Abstract
Background
Patients with pancreatic adenocarcinoma (PDAC) have limited therapeutic options and poor response to the standard gemcitabine (GCB)-based chemotherapy. We investigated the feasibility of non-invasive short-wave RF electric fields to improve cytotoxic effect of GCB on PDAC cells and determined its mechanism of action.
Methods
Cytotoxicity of RF alone and in combination with GCB was studied in vitro on normal pancreatic HPDE cells and different PDAC cell lines by flow cytometry, and in vivo on ectopic and orthotopic human PDAC xenograft models in mice. Mechanism of RF activity was studied by western blot and immunohistochemistry analysis. Toxicity was determined by histopathology.
Results
Exposure of different PDAC cells to 13.56 MHz radiowaves resulted in substantial cytotoxic effect, which was accompanied by induction of autophagy, but not apoptosis. These effects of RF were absent in normal cells. Excessive numbers of autophagosomes in cancer cells persisted 24-48 h after RF exposure and then declined. Addition of a subtoxic dose of GCB to RF treatment inhibited the recovery of cancer cells from the RF-induced autophagy and enhanced cytotoxic effect of the latter on cancer cells. Treatment of PDAC cancer in situ in mice with combination of non-invasive RF and GCB had superior antitumor effect than RF or GCB alone, yet had no evidence of systemic toxicity.
Conclusions
Non-invasive RF treatment induced autophagy, not apoptosis in cancer cells and showed a potential as an enhancer of chemotherapy for treating pancreatic cancer without toxicity to normal cells.
Introduction
In addition to ionizing radiation, physicians have used other physical methods for cancer treatment, such as hyperthermia, cryotherapy, and radiofrequency ablation (RFA). However, their application is limited due to the invasive character of procedures and side effects. RFA is used, though not commonly, for treatment of unresectable liver tumors1 and pancreatic cancer.2 This procedure requires image-guided surgery to insert the electrode probe directly into the tumor, which limits its application for tumors that can be approached by sonographic guidance and excludes lesions that are invisible on imaging or are unattainable, such as micrometastases. High frequency alternating electrical currents generated by the RF probe radiate in an area around the electrode and produce hyperthermia leading to tumor necrosis. As the temperature reaches 100°C and boiling occurs, increased impedance limits further deposition of the electrical current into the tissue.3 Excessive hyperthermia causes tumor and surrounding tissue necrosis that can induce inflammation and produce complications. RFA provides the small zone of active heating around the electrode that makes it unreliable for use in tumors greater than 4-5 cm in diameter due to the enhanced possibility of leaving viable cancer cells.4
We have developed a novel non-invasive RF-based method of cancer. The parameters of the RF field used in our studies is 13.56 MHz frequency and generates power ranging from 100 to 900 W (~ 1 KeV-20 KeV/m2). Electromagnetic energy produced in shortwave frequencies has a low tissue-specific absorption rate and therefore, has excellent whole-body penetration with documented safety in humans.5 However, it remains poorly understood what molecular changes RF treatment can stimulate inside cells and whether they diverge between normal and malignant cells. Few studies indicate on the ability of low intensity electromagnetic fields to cause structural changes in tubulin molecules6-8 or alter the function of ion channels.9 However, mechanisms of RF-induced cell death remain unknown. We focused our study on pancreatic ductal adenocarcinoma (PDAC) due to limited therapeutic options for its treatment and the lowest survival rates for patients. The mainstay drug for PDAC is gemcitabine (GCB). Clinical trials have combined GCB with radiation and other therapeutic modalities but have failed to substantially improve the response rate or overall survival rate of patients treated with GCB alone.10, 11 In this study, we evaluated the feasibility of combining our non-invasive RF treatment with GCB to treat PDAC malignancy in an attempt to determine the molecular changes induced by the RF field inside normal and malignant pancreatic cells.
Materials and Methods
Reagents and Cell Culture
Human cancer cells were acquired from the American Type Culture Collection. Normal human pancreatic ductal epithelial (HPDE) cells were obtained from Dr. Craig Logsdon (M.D. Anderson Cancer Center) and maintained as described elsewhere.12 GCB was from Eli Lilly (Indianapolis, IN).
RF Treatment
For in vitro studies cells were seeded at 0.1 × 106 cells/well in 2 ml of media into 12-well plates. GCB treatment lasted for 24 h and then cells were exposed to the RF field at 600-900W at a frequency of 13.56 MHz (Therm Med LLC, Erie, PA).
In animal experiments, mice were sedated and grounded to receiving plate with conducting copper tape to prevent thermal injury as described previously.13 The RF exposure at 600 W with 13.56 MHz frequency lasted 10 min per mouse.
Apoptosis Measurement by Flow Cytometry
Cells were seeded and received the combination treatment with GCB and RF as described above. After 24 h cells were analyzed using the Annexin V/propidium iodine (PI) apoptosis kit (Invitrogen, Carlsbad, CA) on an FACS LSRII flow cytometer (BD Biosciences, San Jose, CA).
Fluorescent Immunocytochemistry Analysis of Autophagy and Apoptosis
Cells were seeded and treated with GCB and RF as described above. After treatment, cells were returned to the incubator for different times as specified in the Results section. At designated time points, cells were fixed and stained with primary antibodies against LC3B or cleaved caspase 3 proteins (Cell Signaling Technology, Danvers, MA) followed by Alexa 647-conjugated secondary antibody. FITC-labeled phalloidin (Sigma-Aldrich, St. Louis, MO) was used as a marker for the actin cytoskeleton and nuclei were stained with DAPI. Images of cells were taken using an Olympus IX81 fluorescent microscope.
Western Blot
Protein lysates from cells were subjected to 15% SDS-PAGE gel, blotted onto PVDF membrane and probed with primary anti-human LC3B antibody (Cell Signaling Technology, Danvers, MA) followed by Alexa Fluor 680-conjugated secondary antibody (Invitrogen, Carlsbad, CA). Detection was performed with Odyssey Infrared Imaging System (Li-COR Biosciences, Lincoln, NE).
Lentiviral Transduction
Transduction of PDAC cells was performed with LentiBright GFP-LC3 Lentiviral Biosensor from Millipore (Billerica, MA) encoding GFP-tagged LC3 protein, according to the manufacturer's instruction. Briefly, lentiviral stock was added to cells at a ratio of viral particles per cancer cell equal to 40. Infected cells were incubated overnight, washed with fresh medium, exposed for 4 min to the RF field and examined under fluorescent microscope after 24 h.
Animal Studies
SCID mice were purchased from the NCI Mouse Repository. Animals were maintained under pathogen-free conditions and treated in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Mice (10-13 per group) were injected with 2×106 Panc-1 cells suspended in 40 μl of Matrigel directly into the pancreas or with 1×106 cells subcutaneously for orthotopic and ectopic tumor models, respectively. Growth of orthotopic pancreatic cancer in mice was monitored by MRI, and animals started to receive treatment when the tumor size reached 2-4 mm in diameter. Subcutaneous tumors were measured by width (W), length (L) and height (H) and tumor volume (V) was calculated as V=W × L × H. Animals started to receive treatment when average tumor volume reached 100 mm3. Treatment was given once weekly in both cancer models. A GCB dose of 70 mg/kg was given intraperitoneally. The next day, RF exposure for 10 min was performed as described above. Each experiment was repeated three times.
Measurement of Proliferation, Apoptosis, Angiogenesis, and Autophagy in Orthotopic Tumors
Sections of orthotopic Panc-1 tumors were stained with the following primary antibodies: mouse monoclonal anti-human Ki-67 antibody (Dako, Carpinteria, CA), polyclonal rabbit antibody against human cleaved caspase 3 protein (Cell Signaling Technology, Danvers, MA), rabbit polyclonal anti-CD31 (Abcam, Cambridge, MA) and anti-VEGF (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies, rabbit polyclonal anti-LC3B antibody (Abcam, Cambridge, MA). Secondary HRP-labeled IgG antibodies were used for diaminobenzidine (DAB) color staining. Stained tissues were examined under a Leica DMLA F1 microscope. Quantitation of DAB staining was performed on five random areas using Image-Pro software program (Media Cybernetics, Rockville, MA).
Statistical Methods
Results from experiments are presented as means with standard deviations from the mean. The two-tailed Student's t-test was used to measure differences between groups. For analysis of tumor sizes, the second analysis of variance statistical method was applied. P<0.05 was considered statistically significant.
Results
Cytotoxic Effect of RF Treatment on Pancreatic Cancer Cells but not Normal Cells
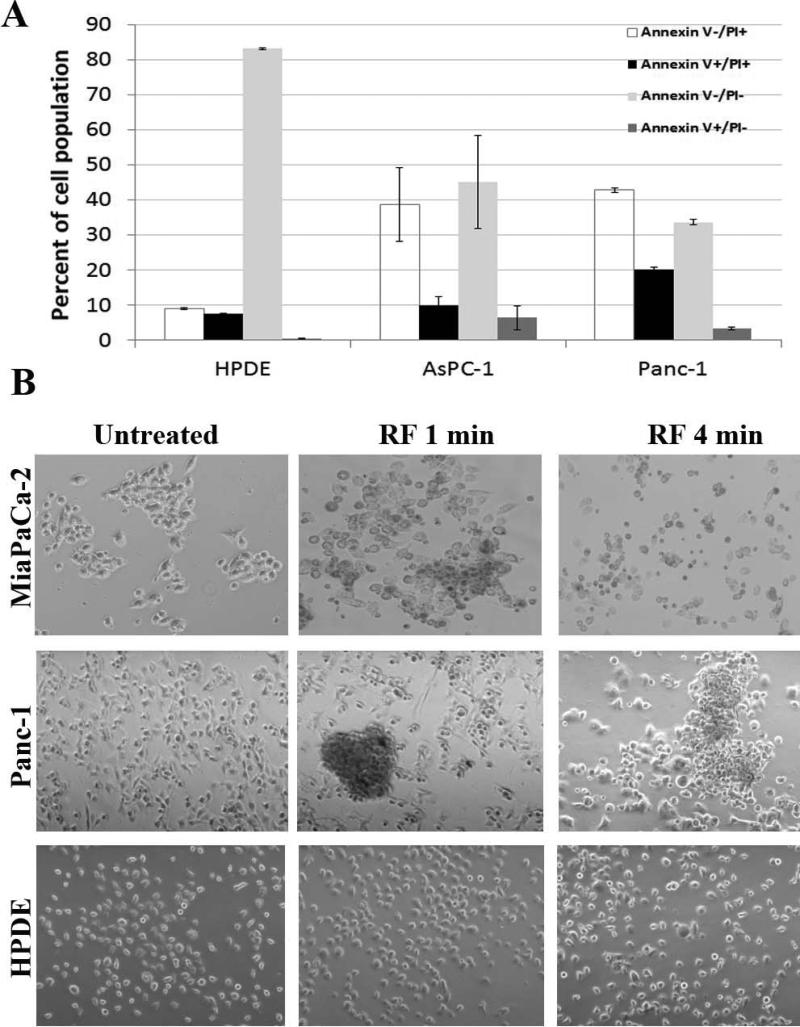
Initially, we verified that exposure of PDAC cells to the RF field causes cell death. As shown in Fig. 1A, FACS analysis with an Annexin V/PI apoptosis kit revealed that exposure of Panc-1 and AsPC-1 PDAC cells for 8 min to the RF field at 600 W was toxic. The number of viable (Annexin V−/PI−) cells declined from 90-95% in untreated control cells to 40% after RF treatment. Fifty percent of cells after RF treatment were necrotic (Annexin V−/PI+) or dead (Annexin V+/PI+) and 10% were apoptotic (Annexin V+/PI−). In contrast to cancer cells, >80% of normal HPDE cells remained viable after RF exposure.
Fig. 1. RF treatment was toxic for pancreatic cancer cells but not normal cells in vitro.
(A) Panc-1, AsPC-1 and HPDE cells were exposed to the RF field (600 W, 13.56 MHZ) for 8 min and analyzed on the following day by FACS with an apoptosis kit. Annexin V−/PI−, viable cells; Annexin V+/PI+, dead cells; Annexin V+/PI−, apoptotic cells; Annexin V−/PI+, necrotic cells. (B) Panc-1, MiaPaCa-2 and HPDE cells were treated for 1-5 min in the RF field, fixed 1 h later and examined under a microscope.
Microscopic analysis showed that RF treatment induced changes in malignant but not normal pancreatic cells (Fig. 1B). The morphology of Panc-1 and MiaPaCa-2 cancer cells began to change 1 h after RF exposure, followed by increased numbers of rounded cells, reduced adherence of cells to the plate surface and formation of cell aggregates, which was most noticeable with Panc-1 cells. Even 1 min of RF exposure was sufficient to induce these changes in cancer cells. In contrast, the morphology of normal HPDE cells remained unchanged.
Induction of Autophagy, but not Apoptosis in Pancreatic Cancer Cells
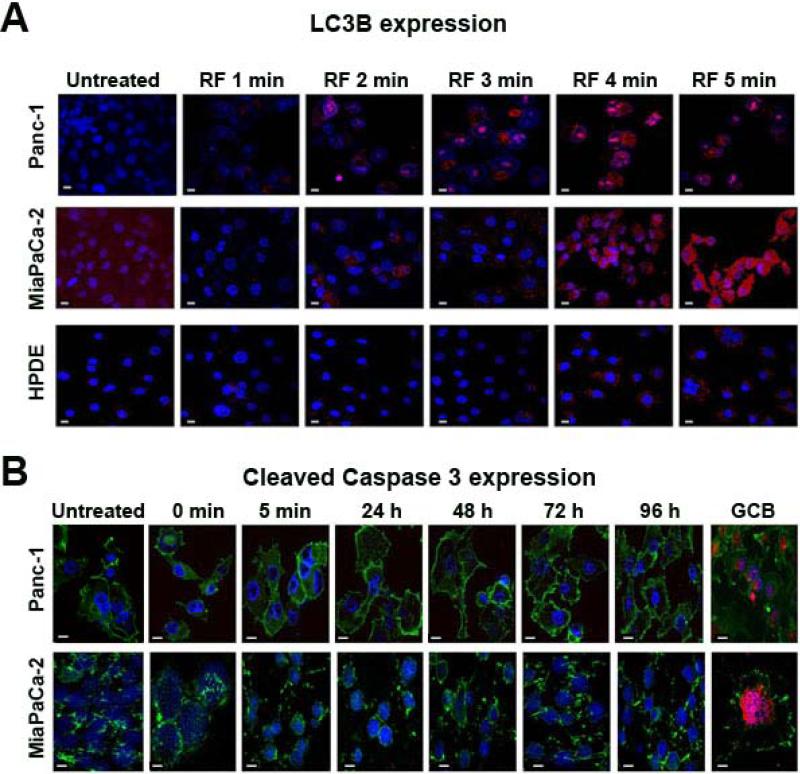
Gradual changes in the morphology and reduced viability of PDAC cells after RF treatment indicates that this treatment can induce programmed cell death. The results shown in Fig. 1A indicate that apoptosis does not seem to be the major mechanism of RF-induced cell death. Therefore, we stained RF-treated cells for LC3B protein, which is used as a hallmark of autophagy, another type of programmed cell death.14 We observed extensive LC3B-positive punctation in PDAC cells exposed to the RF field, indicating the presence of autophagosomes in cells (Fig. 2A). Two minutes of RF treatment was sufficient to detect autophagosomes in cancer cells and the number of these structures increased progressively with increasing time of RF exposure. Normal HPDE cells showed negligible levels of LC3B staining even after 5 min of RF treatment. Staining of cancer cells after RF treatment for cleaved caspase 3 confirmed the lack of apoptosis induction, in contrast to the treatment of these cells with a cytotoxic dose of GCB which induced substantial apoptosis (Fig. 2B).
Fig. 2. RF treatment induced autophagy but not apoptosis in pancreatic cancer cells.
Panc-1, MiaPaCa-2 and HPDE cells were RF treated as described in Fig. 1 for 1-5 min and stained with (A) anti-LC3B antibody (red) to assess autophagy and (B) anti-cleaved caspase 3 antibody (red) to assess apoptosis. GCB treatment at 2 μM was used as a positive control for cleaved caspase 3. Cells were stained with FITC-conjugated phalloidin (green) as a cytoskeleton marker, and with DAPI (blue) as a nuclear marker. Phalloidin staining was not done in Fig. 2B. Magnification 60x. Scale bar is 10 μm.
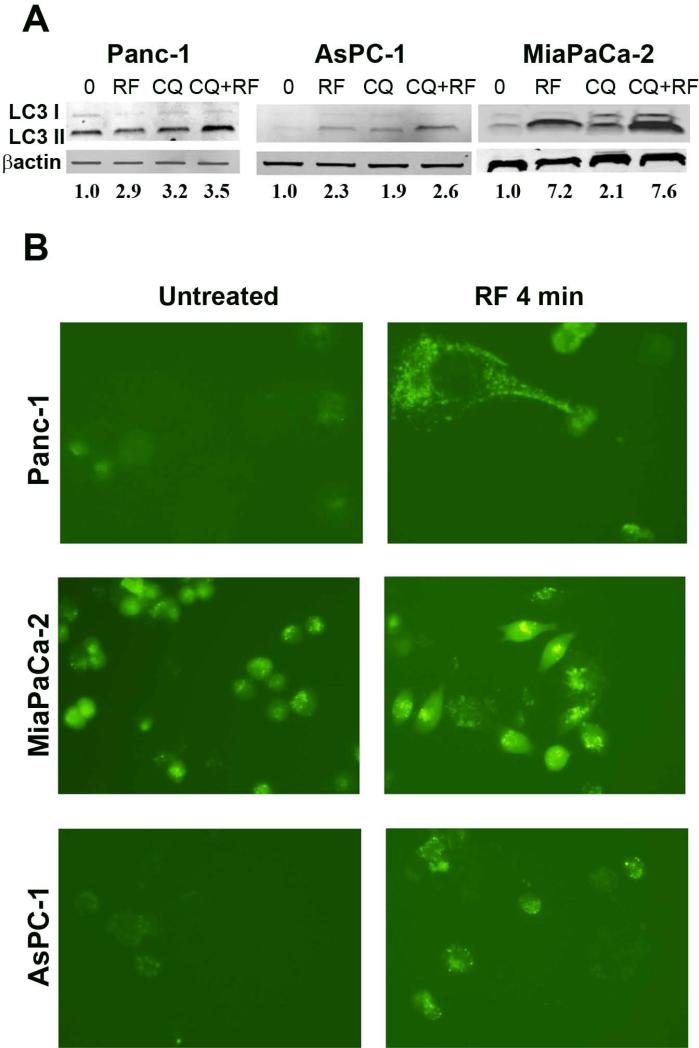
To further verify the ability of RF treatment to trigger de novo formation of autophagic vesicles, we performed analysis of LC3 protein turnover from its cytoplasmic form (LC3-I) to its lipophilic derivative (LC3-II), an essential component of the autophagosome membrane, after RF treatment in the presence of autophagy inhibitor chloroquine (CQ). CQ prevents binding of autophagosomes to lysosomes, thus inhibiting the degradation of autophagosomes in cells. Consequently, this causes the accumulation of autophagosomes inside cells and may be accompanied by elevation of LC3-II expression even after short (2-3 h) CQ exposure, as shown in Fig 3A. In the absence of CQ, RF treatment caused either increase of LC3-II expression as shown in MiaPaCa-2 and AsPC-1 cells, or did not change its expression as shown in Panc-1 cells. The addition of CQ to RF treatment led to superior expression of LC3-II compared to RF or CQ treatment alone in all cell lines. This finding indicates that after RF treatment causes formation of new autophagosomes that accumulated inside cancer cells because CQ prevented their degradation.
Fig. 3. RF treatment increases the LC3-II accumulation and the appearance of punctate GFP-LC3 staining in pancreatic cancer cells after RF field treatment administration.
(A) Due to the variable sensitivity of cells to the duration of RF exposure, Panc-1, AsPC-1 and MiaPaCa-2 cancer cells were exposed for 6, 5 and 4 min, respectively, to the RF field (900W, 13.56 MHz) and incubated for 24 h. Chloroquine (CQ) at 10 μM concentration was added to cells for 3 h prior to lysis. Cells treated with CQ alone were exposed to the drug for 3 h. Cells were then processed for immunoblot analysis using anti-human LC3B antibody to determine the levels of LC3-I and LC3-II expression and antibody against β-actin to verify equal loading of total protein. (B) Cancer cells were transiently transduced with lentiviral GFP-LC3 plasmid and RF treated for 4 min. Images of GFP-LC3 expression in cells were taken 24 h after RF exposure under a fluorescent microscope.
Additionally, PDAC cancer cells transiently transduced with LC3-GFP plasmid showed elevated levels of LC3-GFP protein expression and increased number of fluorescent puncta, typical for autophagosome vesicles, in response to RF treatment (Fig. 3B).
Prevention of Pancreatic Cancer Cell Recovery from RF-Induced Autophagy by Chemotherapy
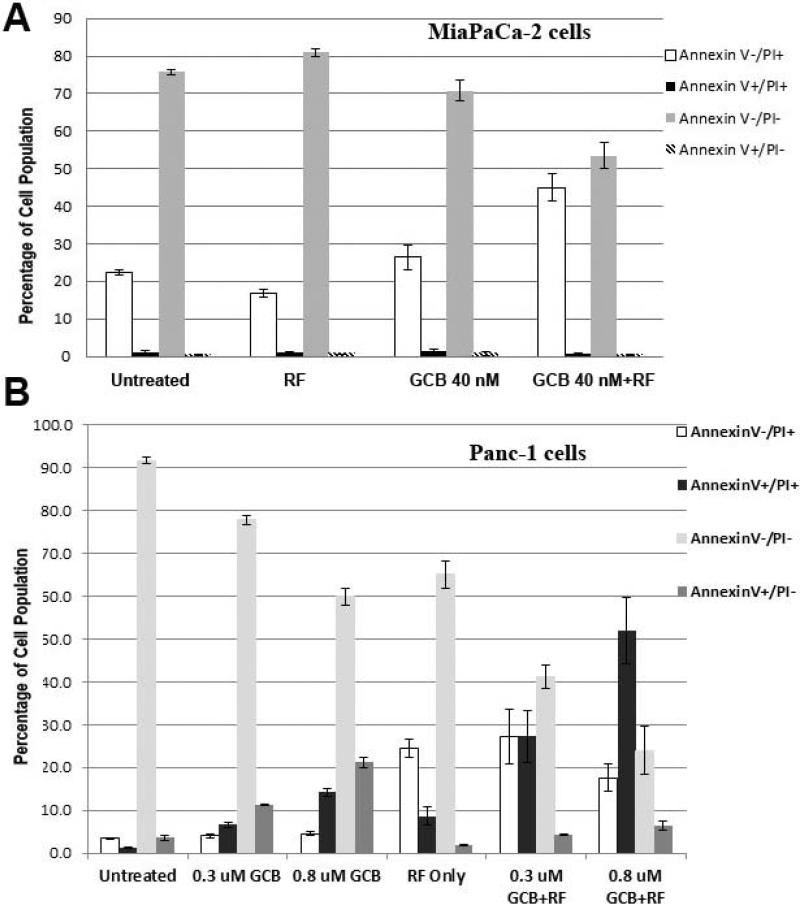
To determine whether RF treatment can enhance anticancer effect of chemotherapy in PDAC cells, we exposed PDAC cells to the RF field with and without GCB treatment followed by FACS apoptosis analysis. MiaPaca-2 cells were used nontoxic doses of RF and GCB that were not able to change viability of cancer cells (Fig. 4A), though combination of these treatments led to significant reduction of viable and elevation of necrotic cells (P<0.005). The number of apoptotic cells did not change in response to any type of treatment.
Fig. 4. Combination treatment with GCB and RF field exposure produced greater pancreatic cancer cell death than does either treatment alone.
(A) MiaPaCa-2 or (B) Panc-1 cells were treated with GCB at the dosages noted for 24 h prior to RF exposure, which lasted for 4 min, and with the same dose of GCB alone or RF alone. Untreated cells were used as controls. Cells were fixed and analyzed 24 h after treatment by FACS using an Annexin V/PI kit as described in Fig.1
Panc-1 cells treated with subtoxic doses of GCB at 0.3 and 0.8 μM showed elevation of apoptotic cells from 10 to 20%, respectively (Fig. 4B). Exposure of Panc-1 cells to the RF field alone was followed by an elevation of necrotic, not apoptotic cells. Addition of GCB treatment to RF caused significant decline of viable cells followed by accumulation of PI+ populations of dead and necrotic cell populations when compared with untreated cells or cells treated with either treatment alone (P<0.001). The amount of apoptotic cells in the RF plus GCB combination group decreased when compared with GCB treatment alone.
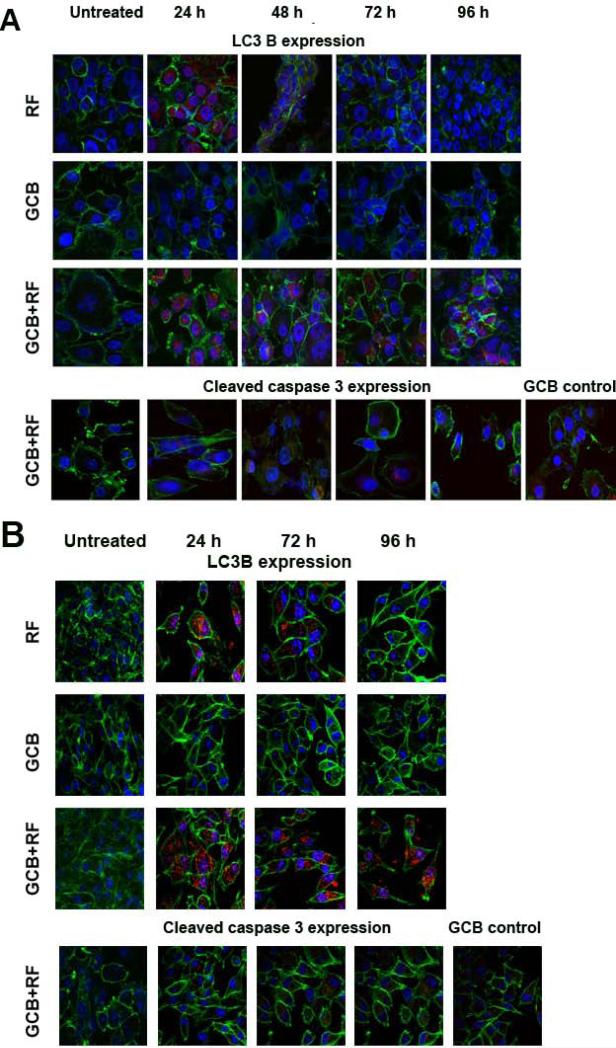
Staining of Panc-1 or MiaPaCa-2 cells with antibody for cleaved caspase 3 showed that low doses of GCB did not induce apoptosis in cells, as they remained negative for activated caspase 3 expression (Fig. 5). These doses of GCB were not able to stimulate LC3B expression over the whole course of the experiment either. In contrast, RF treatment alone increased the number of LC3B-positive puncta in cancer cells, which declined after 24 h and 48 h for Panc-1 and MiaPaCa-2 cells, respectively. The combination of GCB with RF treatment produced a pattern of autophagy levels in cancer cells similar to that produced by RF alone, but addition of GCB extended the duration of RF-induced autophagy in cancer cells and they remained positive for LC3B for 96h after treatment. Expression of cleaved caspase 3 in the combination treatment group of both cell lines was negligible during the entire course of the experiment.
Fig. 5. Autophagy induction in pancreatic cancer cells after RF treatment was extended when combined with GCB.
(A) Panc-1 or (B) MiaPaCa-2 cells were treated with 0.2 or 0.005 μM of GCB, respectively, for 24 h and then exposed to the RF field (900W, 13.56 MHz) for 4 min. Cells were fixed 24, 46, 72, or 96 h after RF treatment and stained with antibodies against LC3B or cleaved caspase 3 (both red), phalloidin (green), and DAPI (blue). Untreated cells and cells treated with RF or GCB alone were used as controls.
Inhibition of Panc-1 Tumor Progression by Combined RF and GCB-based Treatment in Ectopic and Orthotopic Cancer Models
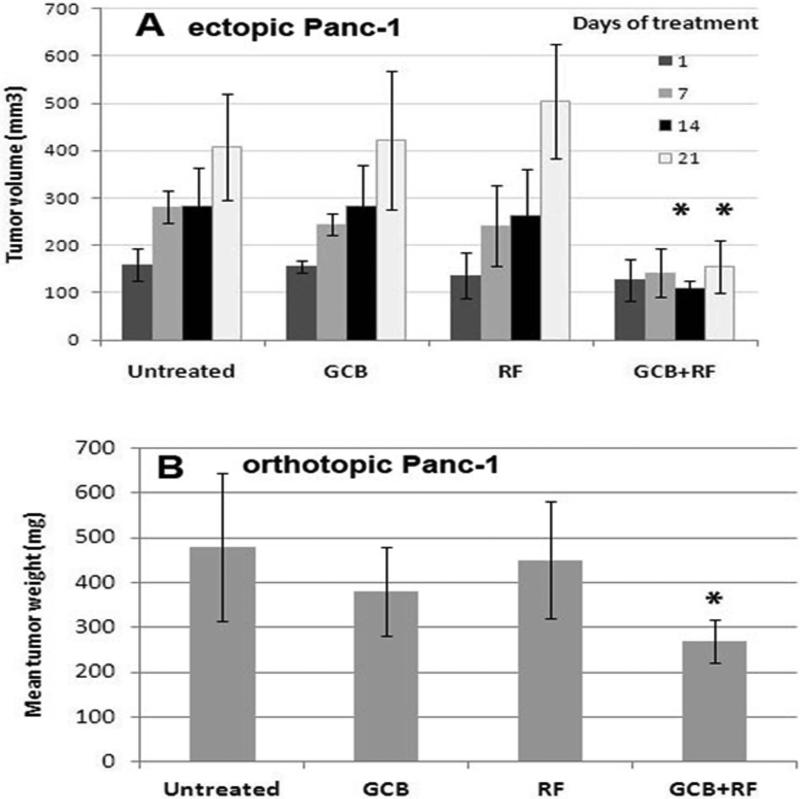
To validate the feasibility of combining GCB chemotherapy with RF treatment in vivo, we used two types of Panc-1 xenograft animal models. We chose to use Panc-1 model because it was reported to be resistant to GCB treatment.15, 16 The use of an ectopic cancer model allowed us to adjust the schedule of treatment during tumor progression. Mice with ectopic Panc-1 tumors that received 70 mg/kg GCB followed by RF exposure for 10 min once weekly, produced complete tumor growth arrest after 2 weeks of treatment (P<0.001) when compared with groups receiving either treatment alone (Fig. 6A).
Fig. 6. Addition of GCB-based treatment to the RF field exposure stimulated anticancer responses in mice with ectopic and orthotopic pancreatic tumors.
Panc-1 cells were injected (A) subcutaneously or (B) orthotopically into mice. When tumors were established, mice started to receive 70 mg/kg of GCB via intraperitoneal injection and 10-min RF treatment the following day once a week. *P < 0.001 (A) and P<0.01 (B) for GCB plus RF group compared with any other group.
This treatment schedule was then studied in mice with established orthotopic Panc-1 tumors. Tumor progression was inhibited only in mice receiving a combination of GCB plus RF treatment (P<0.01) compared with the other groups (Fig. 6B).
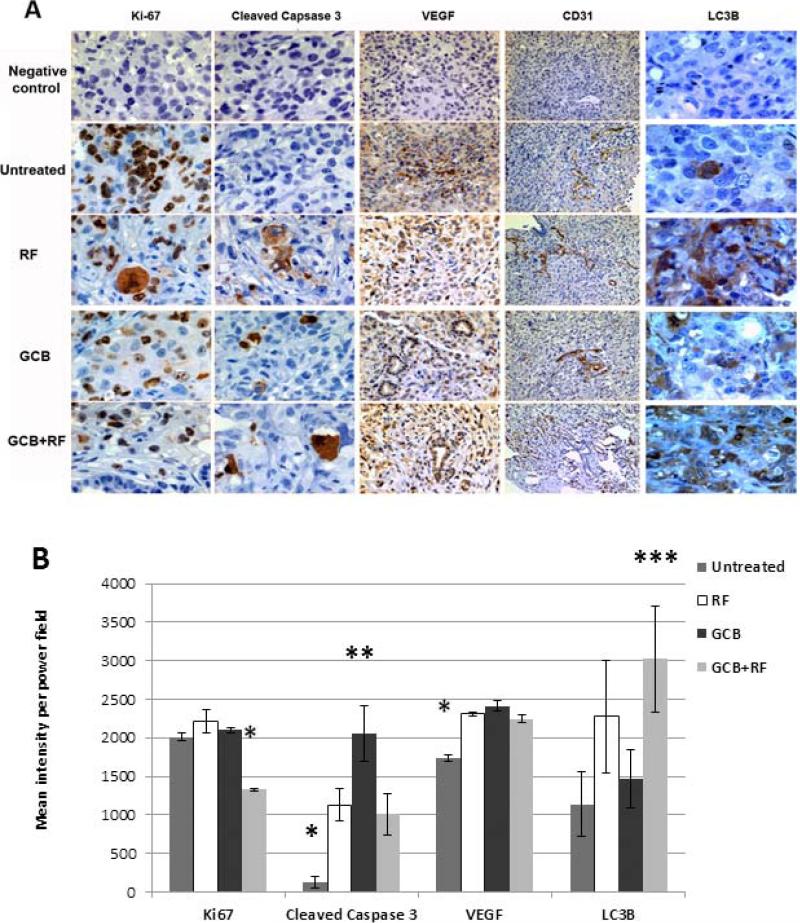
Histological analysis of orthotopic tumor tissues revealed a significant reduction of proliferating Ki-67+ tumor cells only in the group of animals with orthotopic tumors that received combination GCB plus RF treatment (P<0.05), whereas the number of Ki-67+ cells in tumors treated with either treatment alone did not differ from that in the untreated control tumors (Fig. 7A and 7B).
Fig. 7. Immunohistochemical analysis of orthotopic Panc-1 tumors for proliferation, apoptosis, angiogenesis and autophagy after GCB and RF treatments alone and in combination.
Tumor sections were stained with anti-Ki-67, anti-cleaved caspase 3, anti-VEGF, anti-CD31, or anti-LC3B antibodies. Brown staining indicates positivity for the appropriate antigen. Representative areas of tumors were (A) photographed under the microscope or (B) quantified using computer software. Magnification: Ki-67, cleaved caspase 3 and LC3B, 40x; VEGF, 20x; CD31, 10x. *P<0.05 for the treated groups compared with the untreated group; **P<0.02 for the GCB-only group compared with the other treatment groups and untreated group; ***P<0.008 for the GCB plus RF group compared with the other treatment groups and the untreated group.
Pathology analysis of vital organs (liver, kidneys, lungs, heart, normal pancreas, and spleen) obtained from mice 1, 24, and 48 h after RF treatment with and without GCB administration was negative. Mild infrequent (10-20%) multifocal lymphoid aggregates were detected in the pancreas from mice in all treated groups after 1 h of treatment, although this percentage declined with time. Animals with tumors undergoing the 40-day treatment course had good posture and normal behavior during treatment.
Pancreatic Tumor Cell Death Followed by Upregulation of LC3B Expression In Vivo After Treatment with a Combination of RF and GCB
To determine the mechanism of tumor cell death caused by treatment with GCB plus RF exposure in vivo, we stained the orthotopic tumor samples from mice for apoptosis, angiogenesis, and autophagy markers. Staining for apoptosis using antibody for cleaved caspase 3 demonstrated its induction in all treated groups but not in untreated control group (P<0.05, Fig. 7A and 7B). The highest level of cleaved caspase 3 expression was recorded in tumors of mice receiving GCB alone (P<0.02) when compared with all other groups (Fig. 7B). Similar levels of cleaved caspase 3 expression were determined in tumors from mice that received RF alone and GCB plus RF groups.
The structure and density of blood vessels in tumors were similar in treated and untreated groups as demonstrated by CD31 staining (Fig. 7A). Quantitation of VEGF expression showed slight, though statistically significant elevation of its expression in all three treated groups when compared with the untreated group (P<0.05, Fig. 7B), but there was no difference in VEGF expression between treated groups.
Staining of tumor samples for autophagy revealed higher expression of LC3B in the RF alone and GCB plus RF groups than in the GCB and untreated control groups (P<0.05, Fig. 7A) with the highest LC3B expression in the combination GCB plus RF group (Fig. 7B).
Discussion
The effect of RF energy on physiological functions in the human body and its molecular mechanisms of action in cells remains poorly understood. In our present study we were able to show that a few minutes of powerful RF electrical field exposure alone was sufficient to kill cancer cells of pancreatic origin without affecting normal cells in vitro. FACS analysis with apoptosis kit and immunostaining of pancreatic cancer cells with anti-cleaved caspase 3 antibody revealed that the cytotoxic effect of RF field on malignant cells was not mediated by apoptosis, but rather by another type of cell death mechanism. The use of apoptosis inhibitor z-VAD-FMK was not able to rescue cancer cells from the RF-induced cell death. All this demonstrates that apoptosis is not the major cell-death inducing mechanism mediated by RF treatment in cancer cells. Staining of cells with the autophagy marker LC3B indicated that RF treatment resulted in excessive number of LC3B-positive autophagosomes in cancer, but not normal pancreatic cells. Elevation of intracellular expression of LC3 protein is not sufficient to verify the induction of autophagy because treatment of cells with autophagy inhibitors, such as CQ, may be also followed by overexpression of LC3B due to intracellular accumulation of nondegraded autophagosomes.17, 18 Analysis of LC3 protein turnover showed that RF and CQ treatments alone increase expression of the lipidated form of LC3 protein, known as LC3-II, an essential component of the autophagosome membrane. Measurement of LC3-II levels in all tested cells after RF treatment with CQ demonstrated superior expression of LC3-II protein compared to cells receiving any single type of treatment, indicating the ability of RF energy to stimulate new autophagosomes formation in PDAC cells, and subsequent CQ-induced arrest of their binding with lysosomes was followed by excessive autophagosome accumulation. Moreover, studies of GFP-LC3 transduced pancreatic cancer cells showed that RF treatment stimulated translation of the LC3 (atg8) gene that caused elevation of GFP-positive LC3 puncta, demonstraiting the formation of new autophagosomes. The RF-induced autophagy in cancer cells was reversible since the number of LC3B-positive autophagosomes started to decline 24-48 h after RF exposure. The use of subtoxic doses of GCB inhibited cancer cell recovery from RF-induced autophagy and cells remained positive for LC3B even 4 days after RF treatment. This indicates on the ability of chemotherapy to extend the duration of RF-induced autophagy in cancer cells and correlates with the enhanced cytotoxic effect of RF plus GCB combination treatment in vitro. Recent reports indicate the important role of autophagy in the biology of PDAC cells.19 Therefore, disruption of the autophagy mechanism may affect pancreatic cancer cells and lead to their death.
Human Panc-1 PDAC xenografts in mice were used for studying the anticancer effect of RF alone and in combination with low dose chemotherapy in vivo. Of note, we administered 70 mg/kg of GCB once weekly, a dose that was not able to impair the growth of Panc-1 tumors in mice. This correlates with previous reports indicating resistance of Panc-1 cells to GCB.15, 20 Combination of RF treatment with GCB led to significant inhibition of tumor progression not only in ectopic, but also orthotopic cancer models, whereas RF or GCB treatments alone were ineffective. These results confirm the ability of RF energy to reach the tumor in situ and enhance the antitumor effect of chemotherapy. Histopathological analysis did not reveal any significant toxic effects or pathological changes induced by RF treatment alone or in combination with chemotherapy in vital organs of mice. The treatment was well tolerated during the entire course of treatment, which correlates with our in vitro finding concerning the safety of RF treatment to normal cells.
Analysis of orthotopic Panc-1 tumor tissues after staining with Ki-67 antibody for proliferation further confirmed that the number of dividing cells was significantly reduced only in mice receiving the combination of RF plus GCB treatment. Staining of these tumors to assess autophagy revealed higher numbers of LC3B-positive cells in both RF-treated groups compared to the GCB alone and untreated groups, with the highest number occurring in the combined GCB plus RF group. Examination of anti-cleaved caspase 3 expression in tumor samples showed the highest level of apoptosis in tumor samples from the group of mice receiving only GCB treatment, although it was not sufficient to cause tumor regression in vivo. Of note, in orthotopic Panc-1 cancers treated with RF and GCB there was evidence of autophagy and apoptosis, indicating that RF-induced autophagy potentially can progress to apoptotic cell death pathways after multiple treatments. Analysis of blood vessels structure and VEGF levels of expression did not reveal any substantial changes in tumors after treatment.
The role of autophagy in tumors is known to be controversial. Some studies report that cancer cells can respond to drugs by induction of autophagy, resulting in cell death, particularly when the apoptotic pathway is nonfunctional.21, 22 Others indicate that autophagy helps tumor cells to circumvent stress induced by chemotherapy or environmental factors (hypoxia or nutrient deficiency), and thus avoid apoptosis.23, 24 We believe that the fate of cancer cells in response to autophagy depends on the intensity and duration of the autophagy. Our present data indicates that RF treatment induced formation of excessive numbers of autophagosomes in PDAC cells. A significant extension of the presence of autophagic vesicles in these cells became possible with the addition of GCB to the RF treatment. The inability of cancer cells to recover from the RF-induced autophagy due to the presence of GCB correlated with higher levels of cancer cell death and indicated the important role of autophagy in this treatment. We noticed that the levels of LC3-II do not always correlate with the levels of toxicity in cells after RF treatment. Perhaps, different cell lines have different tolerance for autophagy - in some cells addition of few autophagosomes can be sufficient to induce cell death mechanism; for others, even significant elevation of autophagosomes in cytoplasm can be accepted and do not lead to death. Evaluation of details of the contributory role of autophagy in RF treatment requires further investigation and will be performed in our future studies.
In conclusion, we demonstrated the ability of a non-invasive RF field treatment to kill malignant, but not normal, pancreatic cells and to enhance the effect of GCB-based chemotherapy for malignant pancreatic tumors in situ. We observed that exposure of cancer cells to the RF field activated autophagy in pancreatic adenocarcinoma cells. This autophagy became irreversible when the RF treatment was combined with GCB administration, suggesting that autophagy processes drives the anticancer effect of this combined treatment. With further investigation, RF treatment may become a new non-invasive therapeutic approach in combination with standard chemotherapy or with other agents for treating solid tumors at numerous locations in the human body.
Acknowledgements
We thank Warna Kaularachchi for flow cytometry analysis training, Kristine Ash and Rosalind Ramos for administrative assistance. We also thank the staff of institutional Veterinary Medicine and Small Animal Imaging Core Facility; Dr. Jared K. Burks for assistance with fluorescent microscopy and Kenneth Dunner, Jr. for assistance with electron microscopy.
Funding: This work was funded from the NIH (U54CA143837), NIH M. D. Anderson Cancer Center Support grant CA016672, and research grant from the Kanzius Research Foundation (SAC, Erie, PA).
References
- 1.McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990;25(3):267–70. doi: 10.1097/00004424-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Matsui Y, Nakagawa A, Kamiyama Y, Yamamoto K, Kubo N, Nakase Y. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas. 2000;20(1):14–20. doi: 10.1097/00006676-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg SN, Gazelle GS, Compton CC, Mueller PR, McLoud TC. Radio-frequency tissue ablation of VX2 tumor nodules in the rabbit lung. Acad Radiol. 1996;3(11):929–35. doi: 10.1016/s1076-6332(96)80303-5. [DOI] [PubMed] [Google Scholar]
- 4.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239(6):818–25. doi: 10.1097/01.sla.0000128305.90650.71. discussion 25-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adair ER, Blick DW, Allen SJ, Mylacraine KS, Ziriax JM, Scholl DM. Thermophysiological responses of human volunteers to whole body RF exposure at 220 MHz. Bioelectromagnetics. 2005;26(6):448–61. doi: 10.1002/bem.20105. [DOI] [PubMed] [Google Scholar]
- 6.Kirson ED, Dbaly V, Tovarys F, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007;104(24):10152–7. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taghi M, Gholamhosein R, Saeed RZ. Effect of electromagnetic field on the polymerization of microtubules extracted from rat brain. Recent Pat Endocr Metab Immune Drug Discov. 2012;6(3):251–4. doi: 10.2174/187221412802481757. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman JW, Pennison MJ, Brezovich I, et al. Cancer cell proliferation is inhibited by specific modulation frequencies. British Journal of Cancer. 2012;106(2):307–13. doi: 10.1038/bjc.2011.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchionni I, Paffi A, Pellegrino M, et al. Comparison between low-level 50 Hz and 900 MHz electromagnetic stimulation on single channel ionic currents and on firing frequency in dorsal root ganglion isolated neurons. Biochimica et Biophysica Acta. 2006;1758(5):597–605. doi: 10.1016/j.bbamem.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Renouf D, Moore M. Evolution of systemic therapy for advanced pancreatic cancer. Expert Rev Anticancer Ther. 10(4):529–40. doi: 10.1586/era.10.21. [DOI] [PubMed] [Google Scholar]
- 11.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 28(22):3605–10. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Zhai Q, Bharadwaj U, et al. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer. 2006;106(10):2284–94. doi: 10.1002/cncr.21862. [DOI] [PubMed] [Google Scholar]
- 13.Glazer ES, Zhu C, Massey KL, et al. Noninvasive radiofrequency field destruction of pancreatic adenocarcinoma xenografts treated with targeted gold nanoparticles. Clin Cancer Res. 2010;16(23):5712–21. doi: 10.1158/1078-0432.CCR-10-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19(21):5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98(24):1806–18. doi: 10.1093/jnci/djj498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang K, Zhang Z, Bai Z, Ma X, Guo W, Wang Y. Enhancement of gemcitabine sensitivity in pancreatic cancer by co-regulation of dCK and p8 expression. Oncology Reports. 2011;25(4):963–70. doi: 10.3892/or.2011.1139. [DOI] [PubMed] [Google Scholar]
- 17.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3(6):542–5. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 18.Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang S, Wang X, Contino G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25(7):717–29. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang K, Zhang Z, Bai Z, Ma X, Guo W, Wang Y. Enhancement of gemcitabine sensitivity in pancreatic cancer by co-regulation of dCK and p8 expression. Oncol Rep. 25(4):963–70. doi: 10.3892/or.2011.1139. [DOI] [PubMed] [Google Scholar]
- 21.Yu L, Lenardo MJ, Baehrecke EH. Autophagy and caspases: a new cell death program. Cell Cycle. 2004;3(9):1124–6. [PubMed] [Google Scholar]
- 22.Laane E, Tamm KP, Buentke E, et al. Cell death induced by dexamethasone in lymphoid leukemia is mediated through initiation of autophagy. Cell Death Differ. 2009;16(7):1018–29. doi: 10.1038/cdd.2009.46. [DOI] [PubMed] [Google Scholar]
- 23.Abedin MJ, Wang D, McDonnell MA, Lehmann U, Kelekar A. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. 2007;14(3):500–10. doi: 10.1038/sj.cdd.4402039. [DOI] [PubMed] [Google Scholar]
- 24.Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14(2):70–7. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]