Abstract
Background
The handling of experimental animals prior to experimental interventions is often poorly described, even though it may affect the final functional outcome. This study explores how the use of repeated handling of C57BL/6 mice prior to Morris water maze (MWM) tests can affect the performance.
Methods and materials
The handled animals were subjected to the escalating handling protocol, with the investigator spending 5 min per day per cage for 8 days prior to the MWM test. On the last days of handling, the mice were introduced to water and the concept of a hidden platform. The MWM test consisted of four daily trials for 90 s per day for 4 days with a hidden platform. A probe test was performed 4 days after the last learning trial. Control animals were not handled prior to MWM.
Results
Handling reduced the latency to find the platform on the first 2 days of the MWM tests and reduced thigmotaxis. The mice increased their swim speed and elicited more explorative behavior in the learning trials and to some lesser extent in the probe trials.
Conclusions
The improvement in MWM navigation was most likely due to reduced stress and anxiety regarding the investigator and the test. Handled mice displayed less variability than non-handled mice, suggesting that by using a controlled handling protocol prior to the experiments fewer C57BL/6 mice would be needed to achieve statistically significant differences in studies of learning and spatial memory using MWM.
Keywords: Handling, mice, Morris water maze
Introduction
Prior to functional and behavioral tests in rodents many investigators acclimatize the animals to a new factor, e.g. the investigator himself or herself or certain interventions, by handling them. The handling may consist of visits to the animal facility with basic handling, i.e. lifting and moving the animals between cages, marking and weighing them. It could also involve more advanced procedures in order to prepare the animals to intraperitoneal or subcutaneous injections. However, when reporting in the manuscript it is usually briefly described as ‘the animals were handled prior to the experiment’. Even though there are published protocols for basic handling of rodents before behavioral experiments they are rarely referenced. For instance the article by Deacon on housing and handling has only been cited six times despite being published in a very prestigious journal (1).
The Morris water maze (MWM) test was developed by Morris et al. in the early 1980s to measure the functional aspect of lesions in the hippocampus in rats (2-4). It has become one of the most widely used tests for hippocampal function and spatial learning in rodents. The first studies of mice using the MWM were performed by Upchurch and Wehner (5), who also did the first characterization of different mouse strains in the test (6).
In our laboratory MWM has been used to evaluate potential treatments for traumatic brain injury in both rats (7,8) and mice (9,10). We use a protocol that includes both training trials to evaluate the spatial learning and a subsequent probe trial to evaluate the spatial memory a few days after the conclusion of the training trials. In our experiments with Sprague-Dawley rats we have not experienced any learning deficits in naïve or sham operated animals, whereas in experiments with C57BL/6 mice we have found that some non-injured individuals fail to learn to navigate the water maze. This became obvious when we in a recent study added a pre-injury training paradigm to our normal protocol (11). Briefly, mice were trained in four trials a day for 2 days before being subjected to traumatic brain injury or sham surgery and then tested with a probe trial 2 days after injury. In a group of 40 mice with no prior handling by the investigator, 5 failed to find the hidden platform and had to be excluded. Since stress is known to affect MWM performance negatively (12-14) and our mice had been minimally handled before the MWM experiment, we hypothesized that stress by handling and the novelty of the MWM procedure caused some individuals to fail in learning to find the platform.
Stress reduction before subjecting mice or rats to MWM has not been thoroughly studied, though it has been studied in conjunction with enriched environment and exercise (15). In rats, Holscher found that handling improved MWM performance, but unfortunately he did not describe how the handling was done (13). Another variant of handling was devised by Meaney et al. which entailed removing rat pups from their dam and home cage for 15 minutes a day until weaning at day 22, which resulted in improved spatial learning in aged Long-Evans rats (16). Using the same protocol, mice showed improved performance in MWM for the strain Balb/c ByJ, while there was no difference for C57BL/6J mice (17). A similar protocol, but with more time spent per pup, was shown to improve spatial learning in C57BL/6 mice under-expressing the beta-amyloid precursor protein (18). However, handling of neonatal pups is not always possible, especially for laboratories not breeding animals. The effect of gentle handling has been found to neither affect long term potentiation (LTP) significantly, nor elicit stress beyond the first day of contact with the investigator (19).
In this study we present a structured protocol for handling mice prior to Morris water maze experiments. Our hypothesis was that if mice were handled in an escalated fashion, in accordance with the protocol proposed by Deacon (1), the stress imposed by the investigator and the water maze would be reduced, resulting in fewer failures and reduced variation in naïve animals. The handling protocol was designed to involve only a minor increase in workload for the investigator, fit into a busy schedule of other functional tests, and not require the need to acquire new equipment.
Materials and methods
Mice and animal housing
Twenty-eight male C57BL/6 mice (Taconic MS, Ejby, Denmark), aged 6–7 weeks, were housed 3–5 siblings per cage. All inhabitants of the cage were part of the study. The protocol made it necessary to subject all inhabitants of a cage to the same treatment. The cages were randomized to either handling or non-handling. All cages were kept in the same ventilated cabinet in the animal facility, and the animals were habituated to the new animal facility for 11 days prior to handling by the investigator. Food and water were available ad libitum. Fourteen mice were handled prior to MWM trials, and 14 were never handled by the investigator before the MWM trials. The home room was kept on a 12 h light/dark cycle, light 7 a.m. to 7 p.m. The mice were kept in cages of type Makrolon III (42 × 26.5 × 15.5 cm) with bedding material of aspen chips. A paper house and sheets of paper towel were used as basic enrichment. All procedures involving the mice were approved by the Uppsala regional ethical committee for animal experiments (C137/11) and followed the rules and regulations of the Swedish Board of Agriculture.
Handling protocol
The handling protocol was designed to increase the contact with the investigator in a series of escalating steps and was adapted to the animal facility we have access to.
Day 1: The cage was moved out of the ventilated cabinet and moved to a table in the home room. The investigator put a hand in the cage allowing the mice to sniff and explore at will for 5 min.
Day 2: The procedure of day 1 was repeated, but the enrichment material was removed.
Day 3: The procedure of day 2 was repeated for 2 min. The mice were then lifted up by the tail and moved to an empty and clean cage for 30 s before being moved back to the home cage for 30 s. This procedure was repeated three times.
Day 4: The mice were moved from the home room to an antechamber directly outside of the home room. The cage was placed on a ventilated table, and the mice were picked up by the tail and put into a cage on the scales three times for 10 s.
Day 5: Repetition of day 4 with the addition that the mice were kept on the arm of the investigator for 5–10 s, three times before being returned to the cage.
Day 6: The same procedure as for day 5, but the mice were marked with stripes on the tail with a non-toxic permanent marker.
Day 7: The maneuvers of day 6 were repeated, the new addition being to let the mice acclimatize to water in three trials with increasing amounts of water. First trial: mice were put in a cage (Makrolon I, 23.5 × 14 × 13 cm) with 0.5 cm deep water (30°C) for 20–30 s. Second trial: 1 cm deep water for 20–30 s. Third trial: 2 cm deep water for 20–30 s.
Day 8: The same procedure as day 7, but the water challenge was increased in complexity. The same type of cage as used on day 7 was filled with 10 cm of warm water (30°C). A square glass jar (6 × 8 × 5 cm) was used as an invisible platform. In the first trial the mice were put into the water 4 cm from the platform, held by the tail and gently moved to the platform, from where they were allowed to escape on to the arm of the investigator. The second trial was performed in the same way as the first trial, but the mice started 8 cm away from the platform. In the third trial the mouse was put into the water without the investigator holding its tail, instead the hand was used to guide the mouse to the platform. When the mouse was on the platform it was allowed to escape on to the investigator's arm. After each trial the mouse was dried with a cloth towel before being returned to the cage. The time between the trials was 5 min. After the water exposure the mice were moved from the animal house to the MWM room, and the cage was kept there under a heating lamp for 2 hours to familiarize the mice with both the transportation and the MWM room.
Morris water maze trials
MWM learning trials were started on day 9 of the experiment, i.e. the day after handling day 8. The MWM protocol used in this study has been described previously (2,8). The MWM consists of a 1.4 m diameter circular white tank with a 10 cm diameter white platform, placed in the southeast quadrant of the tank. The platform is hidden by submerging it 1 cm below the surface of the water. The temperature of the water was kept at 21–22°C. Simple visual cues to aid navigation were placed on roller curtains surrounding the pool. The learning protocol consisted of four trials a day for four consecutive days. The intervals between the trials were kept strictly to 30 min, and the mice were returned to their home cages between every trial. Each trial was run by placing the mouse in the tank randomly at one of four designated entry points (W, N, E, and S) facing the wall, activating the video-based computer tracking system (HVS Image Ltd, Buckingham, UK), and the trial was terminated when the mouse located the platform. The mouse was allowed to remain undisturbed on the platform for 15 s to acquire the visual cues surrounding the pool. If the mouse failed to locate the platform within 90 s it was led to the platform and allowed to stay there for 15 s. After the trial the mouse was picked up and dried with a towel. The trial was analyzed for latency to find the platform, path length, swim speed, thigmotaxis, and failure to find the platform. The path tracks were investigated to detect any changes in exploratory behavior.
The probe trial to evaluate the spatial memory was performed 4 days following the last learning trial, by removing the platform and analyzing the latency to find the platform area, time spent in the target quadrant of the pool, and number of passes over the platform area. The last two parameters were analyzed for the first 15 s (0–15 s), the first 30 s (0–30 s), and the full 90 s, to spot any changes in explorative behavior over time.
Statistical analysis
Data were analyzed with Statistica® (StatSoft, Tulsa, OK, USA) software. After testing for normality using Shapiro–Wilk w test and finding that data were normally distributed we used parametric tests. Repeated measures two-way ANOVA (split for treatment and trial day) with Fisher LSD post hoc test was used for the latency to find the platform, swim speed, and path length for the learning trials. Thigmotaxis, defined as the amount of time spent within 15 cm from the wall of the pool, was analyzed. The average for the four trials for each day was then calculated and used for the analyses. Student's t test was used for the latency to find the platform area, swim speed, number of passages over the platform area, and the amount of time spent in the quadrant that was used to contain the platform in the probe trials. A p < 0.05 was considered statistically significant.
Results
General observations
The handling protocol led to a rapid habituation of the mice to the investigator. On days 7 and 8 of handling, all of the handled mice sat on the arm of the investigator without trying to escape. The handled mice did not try to escape from the platform in the water maze if they were placed on it, a behavior the non-handled mice did not acquire till day 2 of the learning trials. There was no floating behavior in any of the animals. Both groups steadily gained weight over the MWM trials; non-handled animals increased from 28.6 ± 2.6 g to 30.2 ± 2.4 g (mean ± SEM, n = 14) and handled mice from 27.9 ± 2.2 g to 29.0 ± 3.2 g (mean ± SEM, n = 14). No statistically significant differences in weight between the groups were found at any time point.
MWM learning trials
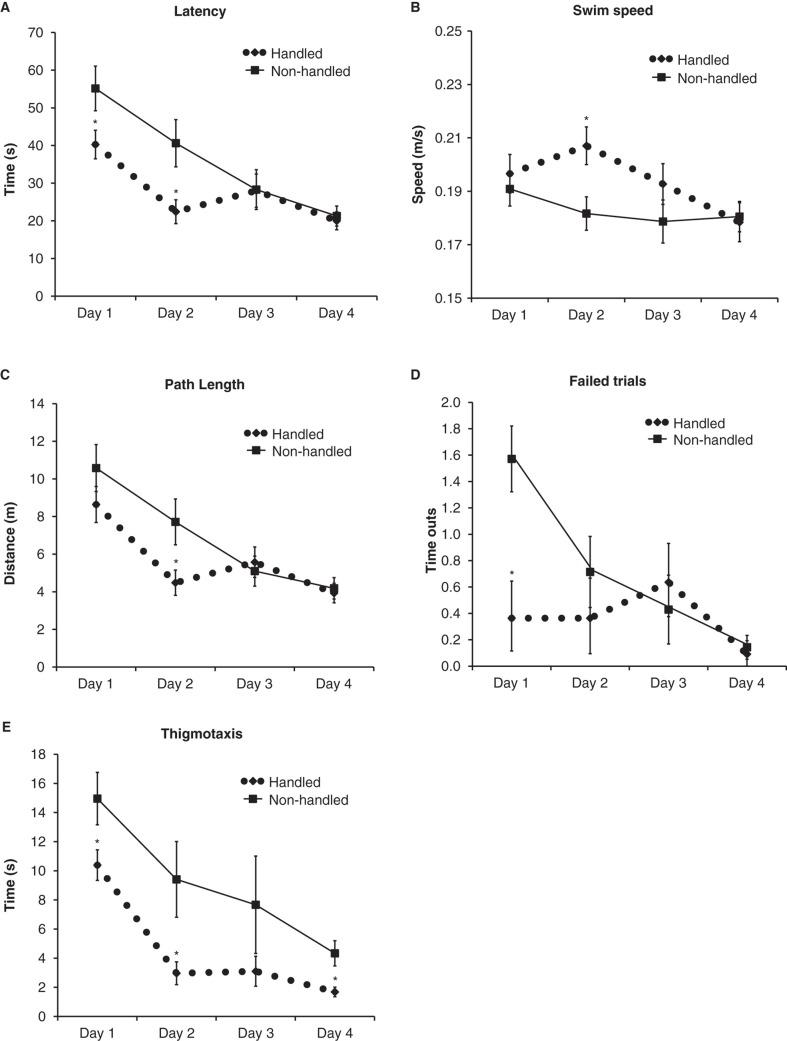
Handling decreased the latency to find the platform the first 2 days of MWM trials (Figure 1A), but failed to reach statistical significance for the mean of all 4 days of trials (Table I).
Figure 1.
Results from the learning trials in the MWM presented as means ± standard error of the mean. *p < 0.05, **p < 0.01, n = 14 per group. A: The mean latency to find the hidden platform for each trial day, where handled animals had shorter latency on days 1 and 2. B: The distance the animal swam (path length). C: The number of failed trials (time-outs). D: The swim speed. E: The thigmotaxis as defined as time spent within 15 cm of the pool wall.
Table I.
Mean and coefficient of variation for latency, path length and swim speed during the learning trials for all 4 days combined with the calculated F ratio for the difference in variance between the groups (n = 14 per group).
| Non-handled |
Handled |
F ratio of variances | p variances | |||
|---|---|---|---|---|---|---|
| Mean | Coefficient of variation | Mean | Coefficient of variation | |||
| Latency (s) | 36.3 | 63.4 | 27.7 | 54.1 | 2.11 | 0.0064* |
| Path length (m) | 6.89 | 65.2 | 5.73 | 57.4 | 1.96 | 0.024* |
| Swim speed (m/s) | 0.183 | 13.1 | 0.194 | 12.4 | 1.22 | 0.47 |
Decrease in variability for the handled mice.
Handling increased the swim speed on day 2 (Figure 1B) and overall for the 4 days of trials (p = 0.048).
The same pattern was seen for the path length, but this parameter only reached statistical significance on day 2 of the daily trial blocks (Figure 1C). Over all 4 days there was no difference between handling and non-handling (p = 0.11). Handled mice had fewer total numbers of failure to find the platform on the first day of trials (Figure 1D). The handling increased the risk-taking behavior in the MWM the first day of trials (Figure 2A) compared with the swimming pattern of a non-handled mouse (Figure 2B). The non-handled mice showed more thigmotaxis on days 1, 2, and 4 of the learning trials, defined as time spent within 15 cm of the wall of the tank (Figure 1E).
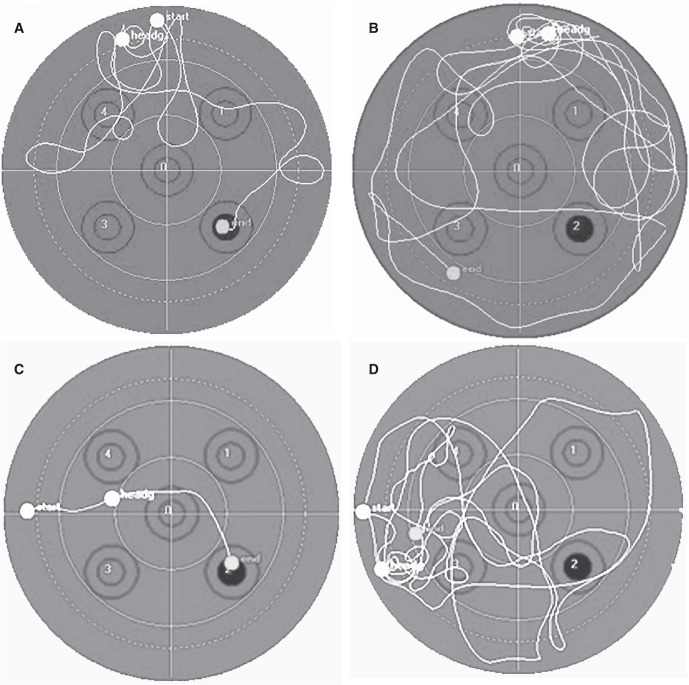
Figure 2.
Swim patterns from the very first trial of a handled mouse (A) and a non-handled mouse (B). The handled mouse quickly left the ‘security’ of the wall to explore the pool, whereas the non-handled mouse displayed more thigmotaxis, as measured by the time spent between the dotted line and the wall of the pool. The black circle represents the position of the hidden platform. The straight lines crossing the pool divide it into four quadrants. The numbered double circles represent different possible placements of the hidden platform. Swim patterns from the third trial on days 2 (C) and 3 (D) for a handled mouse are also shown. On day 2, the mouse took an almost optimal route to the platform, whereas on day 3 it explored the pool and ultimately times out. The platform is shown as a black dot in the south-east quadrant.
The handling resulted in an unexpected increase in latency to find the platform from day 2 to day 3 of the trials. When the swimming patterns were analyzed it was quite evident that the mice remembered the location of the platform but spent more time exploring the pool. Figures 2C, D show the difference in swim pattern for the second trial of the day between days 2 and 3 for a handled mouse.
Looking at the variation within the groups we found that handled animals showed lower coefficients of variation for latency and path length over all 4 days of learning trials (Table I) and for days 1, 2, and 4 of the training trials (data not shown). Calculating the F ratio showed that the variance for both latency to find the platform and path length was lower in the handled group.
Probe trial
In addition to analyzing the full 90 seconds of the probe trial, the first 15 and 30 s were evaluated as well. This was done in order to spot differences in behavior in the early phase of the trial compared with the full duration.
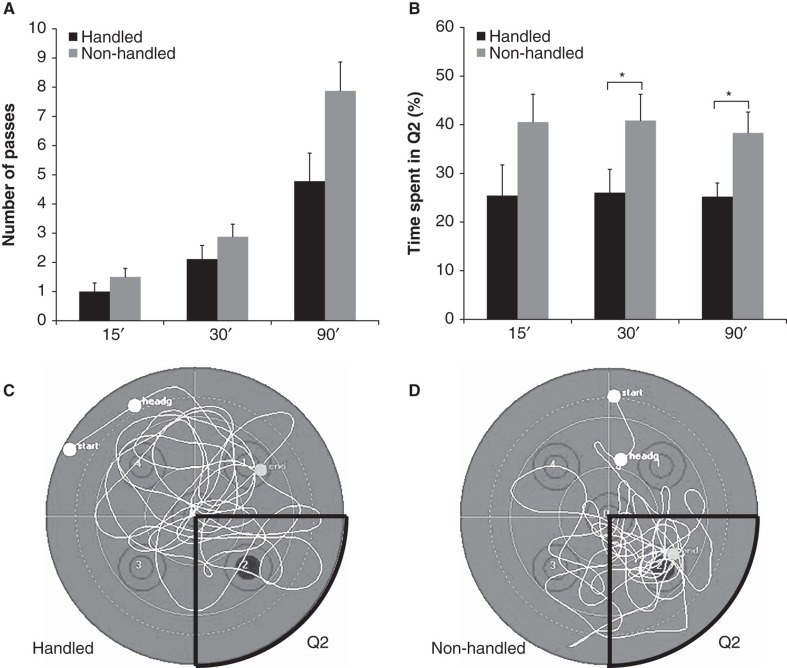
There was no difference between the groups in latency to find the platform area (19.4 ± 3.8 s for handled mice, 20.7 ± 6.3 s for non-handled mice, p = 0.70). Likewise, there was no statistical difference between the groups in number of passes over the platform area in any of the three time slices (Figure 3A), although the non-handled mice passed more times during all 90 s of the trial (7.9 ± 1.0 times for non-handled versus 4.9 ± 0.99 for handled mice, p = 0.100). The non-handled animals spent more time exploring the quadrant where the platform had been placed in the 30 and 90 s time slices, but not in the 15 s slices (Figure 3B). The handled animals showed less thigmotaxis compared with the non-handled mice (handled mice: 7.69 ± 0.89 s, non-handled mice: 16.5 ± 3.1 s, p = 0.028). Figures 3C,D show representative swim patterns from both groups.
Figure 3.
Results from the probe trial for handled and non-handled mice in the MWM presented as means ± standard error of the mean. *p < 0.05, n = 14 per group. A: The number of passes over the platform area for the three time slices analyzed. B: The time spent in south-east where the platform was placed during the learning trials. Non-handled animals spent more time in this quadrant in the 0–30 s and 0–90 s time slices compared with the handled mice. The search pattern of a handled (C) and a non-handled (D) mouse during the 90 s trial in relation to the position of the south-east quadrant (Q2) where the platform was placed during the learning trials (black dot).
Discussion
The handling protocols used before behavioral or motor function tests are seldom well described, with some exceptions (1,20), which is disconcerting since handling most certainly influences the outcome. It has been established that the most important factor for variations in behavioral results is the investigator (21), most probably due to differences in handling of the animals.
The escalated handling protocol had more impact on the learning trials of the MWM test than the memory test in the probe trial, i.e. significantly fewer time-outs on the first day, lower latencies to find the platform the first 2 days, and a marked decrease in thigmotaxis for days 1, 2, and 4. The only statistically significant effect on spatial memory was that handled mice spent less time in the quadrant where the platform had been placed during the learning trials.
The significant decrease in latency to find the platform and number of time-outs show that the handled mice learned to navigate the MWM more quickly than non-handled mice. However, on days 3 and 4 there was no difference between the groups, suggesting that the non-handled mice had learnt to find the platform as proficiently as the handled mice. It is possible that the handled mice were searching for an alternative way to escape the water on day 3, resulting in changes in the swim pattern. It has been previously shown that C57BL/6 mice may have an increased latency at later trial days as well as an increased thigmotaxis (22).
The present findings indicate that handled mice more eagerly entered the open part of the pool, as they have less thigmotaxis than non-handled animals. This increase in explorative behavior could be due to the training in the water-filled cage, where they were exposed to water and the concept of escaping onto a hidden platform. It could also be due to the familiarization of the mice to the investigator, thereby reducing stress, which has been shown to increase the explorative behavior in an open-field test (23). Since reducing stress is less studied than that of inducing stress, there are more data on subjecting C57BL/6 mice to chronic stress or chronic unpredictable stress. Inducing stress has been shown to inhibit exploratory behavior in circular hole board tests (24) and to induce learning deficits in the MWM (25).
Protocols of a behavioral study often include several different tests, which possibly can affect the outcome of the individual tests depending on the order in which the tests are performed. Tests preceding the MWM trials in such a protocol could be considered as handling. However, Voikar et al. evaluated the performance of handled versus non-handled mice in a big battery of tests (26), but found no difference in performance between animals subjected to several other tests before MWM trials compared with non-handled animals.
In the present study, the handled mice showed increased swim speed, possibly due to their more explorative behavior or due to their quicker learning to locate the platform and taking a shorter route. However, the path length of the handled mice was not reduced as much as the latency to find the platform, suggesting that they took a longer route than necessary to reach the platform.
As the handling decreased the variation with the handled mice compared with the non-handled mice, it may be possible to use fewer animals and still achieve statistically significant results in a treatment study. From an animal ethics standpoint the reduction of the number of animals used is highly desirable, and one of the three Rs is Reduction (27). In addition to the reduced number of animals used, the decrease in stress most certainly improves the situation for the mice and is desirable as long as not stress or anxiety is studied (1).
The handling protocol does not require a large increase in workload for the investigator and no or very little additional equipment. Compared with postnatal handling protocols the increased workload is negligible (17,18). This makes it reasonable to include the handling protocol in studies of genetic manipulation, disease, or treatments.
Admittedly this is a small study, and the protocol for escalating handling could be further refined to yield better results. Laboratory tests for stress hormones would further prove that the handling protocol de facto reduced the stress. In this study the mice were lifted up by the tail until they were acclimatized enough to the investigator to climb voluntarily on to the arm. According to a recent report, using cupped hands or a tunnel from the home cage can induce quicker adaptation to the investigator (28). Using either the tunnel or cupped hands approach in order to catch the mice might possibly improve our results even further. Conversely, since the non-handled mice were not as used to being picked up by the tail, they may have been additionally stressed when exposed to the MWM, causing them to perform even worse.
Adding a step where the animals are released into the tank prior to the MWM tests could further reduce the stress of the test, used in other MWM protocols (29,30).
The light and dark cycle of the animal facility made it necessary to do the handling and MWM test during the light (inactive) cycle of the mice; however, data from C129 mice subjected to the same MWM protocol showed no difference in performance between testing in the light or dark cycle (31). ‘Gentle handling’ daily for 3 min during the light cycle for 6 days in C57BL/6J mice resulted in increased plasma concentrations of corticosterone and the NMDA receptor subunit 2A (32). These results are very interesting since the NMDA receptor subunit 2A is critical for spatial learning (33) while corticosterone can be detrimental to MWM performance (14). It should be mentioned that the plasma corticosterone concentrations measured by Longordo and colleagues were higher than those of the controls (32), but not nearly as high as the concentrations found by Harrison and colleagues (14). A certain amount of stress could be beneficial for learning tests with an escape aspect built into them, such as MWM (34).
As described above, the escalating handling protocol could be changed to enhance further the stress relief. It would be of interest to evaluate the effect of the water exposure in our protocol on the swim pattern, by omitting or changing the last 2 days of handling. It is not unreasonable to hypothesize that the brief encounter with a hidden platform primed the shift in search behavior as observed on the first day of learning trials in the handled mice. What is extraordinary is that such a brief exposure resulted in a drastic change in search pattern. It would be of interest to see if the handling effect can be observed in a treatment or traumatic brain injury study.
In conclusion, by using a clearly defined handling protocol the initial performance in MWM was improved, presumably because the stress towards the investigator and the test situation was reduced. The variability within the handled group decreased, something that could reduce the number of animals needed to show differences between experimental groups in the test.
Acknowledgements
The work was supported by grants from the Swedish Brain Foundation, Uppsala University Hospital, Åhlén's Foundation, and funds from Uppsala University.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Deacon RM. Housing, husbandry and handling of rodents for behavioral experiments . Nat Protoc. 2006;1:936–46. doi: 10.1038/nprot.2006.120. [DOI] [PubMed] [Google Scholar]
- 2.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat . J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 3.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions . Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 4.Morris R. Spatial localization does not require the presence of local cues . Learn Motiv. 1981;12:239–60. [Google Scholar]
- 5.Upchurch M, Wehner JM. Effects of chronic diisopropylfluorophosphate treatment on spatial learning in mice . Pharmacol Biochem Behav. 1987;27:143–51. doi: 10.1016/0091-3057(87)90488-6. [DOI] [PubMed] [Google Scholar]
- 6.Upchurch M, Wehner JM. Differences between inbred strains of mice in Morris water maze performance . Behav Genet. 1988;18:55–68. doi: 10.1007/BF01067075. [DOI] [PubMed] [Google Scholar]
- 7.Clausen F, Marklund N, Lewen A, Hillered L. The nitrone free radical scavenger NXY-059 is neuroprotective when administered after traumatic brain injury in the rat . J Neurotrauma. 2008;25:1449–57. doi: 10.1089/neu.2008.0585. [DOI] [PubMed] [Google Scholar]
- 8.Marklund N, Clausen F, McIntosh TK, Hillered L. Free radical scavenger posttreatment improves functional and morphological outcome after fluid percussion injury in the rat . J Neurotrauma. 2001;18:821–32. doi: 10.1089/089771501316919184. [DOI] [PubMed] [Google Scholar]
- 9.Clausen F, Hanell A, Bjork M, Hillered L, Mir AK, Gram H, et al. Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice . Eur J Neurosci. 2009;30:385–96. doi: 10.1111/j.1460-9568.2009.06820.x. [DOI] [PubMed] [Google Scholar]
- 10.Hanell A, Clausen F, Bjork M, Jansson K, Philipson O, Nilsson LN, et al. Genetic deletion and pharmacological inhibition of Nogo-66 receptor impairs cognitive outcome after traumatic brain injury in mice . J Neurotrauma. 2010;27:1297–309. doi: 10.1089/neu.2009.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clausen F, Hanell A, Israelsson C, Hedin J, Ebendal T, Mir AK, et al. Neutralization of interleukin-1beta reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice . Eur J Neurosci. 2011;34:110–23. doi: 10.1111/j.1460-9568.2011.07723.x. [DOI] [PubMed] [Google Scholar]
- 12.Cuadrado-Tejedor M, Ricobaraza A, Del Rio J, Frechilla D, Franco R, Perez-Mediavilla A, et al. Chronic mild stress in mice promotes cognitive impairment and CDK5-dependent tau hyperphosphorylation . Behav Brain Res. 2011;220:338–43. doi: 10.1016/j.bbr.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Holscher C. Stress impairs performance in spatial water maze learning tasks . Behav Brain Res. 1999;100:225–35. doi: 10.1016/s0166-4328(98)00134-x. [DOI] [PubMed] [Google Scholar]
- 14.Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks . Behav Brain Res. 2009;198:247–51. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurfein BT, Stamm AW, Bacchetti P, Dallman MF, Nadkarni NA, Milush JM, et al. The calm mouse: an animal model of stress reduction . Mol Med. 2012;18:606–17. doi: 10.2119/molmed.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus . Science. 1988;239:766–8. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- 17.Zaharia MD, Kulczycki J, Shanks N, Meaney MJ, Anisman H. The effects of early postnatal stimulation on Morris water-maze acquisition in adult mice: genetic and maternal factors . Psychopharmacology (Berl) 1996;128:227–39. doi: 10.1007/s002130050130. [DOI] [PubMed] [Google Scholar]
- 18.Tremml P, Lipp HP, Muller U, Wolfer DP. Enriched early experiences of mice underexpressing the beta-amyloid precursor protein restore spatial learning capabilities but not normal openfield behavior of adult animals . Genes Brain Behav. 2002;1:230–41. doi: 10.1034/j.1601-183x.2002.10405.x. [DOI] [PubMed] [Google Scholar]
- 19.Vecsey CG, Wimmer ME, Havekes R, Park AJ, Perron IJ, Meerlo P, et al. Daily acclimation handling does not affect hippocampal long-term potentiation or cause chronic sleep deprivation in mice . Sleep. 2013;36:601–7. doi: 10.5665/sleep.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adamec R, Head D, Blundell J, Burton P, Berton O. Lasting anxiogenic effects of feline predator stress in mice: sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience . Physiol Behav. 2006;88:12–29. doi: 10.1016/j.physbeh.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive . Neurosci Biobehav Rev. 2002;26:907–23. doi: 10.1016/s0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- 22.Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice . Genes Brain Behav. 2002;1:55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 23.Powell SB, Geyer MA, Gallagher D, Paulus MP. The balance between approach and avoidance behaviors in a novel object exploration paradigm in mice . Behav Brain Res. 2004;152:341–9. doi: 10.1016/j.bbr.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Dalm S, de Kloet ER, Oitzl MS. Post-training reward partially restores chronic stress induced effects in mice . PLoS ONE. 2012;7:e39033. doi: 10.1371/journal.pone.0039033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bisaz R, Schachner M, Sandi C. Causal evidence for the involvement of the neural cell adhesion molecule, NCAM, in chronic stress-induced cognitive impairments . Hippocampus. 2011;21:56–71. doi: 10.1002/hipo.20723. [DOI] [PubMed] [Google Scholar]
- 26.Voikar V, Vasar E, Rauvala H. Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens . Genes Brain Behav. 2004;3:27–38. doi: 10.1046/j.1601-183x.2003.0044.x. [DOI] [PubMed] [Google Scholar]
- 27.Leenaars M, Savenije B, Nagtegaal A, van der Vaart L, Ritskes-Hoitinga M. Assessing the search for and implementation of the Three Rs: a survey among scientists . Altern Lab Anim. 2009;37:297–303. doi: 10.1177/026119290903700312. [DOI] [PubMed] [Google Scholar]
- 28.Hurst JL, West RS. Taming anxiety in laboratory mice . Nat Methods. 2010;7:825–6. doi: 10.1038/nmeth.1500. [DOI] [PubMed] [Google Scholar]
- 29.Wolff M, Savova M, Malleret G, Segu L, Buhot MC. Differential learning abilities of 129T2/Sv and C57BL/6J mice as assessed in three water maze protocols . Behav Brain Res. 2002;136:463–74. doi: 10.1016/s0166-4328(02)00192-4. [DOI] [PubMed] [Google Scholar]
- 30.Bennett JC, McRae PA, Levy LJ, Frick KM. Long-term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice . Neurobiol Learn Mem. 2006;85:139–52. doi: 10.1016/j.nlm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Beeler JA, Prendergast B, Zhuang X. Low amplitude entrainment of mice and the impact of circadian phase on behavior tests . Physiol Behav. 2006;87:870–80. doi: 10.1016/j.physbeh.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 32.Longordo F, Fan J, Steimer T, Kopp C, Luthi A. Do mice habituate to ‘gentle handling?’ A comparison of resting behavior, corticosterone levels and synaptic function in handled and undisturbed C57BL/6J mice . Sleep. 2011;34:679–81. doi: 10.1093/sleep/34.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu M, Sun YJ, Zhou QG, Auberson YP, Chen L, Hu Y, et al. Reduced spatial learning in mice treated with NVP-AAM077 through down-regulating neurogenesis . Eur J Pharmacol. 2009;622:37–44. doi: 10.1016/j.ejphar.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 34.Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action . Neuropsychopharmacology. 2010;35:674–85. doi: 10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]