Abstract
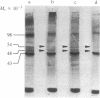
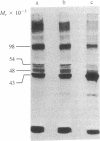
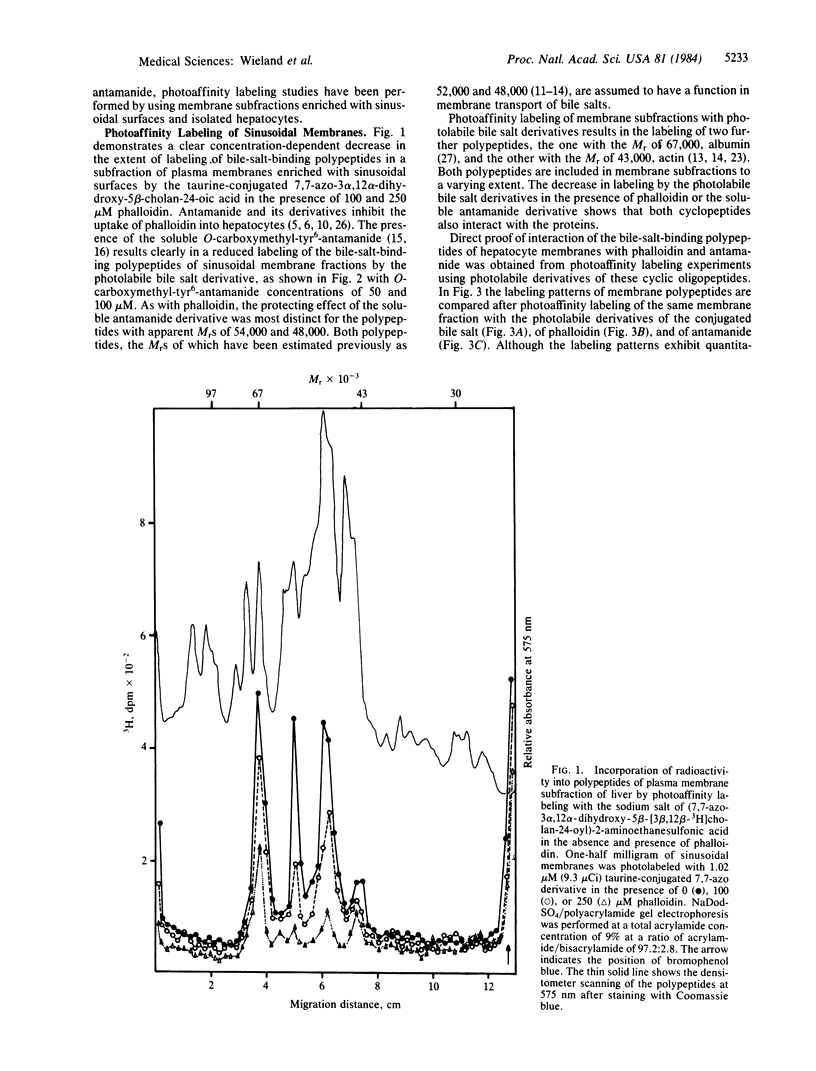
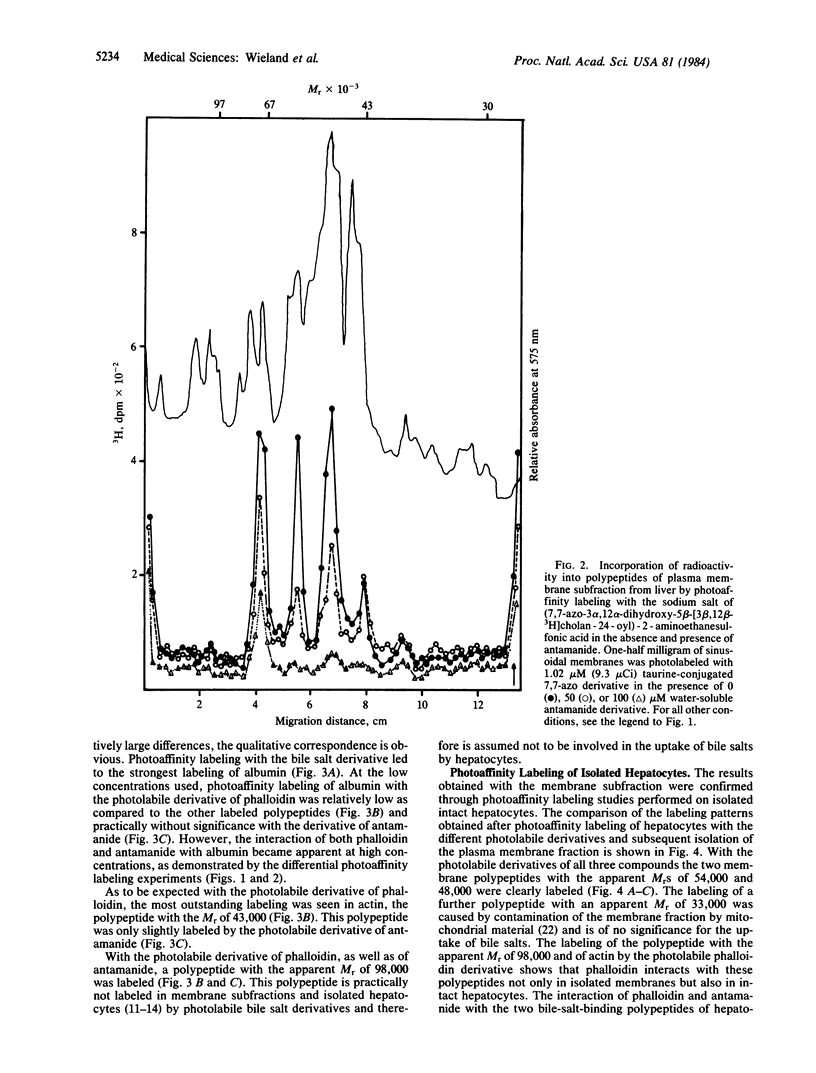
Phalloidin, a bicyclic heptapeptide, and antamanide, a monocyclic decapeptide from the poisonous mushroom Amanita phalloides, interact with bile-salt-binding polypeptides of the hepatocyte membrane, as demonstrated by photoaffinity labeling using the photolabile bile salt derivative 7,7,-azo-3 alpha, 12 alpha-dihydroxy-5 beta-cholan-24-oic acid, either unconjugated or taurine conjugated. With the photolabile derivatives of phalloidin, N-delta-(4-[(1-azi-2,2,2-trifluoroethyl) benzoyl]-beta-alanyl)-delta-aminophalloin, (N epsilon-[4-(1-azi-2,2,2-trifluoroethyl)benzoyl]lys6)-anta manide, the same membrane polypeptides with apparent MrS of 54,000 and 48,000 were labeled as with the photolabile derivatives of unconjugated and conjugated bile salts. The presence of bile salts decreased markedly the extent of labeling of these phalloidin- and antamanide-binding polypeptides. These results indicate that hepatic uptake systems for bile salts, phallotoxins, and the cycloamanide antamanide are identical, thus explaining the organotropism of phallotoxins.
Full text
PDF
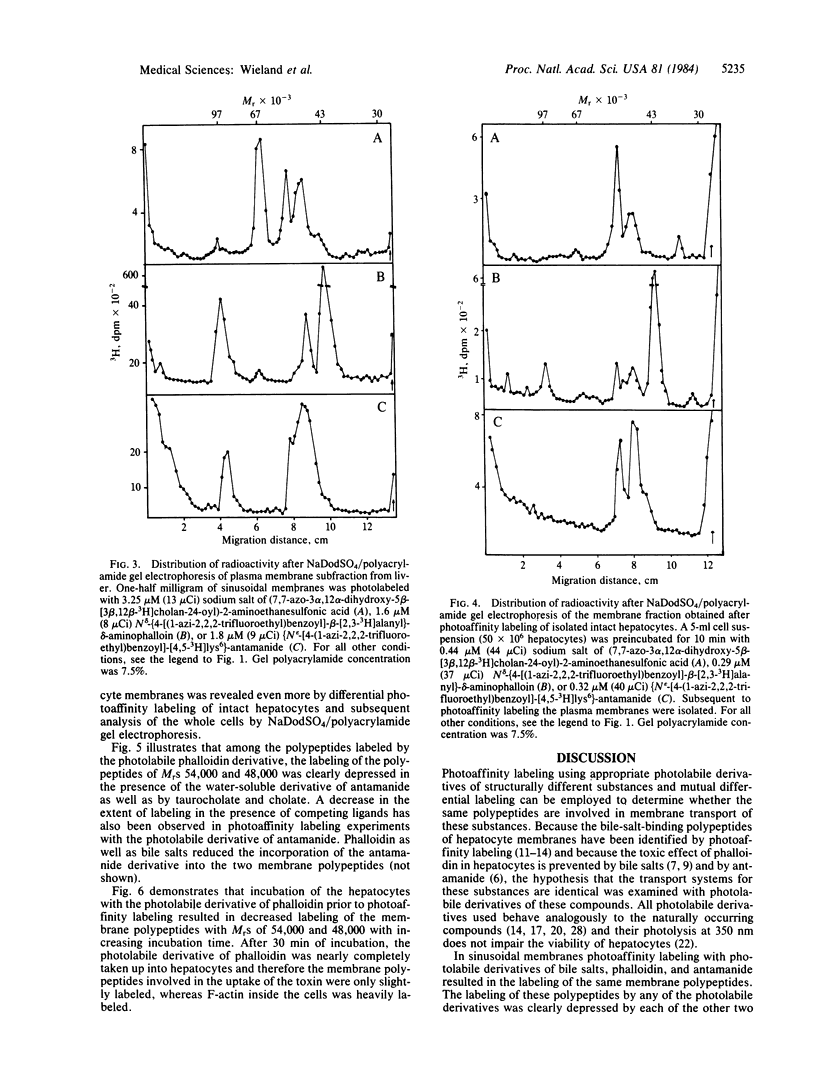

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burckhardt G., Kramer W., Kurz G., Wilson F. A. Inhibition of bile salt transport in brush-border membrane vesicles from rat small intestine by photoaffinity labeling. J Biol Chem. 1983 Mar 25;258(6):3618–3622. [PubMed] [Google Scholar]
- Faulstich H., Wieland T., Walli A., Birkmann K. Anatamanide protects hepatocytes from phalloidin destruction. Hoppe Seylers Z Physiol Chem. 1974 Sep;355(9):1162–1163. [PubMed] [Google Scholar]
- Frimmer M., Petzinger E., Rufeger U., Veil L. B. The role of bile acids in phalloidin poisoning. Naunyn Schmiedebergs Arch Pharmacol. 1977 Dec;301(2):145–147. doi: 10.1007/BF00501430. [DOI] [PubMed] [Google Scholar]
- Frimmer M., Petzinger E., Ziegler K. Protective effect of anionic cholecystographic agents against phalloidin on isolated hepatocytes by competitive inhibition of the phallotoxin uptake. Comparison of the influence on the inward transport of 3H-demethylphalloin and of 14C-cholate. Naunyn Schmiedebergs Arch Pharmacol. 1980 Aug;313(1):85–89. doi: 10.1007/BF00505808. [DOI] [PubMed] [Google Scholar]
- Kramer W., Bickel U., Buscher H. P., Gerok W., Kurz G. Bile-salt-binding polypeptides in plasma membranes of hepatocytes revealed by photoaffinity labelling. Eur J Biochem. 1982 Dec;129(1):13–24. doi: 10.1111/j.1432-1033.1982.tb07015.x. [DOI] [PubMed] [Google Scholar]
- Kramer W., Burckhardt G., Wilson F. A., Kurz G. Bile salt-binding polypeptides in brush-border membrane vesicles from rat small intestine revealed by photoaffinity labeling. J Biol Chem. 1983 Mar 25;258(6):3623–3627. [PubMed] [Google Scholar]
- Kramer W., Buscher H. P., Gerok W., Kurz G. Bile salt binding to serum components. Taurocholate incorporation into high-density lipoprotein revealed by photoaffinity labelling. Eur J Biochem. 1979 Dec;102(1):1–9. doi: 10.1111/j.1432-1033.1979.tb06257.x. [DOI] [PubMed] [Google Scholar]
- Kramer W., Kurz G. Photolabile derivatives of bile salts. Synthesis and suitability for photoaffinity labeling. J Lipid Res. 1983 Jul;24(7):910–923. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Petzinger E., Burckhardt G., Schwenk M., Faulstich H. Lack of intestinal transport of [3H]-demethylphalloin: comparative studies with phallotoxins and bile acids on isolated small intestinal cells and ileal brush border membrane vesicles. Naunyn Schmiedebergs Arch Pharmacol. 1982 Aug;320(2):196–200. doi: 10.1007/BF00506321. [DOI] [PubMed] [Google Scholar]
- Petzinger E., Frimmer M. Comparative studies on the uptake of 14C-bile acids and 3H-demethylphalloin in isolated rat liver cells. Arch Toxicol. 1980 Mar;44(1-3):127–135. doi: 10.1007/BF00303189. [DOI] [PubMed] [Google Scholar]
- Petzinger E., Joppen C., Frimmer M. Common properties of hepatocellular uptake of cholate, iodipamide and antamanide, as distinct from the uptake of bromosulfophthalein. Naunyn Schmiedebergs Arch Pharmacol. 1983 Mar;322(2):174–179. doi: 10.1007/BF00512393. [DOI] [PubMed] [Google Scholar]
- Petzinger E., Ziegler K., Frimmer M. Inhibition of 3H-demethylphalloin uptake in isolated rat hepatocytes under various experimental conditions. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jul;307(3):275–281. doi: 10.1007/BF00505944. [DOI] [PubMed] [Google Scholar]
- REHBINDER D., LOEFFLER G., WIELAND O., WIELAND T. [Studies on the mechanism of the poisonous effect of phalloidin with poisons labelled with radioactive substances]. Hoppe Seylers Z Physiol Chem. 1963 Mar;331:132–142. doi: 10.1515/bchm2.1963.331.1.132. [DOI] [PubMed] [Google Scholar]
- Wieland T., Faulstich H., Jahn W., Govindan M. V., Puchinger H., Kopitar Z., Schmaus H., Schmitz A. Uber Antamanid. XIV. Zur Wirkungsweise des Antamanids. Hoppe Seylers Z Physiol Chem. 1972 Aug;353(8):1337–1345. [PubMed] [Google Scholar]
- Wieland T. Interaction of phallotoxins with actin. Adv Enzyme Regul. 1976;15:285–300. doi: 10.1016/0065-2571(77)90021-8. [DOI] [PubMed] [Google Scholar]
- Wieland T., Rietzel C., Seeliger A. Uber Antamanid. XV. Einige Derivate der phenolischen Seitenkette des Tyrosin 5- und Tyrosin 6- antamanids. Justus Liebigs Ann Chem. 1972 May;759:63–70. doi: 10.1002/jlac.19727590104. [DOI] [PubMed] [Google Scholar]
- Wieland T., Rietzel C. Uber Antamanid. X. Das Tyrosin 6 -Analoge des Antamanids. Justus Liebigs Ann Chem. 1971 Dec;754:107–112. doi: 10.1002/jlac.19717540111. [DOI] [PubMed] [Google Scholar]
- Wieland T. The discovery, isolation, elucidation of structure, and synthesis of antamanide. Angew Chem Int Ed Engl. 1968 Mar;7(3):204–208. doi: 10.1002/anie.196802041. [DOI] [PubMed] [Google Scholar]
- Wieland T. The toxic peptides from Amanita mushrooms. Int J Pept Protein Res. 1983 Sep;22(3):257–276. doi: 10.1111/j.1399-3011.1983.tb02093.x. [DOI] [PubMed] [Google Scholar]