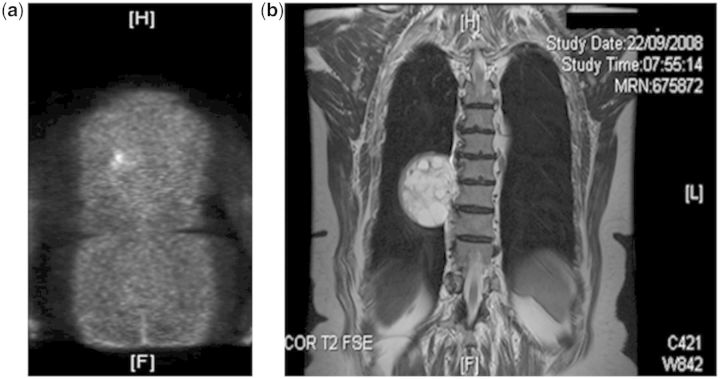
A 78-year-old female presented in 1996 with bone pain, generalized muscular weakness and immobility. She had marked hypophosphataemia and osteomalacic fractures and a clinical diagnosis of oncogernic osteomalacia was made.1 No tumour could be identified at that time. Subsequent management, including partial parathyroidectomy, oral phosphate and 1,25-dihydroxycholecalciferol (1,25-D3), produced good symptomatic improvement. In 2008, elevated levels of fibroblast growth factor (FGF)-23 were found, 18F-Deoxyglucose PET scanning became available and showed a focus of emission in the right chest (Figure 1a). MRI disclosed a paraspinal tumour at the level of T9 vertebral body (Figure 1b). Further investigations showed that she had a renal Fanconi syndrome, comprising low molecular weight proteinuria, (preoperative results in Figure 2), glycosuria of 2.6–25 mmol/mmol creatinine (reference range <0.1) and aminoaciduria characterized by hyperglycinuria and borderline hyperlysinuria. Preoperative low molecular weight proteinuria was shown by very elevated urinary excretion of both retinol-binding protein (RBP) (Figure 2) and Beta-2-microglobulin (data not shown) relative to albumin.2 The ratio of the renal Transport Maximum of Phosphate/Glomerular Filtration rate, TmP/GFR, a measure of the threshold for urine phosphate reabsorption, was markedly reduced preoperatively, being 0.230 and 0.255 in January and March 2009, respectively; the age- and sex-adjusted reference range is 1.03 ± 0.06 mmol/l.3
Figure 1.
(a) 18F-DeoxyGlucose PET Scan showing a large focus of increased uptake in the lower right chest posteriorly close to the rib and lateral to the thoracic vertebra. (b) MRI scan of the thoracic spine showing a mass lesion in the right paraspinal region oppositeT9, corresponding to the increased uptake in (a). The mass measures approximately 5.5 × 5.5 cm. and has a heterogeneous signal intensity showing septation and cyst-like formation.
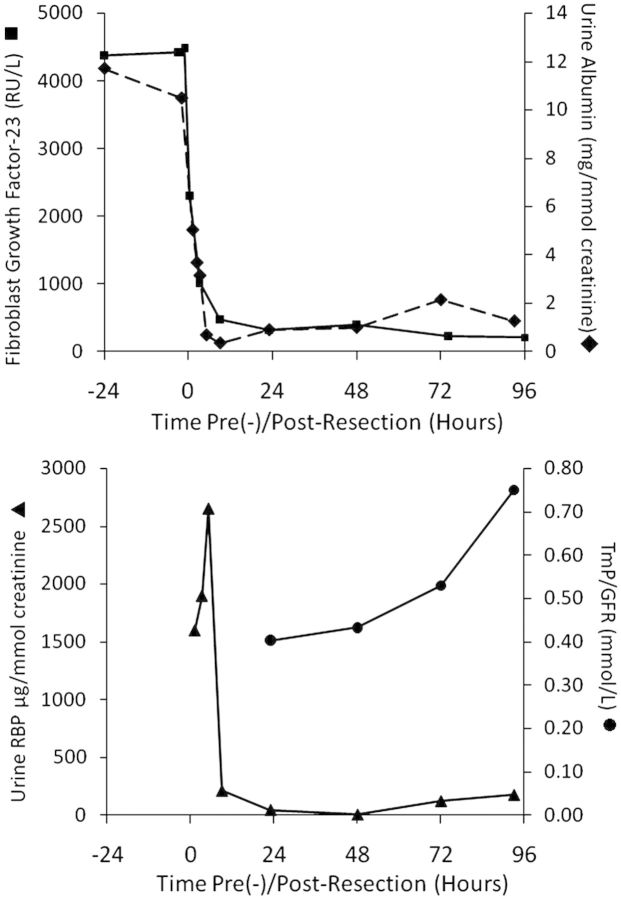
Figure 2.
Measurements of plasma FGF-23 (<100 RU/l), urine albumin (<3.5 mg/mmol creatinine), urine retinol-binding protein (<3 µg/mmol creatinine) and TmP/GFR (1.03 ± 0.06 mmol/l), an estimate of the renal phosphate threshold, from 24 h before mesenchymal tumour resection, peri-operatively and up to 96 h after operation. (Reference ranges are in parentheses).
The patient underwent complete macroscopic resection of the tumour in March 2009. We measured plasma FGF-23, albuminuria and urine RBP to measure low molecular weight proteinuria,2 TmP/GFR, glycosuria and aminoaciduria. All five measurements except aminoaciduria, were made on some 15 occasions, pre-, peri- and post-operatively and, except for urine glucose and aminoacids are shown in Figure 2.
The resected tumour comprised primitive mesenchymal cells without evidence of malignancy. Plasma FGF-23 measured by a C-terminal assay (Immutopics Inc., San Clemente, CA, USA) fell from a preoperative level of 4500 to 314 RU/l within 24 h of tumour resection (Figure 2a) and was undetectable (<100) by 22 days. These results were confirmed by assay for the intact peptide (Kainos Laboratories Inc., Tokyo, Japan) (data not shown). The estimated renal threshold for plasma phosphate reabsorption, rose progressively from some 0.24 mmol/l preoperatively to 0.75 mmol/l (96 h after resection, Figure 2b) and 1.44 mmol/l by 22 days; (reference range 1.03 ± 0.06 mmol/l3). Fasting serum phosphate concentrations were 0.63–0.64 mmol/l preoperatively during treatment with oral phosphate and 1,25-D3 (reference range 1.09 ± 0.02 mmol/l3) and rose to 0.96 mmol/l by 22 days; phosphate and 1,25D3 treatment had been stopped at the time of operation. Unlike the other features of the Fanconi syndrome, marked glycosuria was still present 96 h after resection of the tumour (12.5 mmol/mmol creatinine) but was absent by 22 days. Aminoaciduria was absent when measured 3 weeks post-operatively.
Expression of mRNA for both FGF-23 and matrix extracellular phosphoglycoprotein (MEPE) by the tumour was very elevated. Tumour cells demonstrated a log 6-fold difference in mRNA expression of FGF-23 and a log 4-fold expression of tumour cell MEPE relative to transferrin mRNA in Chinese hamster ovary cells (Pfaffl method4 and log expression of results4,5). Recently, most tumours from patients with oncogenic osteomalacia have been found to express elevated levels of FGF-23 and MEPE mRNA.5
Tumour resection in this patient with oncogenic osteomalacia due to a paraspinal tumour of mesenchymal origin, normalized plasma levels of FGF-23, the reduced threshold for phosphate reabsorption and all features of the renal Fanconi syndrome. These results are consistent with previous reports of three patients with oncogenic osteomalacia who had less complete forms of the Fanconi syndrome than our patient.6–8 The patient reported by Evans and Azzopadi6 showed hyperglycinuria and normoglycaemic glycosuria; the patient of Leehey et al.7 had a generalized hyperaminoaciduria and normoglycaemic glycosuria. Generalized aminoaciduria was also reported in the patient of Drezner and Feinglos8 and resolved after removal of a giant cell tumour of bone. Thus, as in this patient, the pattern of aminoaciduria associated with oncogenic osteomalacia appears variable and not all patients with oncogenic ostomalacia appear to show these urine abnormalities.9
Decreased expression of the sodium-phosphate Type 2 (NaPi-2) co-transporter in the proximal renal tubule is the putative cause of the reduced renal phosphate threshold in patients with oncogenic osteomalacia.1 Two siblings with loss-of-function mutations of the NaPi-2 cotransporter have been reported to show a renal Fanconi syndrome and this raises the possibility that function of this transporter in oncogenic osteomalacia may be directly linked to the Fanconi syndrome.
In this patient with oncogenic osteomalacia, either plasma FGF-23, or other humoral factors highly correlated with FGF-23, caused renal Fanconi syndrome. Measurement of a urine low molecular weight protein such a retinol-binding protein should be a useful biomarker for Fanconi syndrome in patients with oncogenic osteomalacia.
Acknowledgements
Written informed consent was obtained from the patient to report these findings. Mr Keith Burling of the Core Biochemical Assay Laboratory, NIHR Cambridge Biomedical Research Centre, Addenbrooke's Hospital, Cambridge, England is thanked for measurements of urine RBP.
Conflict of interest: None declared.
References
- 1.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–8. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norden AG, Scheinman SJ, Deschodt-Lanckman MM, et al. Tubular proteinuria defined by a study of Dent's (CLCN5 mutation) and other tubular diseases. Kidney Int. 2000;57:240–9. doi: 10.1046/j.1523-1755.2000.00847.x. [DOI] [PubMed] [Google Scholar]
- 3.Cirillo M, Ciacci C, De Santo NG. Age, renal tubular phosphate reabsorption, and serum phosphate levels in adults. N Engl J Med. 2008;359:864–6. doi: 10.1056/NEJMc0800696. [DOI] [PubMed] [Google Scholar]
- 4.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imanishi Y, Hashimoto J, Ando W, Kobayashi K, Ueda T, Nagata Y, et al. Matrix extracellular phosphoglycoprotein is expressed in causative tumors of oncogenic osteomalacia. J Bone Miner Metab. 2011 doi: 10.1007/s00774-011-0290-8. [Epub ahead of print 8 July 2011] [DOI] [PubMed] [Google Scholar]
- 6.Evans DJ, Azzopardi JG. Distinctive tumours of bone and soft tissue causing acquired vitamin-D-resistant osteomalacia. Lancet. 1972;1:353–4. doi: 10.1016/s0140-6736(72)92844-9. [DOI] [PubMed] [Google Scholar]
- 7.Leehey DJ, Ing TS, Daugirdas JT. Fanconi syndrome associated with a non-ossifying fibroma of bone. Am J Med. 1985;78:708–10. doi: 10.1016/0002-9343(85)90419-x. [DOI] [PubMed] [Google Scholar]
- 8.Drezner MK, Feinglos MN. Osteomalacia due to 1alpha,25-dihydroxycholecalciferol deficiency. Association with a giant cell tumor of bone. J Clin Invest. 1977;60:1046–53. doi: 10.1172/JCI108855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clunie GP, Fox PE, Stamp TC. Four cases of acquired hypophosphataemic (oncogenic) osteomalacia. Problems of diagnosis, treatment and long-term management. Rheumatology. 2000;39:1415–21. doi: 10.1093/rheumatology/39.12.1415. [DOI] [PubMed] [Google Scholar]
- 10.Magen D, Berger L, Coady MJ, Ilivitzki A, Militianu D, Tieder M, et al. A loss-of-function mutation in NaPi-IIa and renal Fanconi's syndrome. N Engl J Med. 2010;362:1102–9. doi: 10.1056/NEJMoa0905647. [DOI] [PubMed] [Google Scholar]