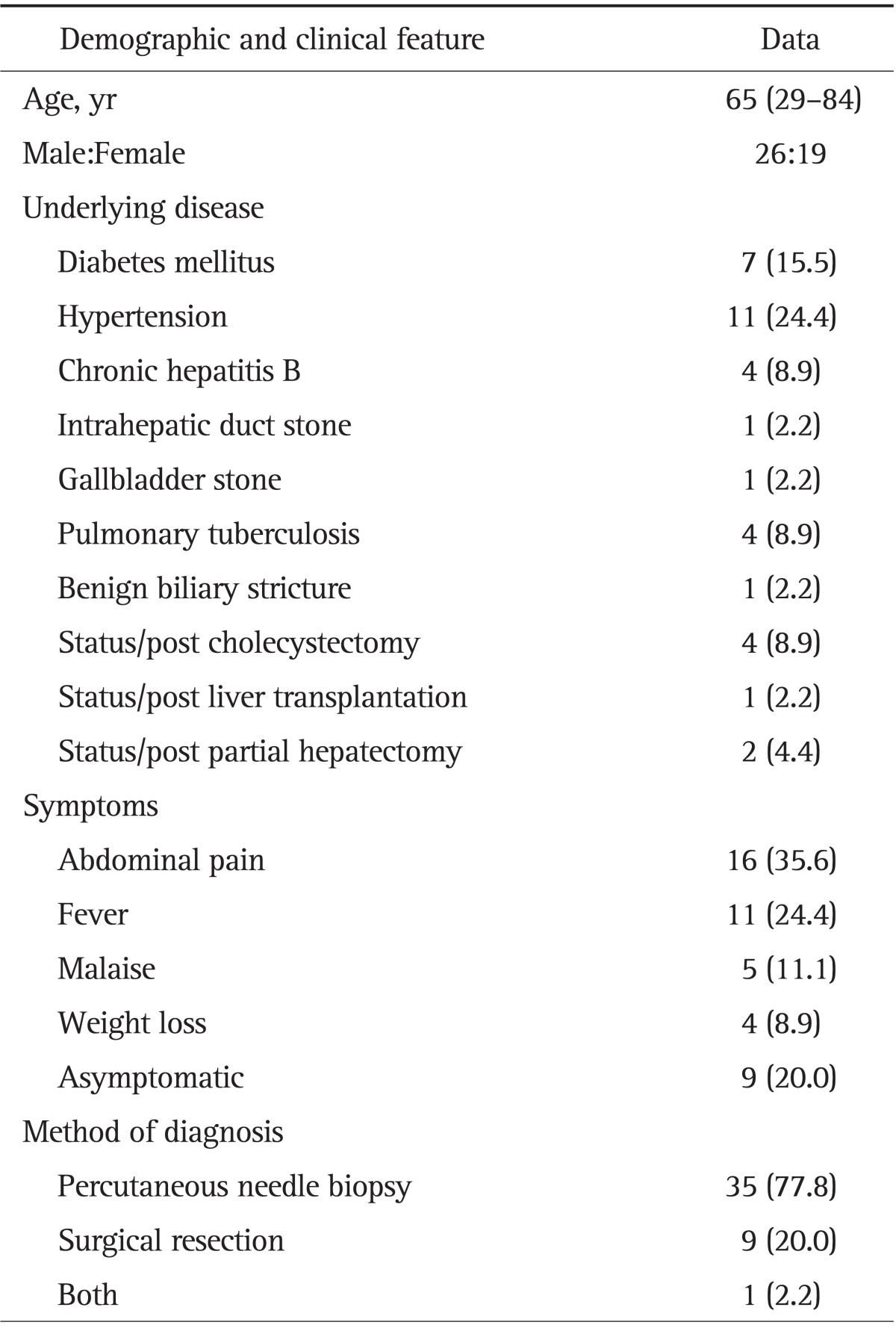
Our study population consisted of 26 men and 19 women with median age of 65 years (range, 29 to 84 years). They complained of abdominal pain (n=16, 35.6%), fever (n=11, 24.4%), malaise (n=5, 11.1%), and weight loss (n=4, 8.9%) while 9 patients (20.0%) were asymptomatic. Seven patients (15.5%) had history of surgery including cholecystectomy, liver transplantation, and partial hepatectomy. Two (4.4%) had asymptomatic intrahepatic duct stone or gallbladder stone. One (2.2%) showed benign biliary stricture (Table 1).
Table 1.
Demographic and Clinical Features of 45 Patients with Inflammatory Pseudotumor of the Liver
Data are presented as median (range) or number (%).
2. Laboratory findings
Most common abnormal laboratory findings were increased serum erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP) level (n=31, 68.9%) followed by increased serum alkaline phosphatase (n=11, 24.4%) and serum alanine aminotransferase level (n=11, 24.4%). Serum tumor marker was normal in 42 patients (93.3%); Serum α-fetoprotein (AFP; n=1, 2.2%), carbohydrate antigen 19-9 (CA 19-9; n=1, 2.2%), and carcinoembryonal antigen (CEA; n=1, 2.2%) level was elevated in each of three patients. Hepatitis B surface antigen was negative in 41 patients (91.1%) and hepatitis C virus antibody was negative in all patients. Fourteen patients (31.1%) showed anemia, 10 (22.2%) presented leukocytosis, and five (11.1%) had thrombocytopenia. No pathogen was identified among 26 patients in whom peripheral blood culture was done. Tissue culture was obtained with specimens from five patients. Among them, Klebsiella pneumoniae was proved in only one sample.
3. Radiologic and histologic findings
The number of tumors was one in 38 patients (84.4%), two in four patients, and three or more in three patients. Median tumor size was 4.4 cm (range, 1 to 9.7 cm). Tumors were most commonly located in right lobe (n=27, 60%), followed by left lobe (n=14, 31.1%), and both lobe (n=4, 8.9%).
CT scan of liver was done in all patients. Nonenhanced CT scan showed ill-defined hypoattenuating lesions in 40 patients (97.6%). Enhanced CT scan showed poorly defined peripheral enhancement at arterial phase and poorly defined hyperattenuating lesions with internal hypoattenuating area at equilibrium phase in most patients (82.5% and 77.0%, respectively) (Table 2, Fig. 1A-C).
Table 2.
Computed Tomography Findings of Inflammatory Pseudotumors of the Liver (n=45)
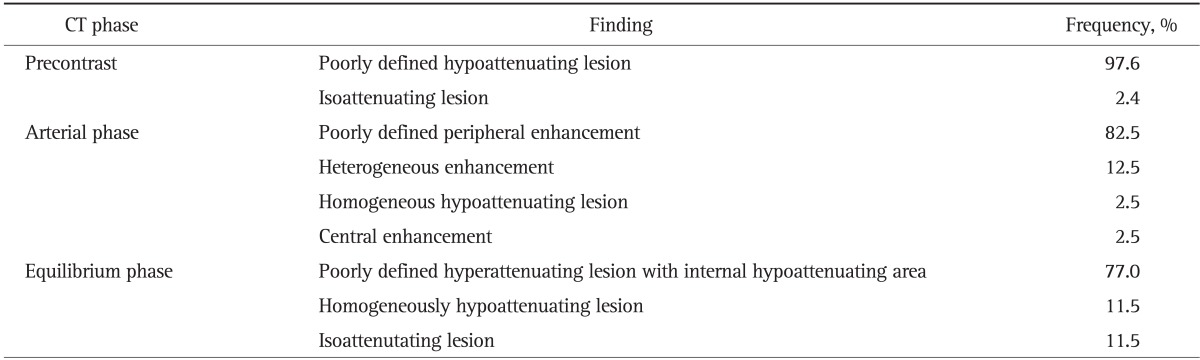
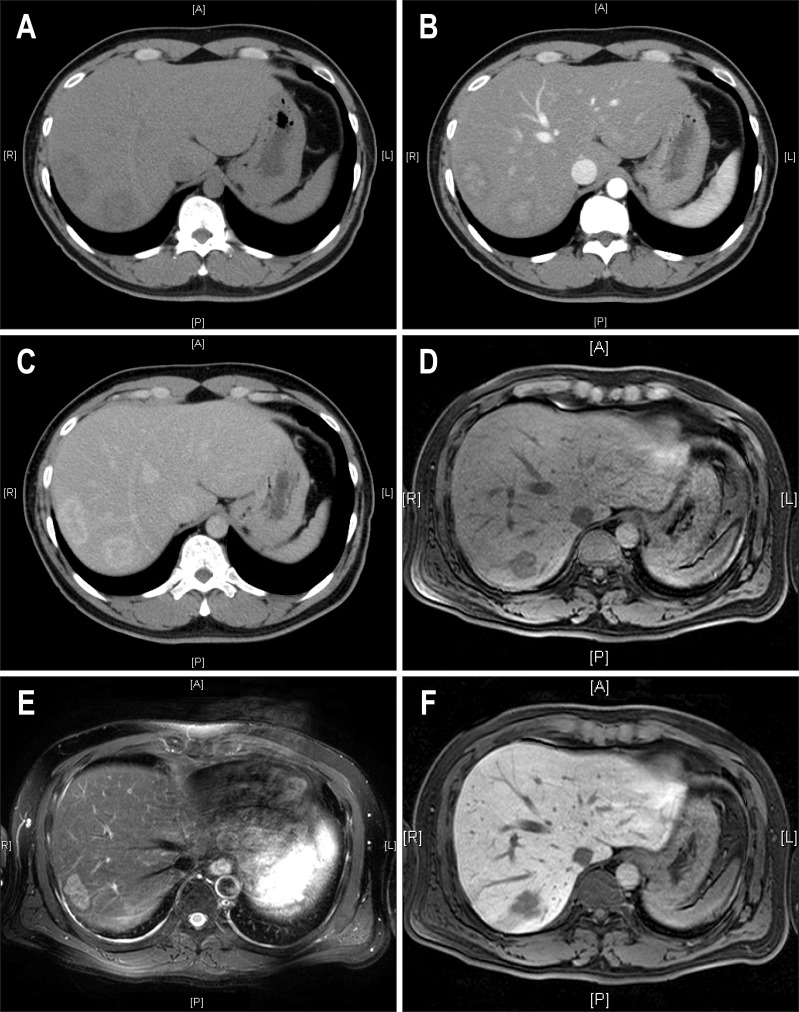
Fig. 1.
Image findings in a 41-year-old man with two surgically proven inflammatory pseudotumors of the liver. (A) A 3.8-cm mass (S7) and another 4.7-cm-sized mass (S8) of low attenuation were noted on precontrast computed tomographic (CT) images. (B) On contrast-enhanced CT imaging, the masses exhibited central enhancement in the arterial phase and (C) peripheral enhancement in the delayed phase. (D) In gadolinium-enhanced magnetic resonance imaging, the masses exhibit low signal intensity in T1-weighted images, (E) intermediate high signal intensity in T2-weighted images, (F) and low signal intensity in the central portion with ill-defined hyperintensity in the delayed phase.
Among 23 patients with available gadolinium (Gd)-enhanced MRI, precontrast MRI showed low signal intensity lesion at T1-weighted image in 86.4% and relatively homogenous high signal intensity lesion at T2-weighted image in 76.2%. Gd-enhancement MRI showed poorly defined peripheral rim-like enhancement at arterial phase in 77.8% (Table 3, Fig. 1D and E).
Table 3.
Gd-Enhanced Magnetic Resonance Imaging Findings of Inflammatory Pseudotumors of the Liver (n=23)
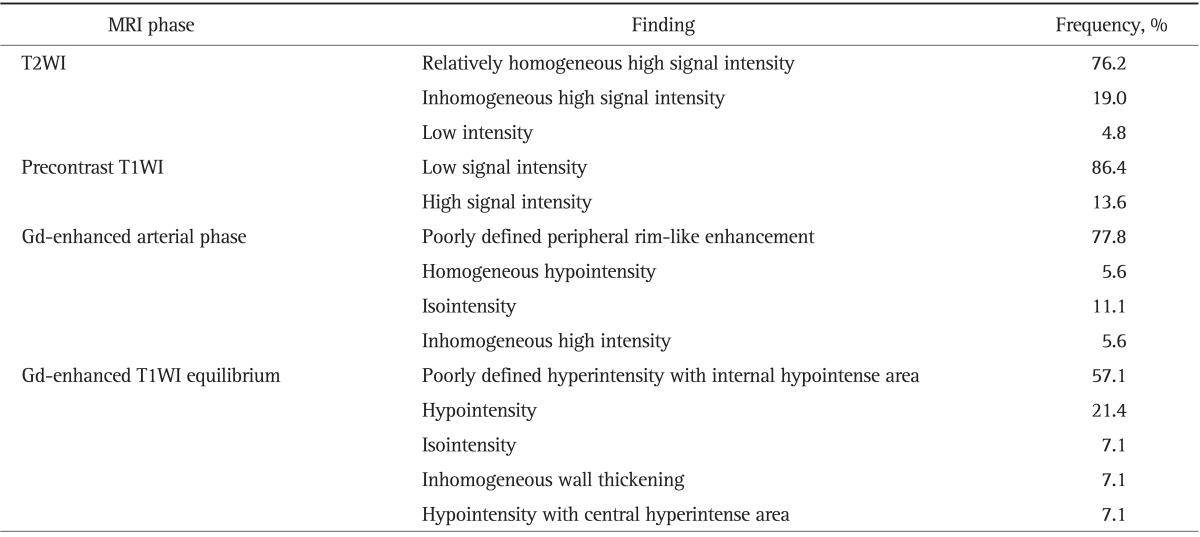
Gd, gadolinium; T2WI, T2-weighted image; T1WI, T1-weighted image.
Histologic examination of percutaneous needle biopsy and surgically resected specimen showed chronic infiltration of various inflammatory cells (plasma cells, lymphocytes, neutrophils, and eosinophils) and fibrous stroma (Fig. 2).
Fig. 2.
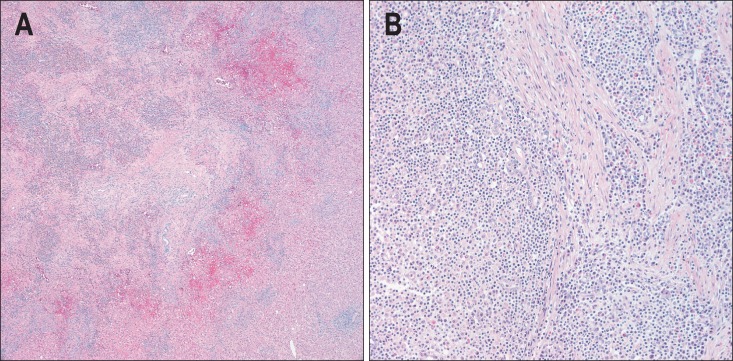
Histological findings of a 41-year-old man with two surgically proven inflammatory pseudotumors of the liver. (A) The lesions contained a mixture of inflammatory cells with a predominance of mature plasma cells (H&E stain, ×50). (B) Lymphocytes with lymphoid follicles, neutrophils, and eosinophils were also noted. These inflammatory cells infiltrated the stroma, composed of interlacing bundles of fibroblasts and collagen bundles (H&E stain, ×200).
4. Clinical course
Most common initial clinical diagnosis based on laboratory and image was malignant tumor (cholangicarcinoma or HCC; n=23, 51.1%) followed by abscess (n=8, 17.8%), IPT versus malignancy (n=2), IPT versus abscess (n=2), lymphoma (n=2), and abscess versus malignancy (n=1). Overall, malignancy was suggested in 26 patients (57.8%) and abscess in 11 patients (24.4%).
Among 36 patients diagnosed as IPT on percutaneous needle biopsy, one patient underwent surgery since possible coexistence of malignancy was not ruled out. Overall, surgical resection was performed in 10 patients due to possibility of malignancy including this patient described above and another nine patients without percutaneous needle biopsy. Remaining 35 patients were treated conservatively with or without antibiotics (n=20 and n=15, respectively).
No recurrence was noted after surgical resection during median follow-up of 8 months (range, 1 to 48 months). In all patients with conservative treatment and available follow-up images (n=27), complete resolution or size reduction was noted during median follow-up period of 7.4 months (range, 1 to 192 months).
DISCUSSION
IPT of the liver is a rare disease which lacks specific symptoms, laboratory or radiologic findings. We assessed the clinical features, laboratory and image findings, treatments, and prognosis of a large number of patients with histologically proven IPT of liver. Our data suggested that dynamic CT and Gd-enhanced MRI could provide clues to the diagnosis of IPT in patients with liver mass and normal serum CA 19-9 and CEA level. Clinical symptom or laboratory marker suggesting inflammation would be helpful but not specific for diagnosis of IPT. Prognosis of IPT patients would be favorable with conservative treatment. However, due to lack of pathognomonic findings, clinical suspicion and active histological diagnosis is warranted to make accurate diagnosis for IPT of liver.
Previous studies from Western countries reported that IPT of the liver is common in males of mid-thirties.5 In contrast, patients with IPT of the liver from Eastern countries tend to show older age of onset.19 In this study, the male to female ratio was about 2:1 as previously reported. Mean age of 45 patients was 65 year, which is older than that of reports from Western countries but similar to those from Eastern countries.20,21
The etiology of IPT is unclear, but predominantly inflammatory pattern of pathology and the associated laboratory findings suggest an underlying infection via hepatobiliary tract in several studies.2,5 Previous studies reported that patients had hepatobiliary tract disease such as biliary stones, history of liver resection, cholangitis, liver abscess, hepatobiliary malignancy in 68% to 80%,17,20-23 and abdominal pain, fever, elevated inflammatory markers including ESR, CRP, leukocyte count were common in cases with IPTs of liver.2,17,20,21 These findings support the underlying infectious condition as etiology of IPTs. In this study, abdominal pain, fever, leukocytosis and a raised ESR and CRP level were also evident. However, hepatobiliary tract diseases were found in only 10 patients (22.2%). Thus, IPTs of the liver should be considered even if there was no underlying biliary tract disease. Serum AFP, CEA and CA 19-9 levels were normal in most patients.
Several previous studies reported Bacteroides caccae, Klebsiella pneumoniae, Escherichia coli, gram-positive cocci as microorganisms responsible for IPT.8,24,25 However, no causative organism were discovered in blood or tissue culture in other studies.20,26,27 In our study, K. pneumonia was found from tissue culture in only one patient.
IPTs of the liver commonly present as large, solitary mass and occur predominantly in right lobe although multicentricity has been also described.2,20,26-28 Our study showed that mean size of mass was 4.4 cm and most were solitary (84.4%) with right lobe predominance (60%), which is consistent with results of previous studies.2,20,26-28
According to previous reports, IPTs revealed inconsistent appearance on radiologic studies and no specific image findings have been reported yet.2,10,20 In our study, most patients showed poorly defined hypoattenuating lesion in precontrast CT (97.6%), poorly defined peripheral enhancement at arterial phase (82.5%), and poorly defined hyperattenuating lesions with internal hypoattenuating area at equilibrium phase (77.0%) during dynamic CT imaging. Precontrast MRI showed low signal intensity lesion at T1-weighted image (86.4%) and relatively homogenous high signal intensity lesion at T2-weighted image (76.2%). Gd-enhancement MRI showed poorly defined peripheral rim-like enhancement at arterial phase (77.8%). However, other focal lesions such as atypical HCC, intrahepatic CC, metastatic tumors, or abscess might show similar enhancement patterns during CT or MRI.11,12 Previous studies reported IPTs of the liver mimicking malignant lesions such as intrahepatic CC, lymphoma, metastatic cancer, and HCC.11-13 Likewise, in this study, most common presumptive initial diagnosis based on laboratory and image findings were hepatic malignancy (51.1%), followed by liver abscess (17.8%). Hence, active histological diagnosis is warranted for accurate diagnosis of IPT. The clinical course and prognosis of IPTs have suggested to be favorable with conservative treatment.2,5,6,10,17,20,28
Our study also demonstrated good long-term results without recurrence. During median follow-up period of 7 months (range, 1 to 192 months), all patients with conservative treatment showed complete resolution or size reduction of mass. In addition, no recurrence was noted after surgical resection of IPT during median follow-up period of 8 months (range, 1 to 48 months).
We acknowledge that our study has a few weak points as retrospective study involving patients from three different institutions for 16 years. Especially, Gd-enhanced MRI was performed in 40% of the patients. However, considering the rarity and very variable presentation of IPT, our data involving a large number of patients could provide useful clinical information for diagnosis and management of IPT of the liver.
In conclusion, IPTs of the liver have good prognosis with conservative management, accurate diagnosis is important to avoid unnecessary surgery. Dynamic CT and Gd-enhanced MRI could provide clues to the diagnosis of IPT in patients with liver mass and normal serum AFP, CA 19-9 and CEA level, especially in those with clinical or laboratory findings of inflammation. Active histological diagnosis is warranted for patients with suspected IPT of the liver to avoid unnecessary operation.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Torzilli G, Inoue K, Midorikawa Y, Hui AM, Takayama T, Makuuchi M. Inflammatory pseudotumors of the liver: prevalence and clinical impact in surgical patients. Hepatogastroenterology. 2001;48:1118–1123. [PubMed] [Google Scholar]
- 2.Horiuchi R, Uchida T, Kojima T, Shikata T. Inflammatory pseudotumor of the liver. Clinicopathologic study and review of the literature. Cancer. 1990;65:1583–1590. doi: 10.1002/1097-0142(19900401)65:7<1583::aid-cncr2820650722>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto H, Yamaguchi H, Aishima S, et al. Inflammatory myofibroblastic tumor versus IgG4-related sclerosing disease and inflammatory pseudotumor: a comparative clinicopathologic study. Am J Surg Pathol. 2009;33:1330–1340. doi: 10.1097/pas.0b013e3181a5a207. [DOI] [PubMed] [Google Scholar]
- 4.Zen Y, Fujii T, Sato Y, Masuda S, Nakanuma Y. Pathological classification of hepatic inflammatory pseudotumor with respect to IgG4-related disease. Mod Pathol. 2007;20:884–894. doi: 10.1038/modpathol.3800836. [DOI] [PubMed] [Google Scholar]
- 5.White JE, Chase CW, Kelley JE, Brock WB, Clark MO. Inflammatory pseudotumor of the liver associated with extrahepatic infection. South Med J. 1997;90:23–29. doi: 10.1097/00007611-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Coffin CM, Humphrey PA, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor: a clinical and pathological survey. Semin Diagn Pathol. 1998;15:85–101. [PubMed] [Google Scholar]
- 7.Melloni G, Carretta A, Ciriaco P, et al. Inflammatory pseudotumor of the lung in adults. Ann Thorac Surg. 2005;79:426–432. doi: 10.1016/j.athoracsur.2004.07.077. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi T, Mochizuki K, Kizu T, et al. Inflammatory pseudotumor of the liver and spleen diagnosed by percutaneous needle biopsy. World J Gastroenterol. 2012;18:90–95. doi: 10.3748/wjg.v18.i1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang L, Lai EC, Cong WM, et al. Inflammatory myofibroblastic tumor of the liver: a cohort study. World J Surg. 2010;34:309–313. doi: 10.1007/s00268-009-0330-x. [DOI] [PubMed] [Google Scholar]
- 10.Fukuya T, Honda H, Matsumata T, et al. Diagnosis of inflammatory pseudotumor of the liver: value of CT. AJR Am J Roentgenol. 1994;163:1087–1091. doi: 10.2214/ajr.163.5.7976880. [DOI] [PubMed] [Google Scholar]
- 11.Kitajima K, Shiba H, Nojiri T, et al. Intrahepatic cholangiocarcinoma mimicking hepatic inflammatory pseudotumor. J Gastrointest Surg. 2007;11:398–402. doi: 10.1007/s11605-006-0071-1. [DOI] [PubMed] [Google Scholar]
- 12.Ishida H, Tatsuta M, Furukawa H, et al. Multiple inflammatory pseudotumors mimicking liver metastasis from colon cancer: report of a case. Surg Today. 2000;30:530–533. doi: 10.1007/s005950070121. [DOI] [PubMed] [Google Scholar]
- 13.Nam KJ, Kang HK, Lim JH. Inflammatory pseudotumor of the liver: CT and sonographic findings. AJR Am J Roentgenol. 1996;167:485–487. doi: 10.2214/ajr.167.2.8686633. [DOI] [PubMed] [Google Scholar]
- 14.Yoon KH, Ha HK, Lee JS, et al. Inflammatory pseudotumor of the liver in patients with recurrent pyogenic cholangitis: CT-histopathologic correlation. Radiology. 1999;211:373–379. doi: 10.1148/radiology.211.2.r99ma36373. [DOI] [PubMed] [Google Scholar]
- 15.Di Vita G, Soresi M, Patti R, et al. Concomitant inflammatory pseudotumor of the liver and spleen. Liver. 2001;21:217–222. doi: 10.1034/j.1600-0676.2001.021003217.x. [DOI] [PubMed] [Google Scholar]
- 16.Vassiliadis T, Vougiouklis N, Patsiaoura K, et al. Inflammatory pseudotumor of the liver successfully treated with nonsteroidal anti-inflammatory drugs: a challenge diagnosis for one not so rare entity. Eur J Gastroenterol Hepatol. 2007;19:1016–1020. doi: 10.1097/MEG.0b013e32821acdd2. [DOI] [PubMed] [Google Scholar]
- 17.Koea JB, Broadhurst GW, Rodgers MS, McCall JL. Inflammatory pseudotumor of the liver: demographics, diagnosis, and the case for nonoperative management. J Am Coll Surg. 2003;196:226–235. doi: 10.1016/S1072-7515(02)01495-3. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi J, Sakamoto Y, Sano T, Shimada K, Kosuge T. Spontaneous regression of inflammatory pseudotumor of the liver: report of three cases. Surg Today. 2007;37:525–529. doi: 10.1007/s00595-006-3433-0. [DOI] [PubMed] [Google Scholar]
- 19.Schmid A, Jänig D, Bohuszlavizki A, Henne-Bruns D. Inflammatory pseudotumor of the liver presenting as incidentaloma: report of a case and review of the literature. Hepatogastroenterology. 1996;43:1009–1014. [PubMed] [Google Scholar]
- 20.Park KS, Jang BK, Chung WJ, et al. Inflammatory pseudotumor of liver: a clinical review of 15 cases. Korean J Hepatol. 2006;12:429–438. [PubMed] [Google Scholar]
- 21.Ahn KS, Kang KJ, Kim YH, et al. Inflammatory pseudotumors mimicking intrahepatic cholangiocarcinoma of the liver; IgG4-positivity and its clinical significance. J Hepatobiliary Pancreat Sci. 2012;19:405–412. doi: 10.1007/s00534-011-0436-z. [DOI] [PubMed] [Google Scholar]
- 22.Sakai T, Shiraki K, Yamamoto N, et al. Diagnosis of inflammatory pseudotumor of the liver. Int J Mol Med. 2002;10:281–285. [PubMed] [Google Scholar]
- 23.Toda K, Yasuda I, Nishigaki Y, et al. Inflammatory pseudotumor of the liver with primary sclerosing cholangitis. J Gastroenterol. 2000;35:304–309. doi: 10.1007/s005350050351. [DOI] [PubMed] [Google Scholar]
- 24.Lupovitch A, Chen R, Mishra S. Inflammatory pseudotumor of the liver. Report of the fine needle aspiration cytologic findings in a case initially misdiagnosed as malignant. Acta Cytol. 1989;33:259–262. [PubMed] [Google Scholar]
- 25.Standiford SB, Sobel H, Dasmahapatra KS. Inflammatory pseudotumor of the liver. J Surg Oncol. 1989;40:283–287. doi: 10.1002/jso.2930400416. [DOI] [PubMed] [Google Scholar]
- 26.Anthony PP. Inflammatory pseudotumour (plasma cell granuloma) of lung, liver and other organs. Histopathology. 1993;23:501–503. doi: 10.1111/j.1365-2559.1993.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 27.Anthony PP, Telesinghe PU. Inflammatory pseudotumour of the liver. J Clin Pathol. 1986;39:761–768. doi: 10.1136/jcp.39.7.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shek TW, Ng IO, Chan KW. Inflammatory pseudotumor of the liver. Report of four cases and review of the literature. Am J Surg Pathol. 1993;17:231–238. doi: 10.1097/00000478-199303000-00003. [DOI] [PubMed] [Google Scholar]