Abstract
Background/Aims
Epithelial-mesenchymal transition (EMT)-related proteins may exhibit differential expression in intestinal type or pancreatobiliary type ampulla of Vater carcinomas (AVCs). We evaluated the expression of E-cadherin, β-catenin, and S100A4 in intestinal and nonintestinal type AVCs and analyzed their relationships with clinicopathological variables and survival.
Methods
A clinicopathological review of 105 patients with AVCs and immunohistochemical staining for E-cadherin, β-catenin, and S100A4 were performed. The association between clinicopathological parameters, histological type, and expression of EMT proteins and their effects on survival were analyzed.
Results
Sixty-five intestinal type, 35 pancreatobiliary type, and five other types of AVCs were identified. The severity of EMT changes differed between the AVC types; membranous loss of E-cadherin and β-catenin was observed in nonintestinal type tumors, whereas aberrant nonmembranous β-catenin expression was observed in intestinal type tumors. EMT-related changes were more pronounced in the invasive tumor margin than in the tumor center, and these EMT-related changes were related to tumor aggressiveness. Among the clinicopathological parameters, a desmoplastic reaction was related to overall survival, and the reaction was more severe in nonintestinal type than in intestinal type AVCs.
Conclusions
Dysregulation of E-cadherin, β-cadherin, and S100A4 expression may play a role in the carcinogenesis and tumor progression of AVCs.
Keywords: Ampullary adenocarcinoma, Intestinal type, Pancreatobiliary type, Epithelial-mesenchymal transition
INTRODUCTION
Most of ampulla of Vater carcinomas (AVCs) can be classified as intestinal type or pancreatobiliary type based on histologic characteristics.1 Intestinal AVCs behave like their duodenal counterparts, while pancreatobiliary ones are more aggressive and behave like pancreatic adenocarcinomas.2 AVCs of different histologic types express different specific markers and have different modes of tumor spread, extent of and interaction with the extracellular matrix, and premalignant lesions.3 Patients with intestinal AVCs have a much better prognosis than those with pancreatobiliary AVCs.4 However, other studies have reported that histologic subgroup does not correlate with clinical outcomes.5-7
The epithelial-mesenchymal transition (EMT) is a process in which mature epithelial cells change in appearance and lose cell-cell contacts and epithelial protein expression while at the same time acquiring the phenotypic characteristics of mesenchymal cells.8 Immunohistochemical (IHC) staining studies of tissues from patients with AVCs have demonstrated that the loss of epithelial markers and the gain of mesenchymal markers are related to tumor progression.9-11 Loss of E-cadherin expression in AVCs correlates with clinicopathologic features of tumor progression, and predicts poor prognosis.9 Loss of membranous β-catenin expression9 and gain of cytoplasmic or nuclear β-catenin expression in neoplastic glands is related to carcinogenesis and tumor progression in gastrointestinal cancers.12 S100A4 promotes metastasis in several tumors. Nuclear or cytoplasmic expression of this protein is related to carcinogenesis of AVCs, but its relation to prognosis and clinicopathological parameters has not been evaluated.11 EMT-related changes in E-cadherin, β-catenin, and S100A4 expression in different histologic types of AVCs have not been evaluated, nor has the relationship between the expression of these proteins and various clinicopathological parameters.
In this study, we analyzed the expression patterns of E-cadherin, β-catenin, and S100A4 in intestinal and nonintestinal type AVCs and evaluated whether the expression of these proteins was associated with clinicopathological parameters and overall survival.
MATERIALS AND METHODS
1. Patients and specimens
Paraffin-embedded tissues from AVCs were obtained from five hospitals located in Chungcheong province in Korea with the approval of the Ethics Committee of Chungbuk National University Hospital (IRB approval ID: 2011-06-042). One hundred five patients with AVCs (mean age, 70.3±9.9 years; male:female=58:47) who underwent curative resection were enrolled in this study. The patients did not receive any chemotherapy or radiation therapy before surgery.
2. Histopathological study
Each tumor was re-evaluated by retrospective analysis of the medical records and tissue slide files by two experienced pathologists based on hematoxylin and eosin staining according to previously described criteria.13 Tumors essentially indistinguishable from colorectal carcinomas were classified as intestinal type, whereas carcinomas with a dense desmoplastic stroma surrounding small glands or solid nests of tumor cells were referred to as pancreatobiliary type. In addition, invasive papillary carcinomas, signet-ring cell type, and mucinous type AVCs were identified.
The degree of differentiation, T stage, lymph node, or distant metastasis, desmoplasia, neural or lymphovascular invasion, and tumor budding were assessed. Stages were defined according to the tumor node metastasis (TNM) staging system of the American Joint Committee on Cancer. The number of tumor buds in clusters of less than five tumor cells was counted in five regions at ×200 magnification. We divided cases into those with low budding (median, <5) and high budding (median, ≥5).14
3. Tissue microarray construction and IHC studies
After all slide reviews, 3-mm sized tissue microarrays were constructed. Areas from the center of the tumor and the invasive margin of the tumor with the lowest degree of differentiation but abundant in cells with high mitotic activity were chosen from the original blocks. IHC staining was carried out using anti-E-cadherin (1:400, DakoCytomation; Glostrup, Denmark), anti-β-catenin (1:400, DakoCytomation), and anti-S100A4 (1:800, DakoCytomation) primary antibodies. Immunostaining of E-cadherin was evaluated as follows: no staining, weak staining, and strong staining as reference to adjacent nonneoplastic epithelium. For β-catenin, membrane, and cytoplasmic/nuclear expression were recorded separately as either no staining, weak staining, or strong staining. Cases with more than 5% of nuclei stained were considered nuclear staining while cases with more than 50% of the cytoplasm stained were considered cytoplasmic staining. For S100A4, staining intensity was recorded as no staining, weak staining, or strong staining as reference to adjacent inflammatory cells.
4. Statistical analysis
The relationships between semiquantitative EMT related protein staining intensity and clinicopathological parameters were analyzed using Fisher exact test, chi-square test, or the Mann-Whitney U test. Prognostic factors among clinicopathologic parameters were examined by univariate and multivariate survival analyses using Cox proportional hazards model. All statistical tests were two-sided, and statistical significance was accepted at the p<0.05 level. All analyses were performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Clinicopathological features according to tumor subtype
Representative histopathologic features and immunostaining images of AVCs were shown in Figs 1-3. One hundred five AVCs were classified 65 as intestinal type (61.9%), 35 as pancreatobiliary type (33.3%), three as invasive papillary type (2.9%), one as a mucinous cancer, and one as a signet-ring cell cancer. Compared to intestinal type, nonintestinal type AVCs were characterized by poor differentiation, severe desmoplastic reaction, high tumor budding, neural invasion, and advanced TNM stage. Precursor lesions were also different between the two major types. Adenomas, comprising 40 tubular cases, eight tubulo-villous cases, and five villous cases were identified in 61.5% of intestinal type and 32.5% of nonintestinal type AVCs. Epithelial dysplasia occurred in only 22.5% of nonintestinal type AVCs (Table 1).
Fig. 1.
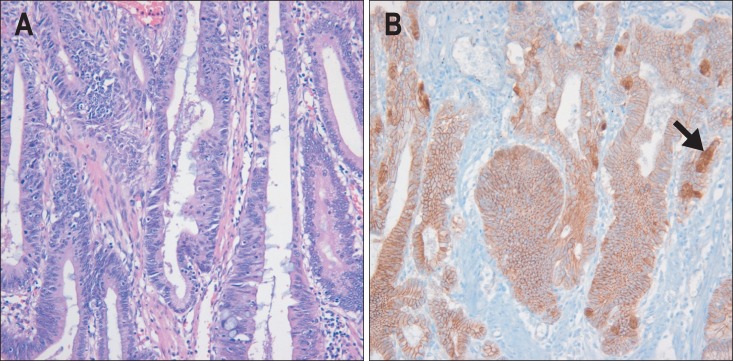
Well-differentiated intestinal type adenocarcinoma. (A) The microscopic features are indistinguishable from those of primary adenocarcinoma of the large intestine. Tumor cells are tall, columnar, and cohesive. Tumor nuclei are elongated, hyperchromatic, and pseudostratified (H&E stain, ×200). (B) Membranous, cytoplasmic, and focal nuclear expression (arrow) of β-catenin (β-catenin immunostaining, ×200).
Fig. 3.
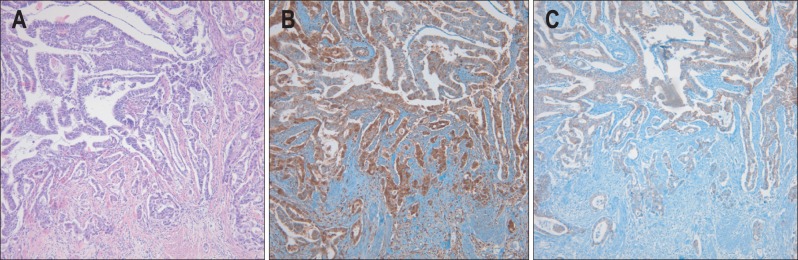
Moderately differentiated pancreatobiliary type adenocarcinoma at the invasive margin. (A) Desmoplasia is prominent around the invasive tumor and tumor buds (H&E stain, ×100). (B) S100A4 is more highly expressed in invasive tumor cells than in noninvasive tumor cells (S100A4 immunostaining, ×100). (C) Membranous E-cadherin is more frequently lost in invasive tumor cells than in noninvasive tumor cells (E-cadherin immunostaining, ×100).
Table 1.
Clinicopathological Parameters between Intestinal and Nonintestinal Types of Ampulla of Vater Carcinomas
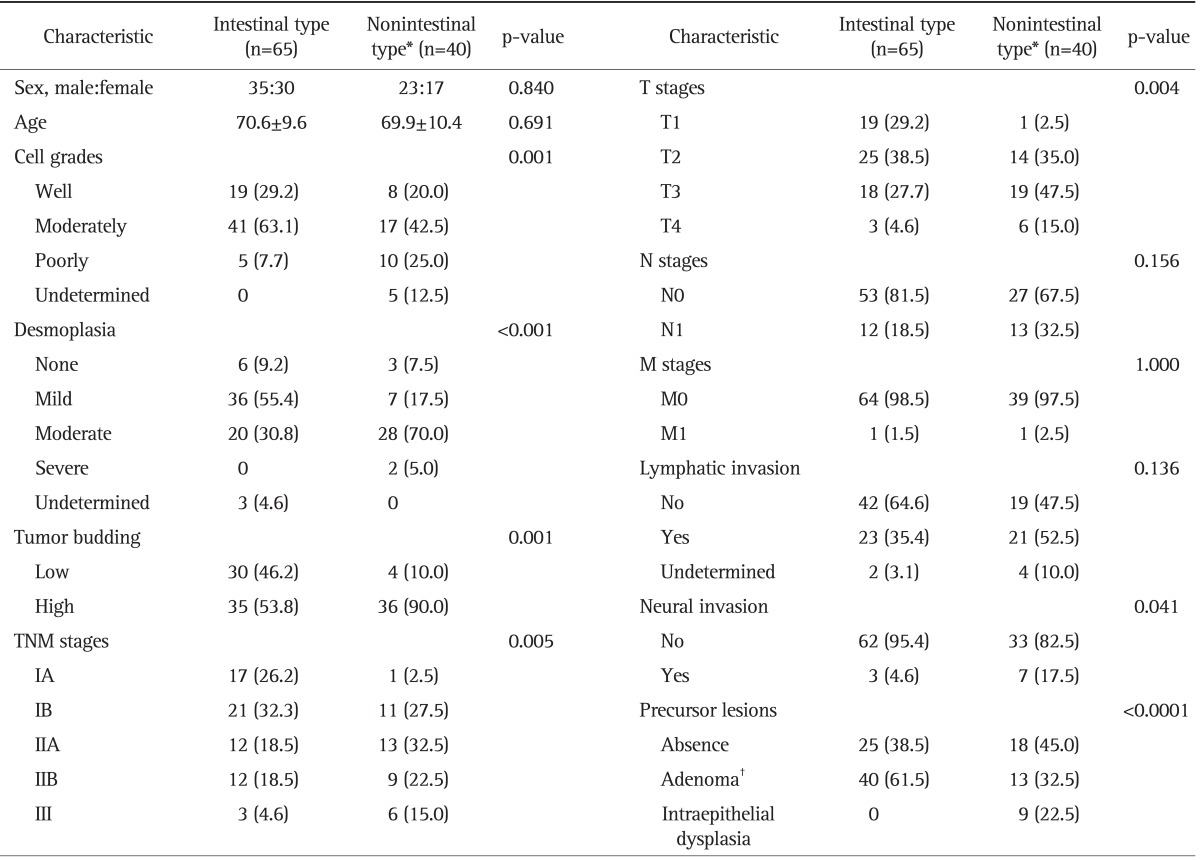
Data are presented as mean±SD or number (%).
TNM, tumor node metastasis.
*Nonintestinal type includes 35 cases of P-B type, three cases of invasive papillary type, one case of mucinous cancer, and one case of signet-ring cell cancer; †Adenoma consists of 42 cases of tubular type, eight cases of tubule-villous type, and five cases of villous type.
2. E-cadherin, β-catenin, and S100A4 expression in intestinal type versus nonintestinal type AVCs
Loss or attenuated membranous expression of E-cadherin was seen more frequently in nonintestinal type AVCs than intestinal type AVCs. Although loss or attenuated membranous expression of E-cadherin was more severe in nonintestinal type AVCs, nonmembranous expression of β-catenin was more severe in intestinal type AVCs. S100A4 expression was not significantly different between histologic subtypes. Comparison of EMT-related protein expression in the tumor center and invasive margin revealed that loss of membranous expression of E-cadherin and β-catenin and gain of S100A4 expression were more prominent in the invasive margin than the tumor center (all p<0.0001). In contrast, cytoplasmic or nuclear expression of β-catenin was more prominent in the tumor center than the invasive margin (p<0.000).
EMT changes were also apparent in adenomas and epithelial dysplasia (Fig. 4). Weak E-cadherin expression was observed in 13.0% of precursors, and was more frequent in epithelial dysplasia cases than adenoma cases. Membranous expression of β-catenin was lost in 4.2% of precursors and reduced in 64.6% of them. Weak nonmembranous expression of β-catenin was observed in 81.1% of precursors, while strong staining was observed in 13.5% of them. Weak S100A4 protein expression was detected in 28.3% of precursors, while strong S100A4 staining was observed in 2.2% of them (Table 2).
Fig. 4.
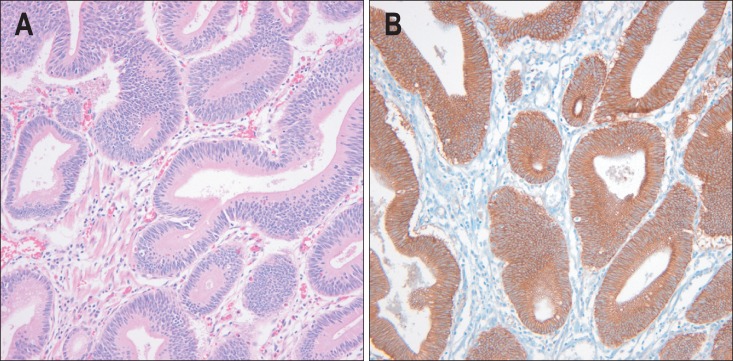
Intestinal type adenoma. (A) Microscopically, the tumor is not distinguishable from adenoma of the intestine (H&E stain, ×200). (B) Both the tumor cell membrane and cytoplasm express β-catenin (β-catenin immunostaining, ×200).
Table 2.
E-Cadherin, β, and S100A4 Expression in the Ampulla of Vater Carcinomas and Precursor Lesions
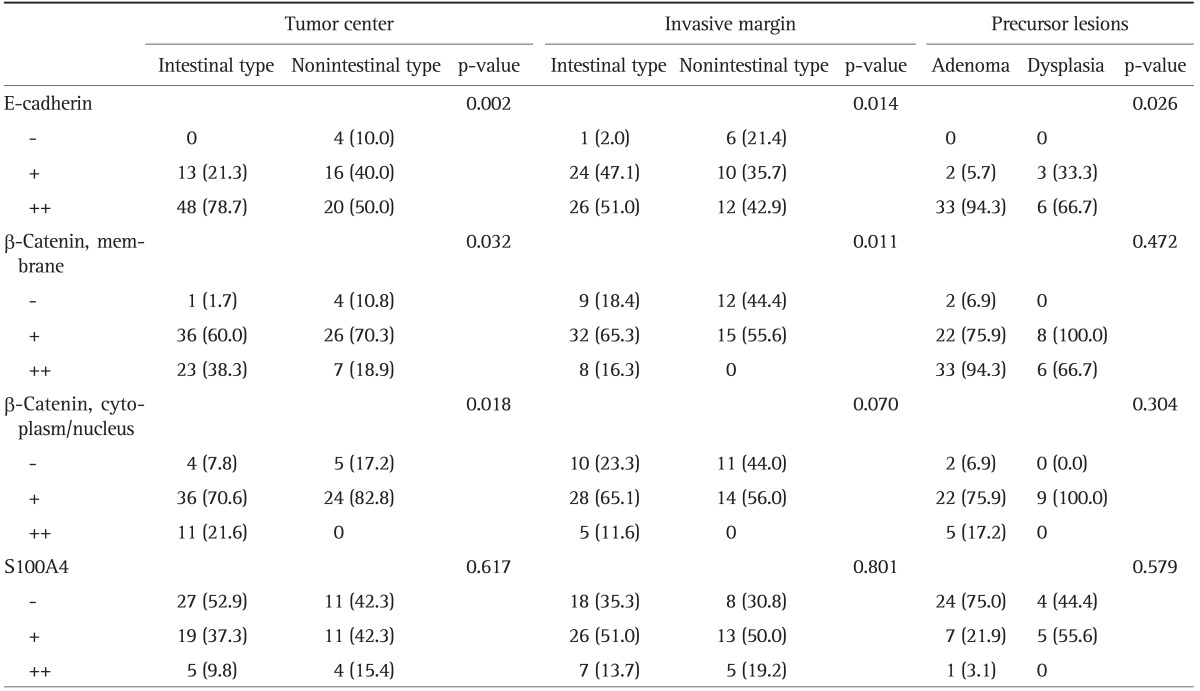
Data are presented as number (%). Number of tested cases (tumor center, invasive margin): E-cadherin (101, 79), β-catenin (80, 76), and S100A4 (77, 77).
The relationships between EMT-related protein expression in the invasive margin and clinicopathological parameters were analyzed. Membranous expression of E-cadherin was related to cell grade, tumor budding, and TNM stage. Membranous expression of β-catenin was related to cell grade, tumor budding, desmoplasia, TNM stage, lymphatic invasion, and neural invasion. S100A4 expression was related to TNM stage and lymphatic invasion (Table 3). Nuclear or cytoplasmic β-catenin expression was related to lymphatic invasion (p=0.003).
Table 3.
Clinical and Pathological Parameters Related to Epithelial-Mesenchymal Transition-Related Protein Expression at the Invasive Margin
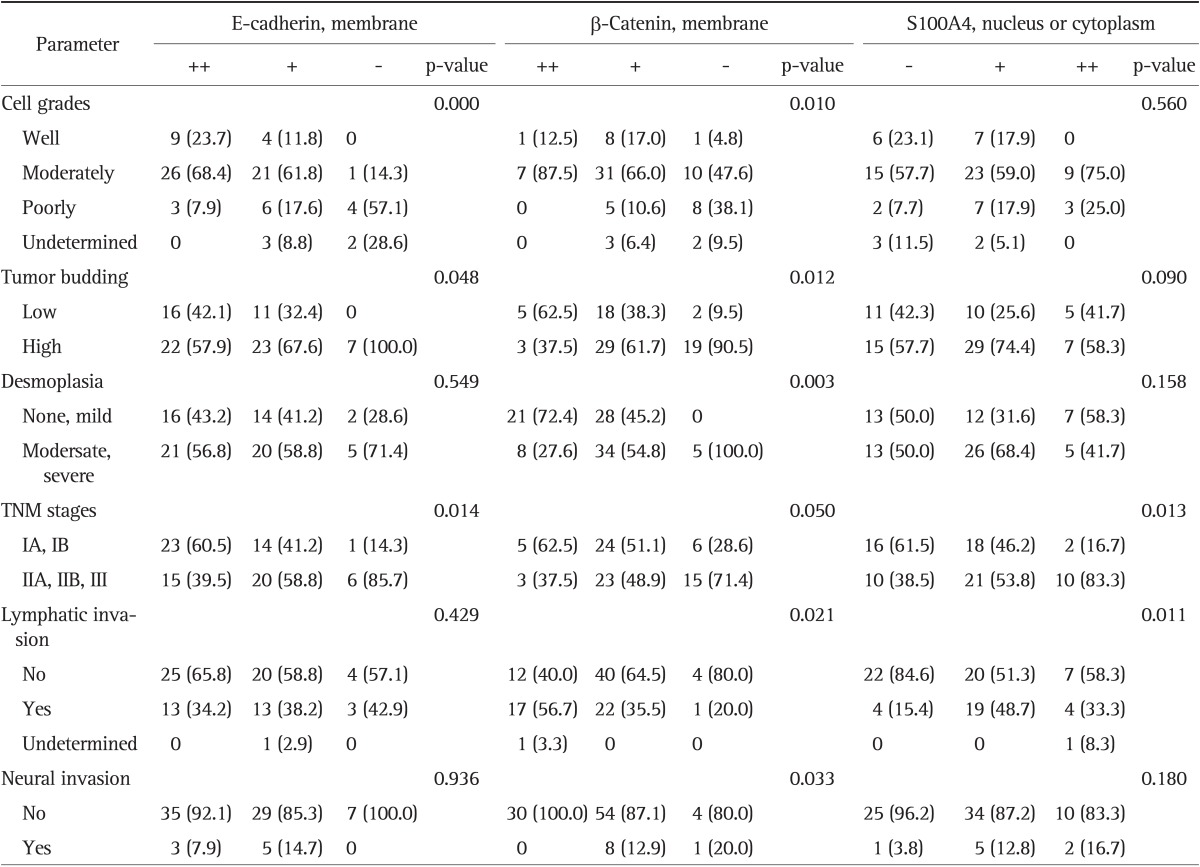
Values are presented as number (%).
TNM, tumor node metastasis.
3. Parameters related to survival time
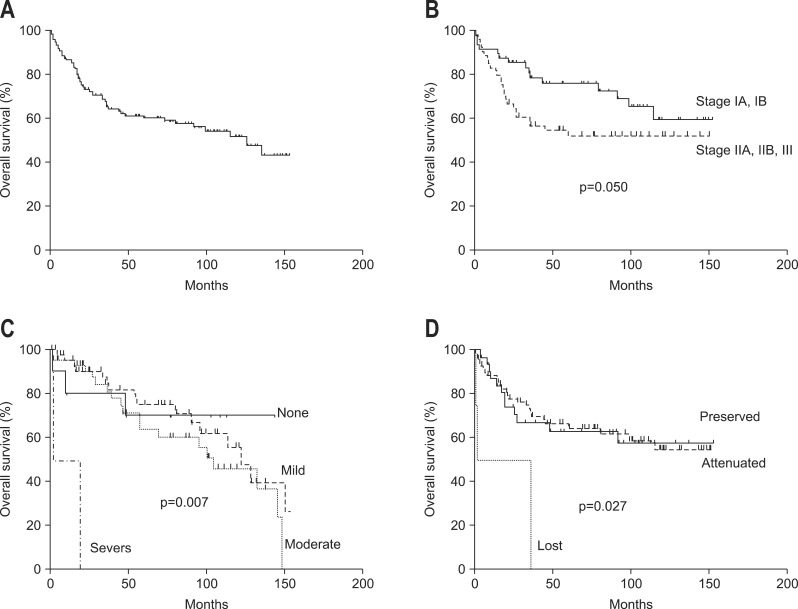
Median survival time was 126 months. Five-year and 10-year survival rates were 61.2% and 43.1%, respectively (Fig. 5A). Parameters related to survival were TNM stage (p=0.05) (Fig. 5B), desmoplasia (p=0.007) (Fig. 5C), and E-cadherin expression (p=0.027) (Fig. 5D). Cox regression analysis showed that only desmoplastic reaction was related to survival time.
Fig. 5.
The survival analysis according to (A) overall, (B) TNM stage, (C) desmoplasia, and (D) E-cadherin expression.
DISCUSSION
We demonstrated that intestinal type and nonintestinal type AVCs have different histopathologic features and different expression patterns of E-cadherin and β-catenin. Nonintestinal type AVCs showed aggressive tumor behavior and frequent loss or reduced expression of E-cadherin and β-catenin in the cell membrane. In contrast, cytoplasmic or nucleus expression of β-catenin was more severe in intestinal type AVCs. These EMT-related proteins were also expressed in precursor lesions, namely adenomas and intraepithelial dysplasia. Aberrant expression of EMT-related proteins was more severe in carcinomas than precursor lesions and advanced AVCs, but was not related with overall survival. These results suggest that EMT phenotype is related to carcinogenesis and tumor progression in AVCs.
A previous study showed that loss of membranous expression of E-cadherin and β-catenin was associated with pancreatic invasion, recurrence, and poor prognosis.9 We showed that EMT-related changes in E-cadherin expression were associated with nonintestinal type AVCs and that this was a prognostic factor in univariate analysis.
β-Catenin was first identified as a protein that functions together with E-cadherin to maintain cell to cell adhesion. However, β-catenin also functions as a transcription factor. If the Wnt signaling pathway is perturbed, Wnt ligands block β-catenin degradation, and excess β-catenin enters the nucleus, resulting in the transcription of target genes. Previous study reported that dysfunction of β-catenin might contribute to the development of AVCs15 and nuclear accumulation of β-catenin was associated with tumor cell proliferation and a worse prognosis in AVC patients.9,16 We demonstrated that loss of membranous expression or gain of nonmembranous expression of β-catenin was associated with different clinicopathological parameters. These findings suggest that dysregulation of the Wnt signaling pathway leads to the nuclear accumulation of β-catenin and plays an important role in the carcinogenesis of intestinal type AVCs.
S100A4 is localized in the nucleus, cytoplasm, and extracellular space and possesses a wide range of biological functions.17 Gain of the S100A4 protein was significantly increased in normal mucosa, adenoma, and carcinoma in ascending order in patients with AVCs.11 In our series, S100A4 expression was related to TNM stage and lymphovascular invasion. However, S100A4 expression was not related to histologic types or prognosis, consistent with previous study.11
Because the tumor biology of the invasive margin is different from that of the tumor center, the patterns of EMT protein expression may differ between these two sites. Our result of that EMT-related changes were more severe in the invasive margin than the tumor center suggest that those play a role in cancer invasion and metastasis of AVCs.
The EMT is an early event that can be reliably detected in colonic adenomas; loss of E-cadherin expression in 6.1% and aberrant β-catenin expression in 8.2%.18 Gain of S100A4 expression was observed in 25% of ampullary adenomas.11 Our series showed that changes of E-cadherin expression and S100A4 expression was observed in 13% and in 32% of them, respectively. However, changes in membranous or nonmembranous expression of β-catenin were observed in 68.8% and 94.6% of ampullary adenomas, respectively. These results suggest that Wnt signaling dysfunction plays a major role in the carcinogenesis of AVCs.
Tumor budding is a hallmark of the EMT and serve as a prognostic factor in patients with AVCs.14 We demonstrated that high tumor budding was associated with nonintestinal AVCs and loss of membranous staining of E-cadherin and β-catenin in the invasive margin, but was not related to overall survival.
Carcinogenesis of AVCs may differ between intestinal and pancreatobiliary types. Intestinal epithelia transform to carcinomas via the adenoma-carcinoma sequence, whereas the epithelium of the distal bile duct, pancreatic duct, or ampullary channel progress from intraepithelial neoplasia to adenocarcinoma.19-21 We showed that adenomas were more frequent in intestinal type AVCs and dysplasia was more frequent in nonintestinal type AVCs.
This study showed that nonintestinal type adenocarcinomas have more malignant features than intestinal type AVCs. Although survival was not different between the two histologic subgroups, desmoplasia, which was the only prognostic factor for overall survival, was the dominant feature of pancreatobiliary type AVCs. In conclusion, loss of membranous expression of E-cadherin/β-catenin and gain of S100A4 expression were related to carcinogenesis and tumor progression in AVCs. Aberrant nonmembranous expression of β-catenin was closely related to intestinal type AVCs. Further studies are needed to establish standard criteria for histologic subgroups and to determine treatment guidelines according to prognostic parameters.
Fig. 2.
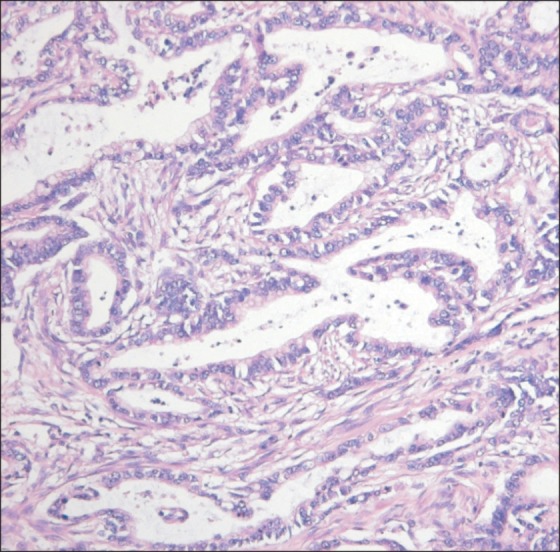
Well-differentiated pancreatobiliary type adenocarcinoma. Low columnar tumor cells are arranged in a single layer without nuclear pseudostratification. The nuclei are vesicular and more rounded than those of intestinal type tumors (H&E stain, ×200).
ACKNOWLEDGEMENTS
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1120330).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kimura W, Futakawa N, Yamagata S, et al. Different clinicopathologic findings in two histologic types of carcinoma of papilla of Vater. Jpn J Cancer Res. 1994;85:161–166. doi: 10.1111/j.1349-7006.1994.tb02077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter JT, Grenert JP, Rubenstein L, Stewart L, Way LW. Tumors of the ampulla of vater: histopathologic classification and predictors of survival. J Am Coll Surg. 2008;207:210–218. doi: 10.1016/j.jamcollsurg.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Schirmacher P, Büchler MW. Ampullary adenocarcinoma: differentiation matters. BMC Cancer. 2008;8:251. doi: 10.1186/1471-2407-8-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westgaard A, Tafjord S, Farstad IN, et al. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer. 2008;8:170. doi: 10.1186/1471-2407-8-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou H, Schaefer N, Wolff M, Fischer HP. Carcinoma of the ampulla of Vater: comparative histologic/immunohistochemical classification and follow-up. Am J Surg Pathol. 2004;28:875–882. doi: 10.1097/00000478-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 6.de Paiva Haddad LB, Patzina RA, Penteado S, et al. Lymph node involvement and not the histophatologic subtype is correlated with outcome after resection of adenocarcinoma of the ampulla of vater. J Gastrointest Surg. 2010;14:719–728. doi: 10.1007/s11605-010-1156-4. [DOI] [PubMed] [Google Scholar]
- 7.Sessa F, Furlan D, Zampatti C, Carnevali I, Franzi F, Capella C. Prognostic factors for ampullary adenocarcinomas: tumor stage, tumor histology, tumor location, immunohistochemistry and microsatellite instability. Virchows Arch. 2007;451:649–657. doi: 10.1007/s00428-007-0444-1. [DOI] [PubMed] [Google Scholar]
- 8.Park SM, Kim SM, Han JH. The role of epithelial-mesenchymal transition in the gastroenterology. Korean J Gastroenterol. 2010;56:69–77. doi: 10.4166/kjg.2010.56.2.69. [DOI] [PubMed] [Google Scholar]
- 9.Hsu HP, Shan YS, Jin YT, Lai MD, Lin PW. Loss of E-cadherin an beta-catenin is correlated with poor prognosis of ampullary neoplasms. J Surg Oncol. 2010;101:356–362. doi: 10.1002/jso.21493. [DOI] [PubMed] [Google Scholar]
- 10.Hamada Y, Nakayama Y, Mizoguchi M, Ikeda S, Kuroki M, Iwasaki H. MK-1 expression in carcinoma of the ampulla of Vater as a predictor of improved prognosis after surgical resection. Cancer Lett. 2006;243:211–216. doi: 10.1016/j.canlet.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 11.Baumhoer D, Riener MO, Zlobec I, et al. Expression of CD24, P-cadherin and S100A4 in tumors of the ampulla of Vater. Mod Pathol. 2009;22:306–313. doi: 10.1038/modpathol.2008.192. [DOI] [PubMed] [Google Scholar]
- 12.Cheah PY, Choo PH, Yao J, Eu KW, Seow-Choen F. A survival-stratification model of human colorectal carcinomas with beta-catenin and p27kip1. Cancer. 2002;95:2479–2486. doi: 10.1002/cncr.10986. [DOI] [PubMed] [Google Scholar]
- 13.Albores-Saavedra J, Henson DE, Klimstra DS . Armed Forces Institute of Pathology (U.S.); Universities Associated for Research and Education in Pathology; Armed Forces Institute of Pathology (U.S.), editors; Universities Associated for Research and Education in Pathology, editors. Tumor of the gallbladder, extrahepatic bile duct, and ampulla of Vater. In: Albores-Saavedra J, Henson DE, Klimstra DS, editors. Malignant epithelial tumors of the ampullar. Washington, DC: Armed Forces Institute of Pathology; 2000. pp. 259–316. [Google Scholar]
- 14.Ohike N, Coban I, Kim GE, et al. Tumor budding as a strong prognostic indicator in invasive ampullary adenocarcinomas. Am J Surg Pathol. 2010;34:1417–1424. doi: 10.1097/PAS.0b013e3181f0b05a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawakami M, Kimura Y, Furuhata T, et al. beta-Catenin alteration in cancer of the ampulla of Vater. J Exp Clin Cancer Res. 2002;21:23–27. [PubMed] [Google Scholar]
- 16.Yamazaki K, Hanami K, Nagao T, Asoh A, Sugano I, Ishida Y. Increased cyclin D1 expression in cancer of the ampulla of Vater: relevance to nuclear beta catenin accumulation and k-ras gene mutation. Mol Pathol. 2003;56:336–341. doi: 10.1136/mp.56.6.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. Am J Pathol. 2010;176:528–535. doi: 10.2353/ajpath.2010.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elzagheid A, Buhmeida A, Laato M, et al. Loss of E-cadherin expression predicts disease recurrence and shorter survival in colorectal carcinoma. APMIS. 2012;120:539–548. doi: 10.1111/j.1600-0463.2011.02863.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser A, Jurowich C, Schönekäs H, Gebhardt C, Wünsch PH. The adenoma-carcinoma sequence applies to epithelial tumours of the papilla of Vater. Z Gastroenterol. 2002;40:913–920. doi: 10.1055/s-2002-35414. [DOI] [PubMed] [Google Scholar]
- 20.Takashima M, Ueki T, Nagai E, et al. Carcinoma of the ampulla of Vater associated with or without adenoma: a clinicopathologic analysis of 198 cases with reference to p53 and Ki-67 immunohistochemical expressions. Mod Pathol. 2000;13:1300–1307. doi: 10.1038/modpathol.3880238. [DOI] [PubMed] [Google Scholar]
- 21.Baczako K, Büchler M, Beger HG, Kirkpatrick CJ, Haferkamp O. Morphogenesis and possible precursor lesions of invasive carcinoma of the papilla of Vater: epithelial dysplasia and adenoma. Hum Pathol. 1985;16:305–310. doi: 10.1016/s0046-8177(85)80018-6. [DOI] [PubMed] [Google Scholar]