Abstract
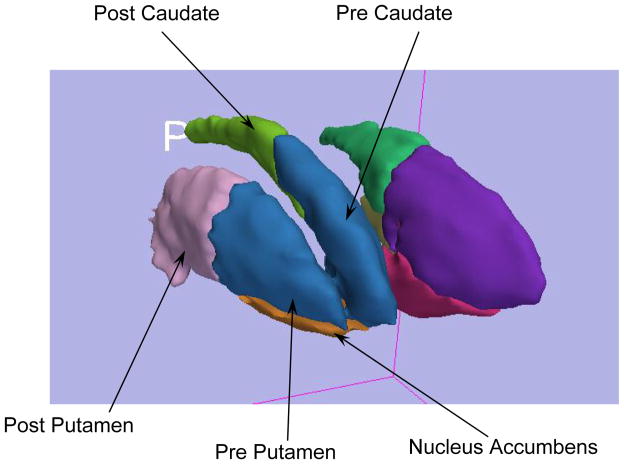
The striatum is the input component of the basal ganglia from the cerebral cortex. It includes the caudate, putamen, and nucleus accumbens. Thus, the striatum is an important component in limbic frontal-subcortical circuitry and is believed to be relevant both for reward-guided behaviors and for the expression of psychosis. The dorsal striatum is composed of the caudate and putamen, both of which are further subdivided into pre- and post-commissural components. The ventral striatum (VS) is primarily composed of the nucleus accumbens. The striatum can be functionally divided into three broad regions: 1) a limbic; 2) a cognitive and 3) a sensor-motor region. The approximate corresponding anatomic subregions for these 3 functional regions are: 1) the VS; 2) the pre/post-commissural caudate and the pre-commissural putamen and 3) the post-commissural putamen.
We believe assessing these subregions, separately, in disorders with limbic and cognitive impairment such as schizophrenia may yield more informative group differences in comparison with normal controls than prior parcellation strategies of the striatum such as assessing the caudate and putamen. The manual parcellation of the striatum into these subregions is currently defined using certain landmark points and geometric rules. Since identification of these areas is important to clinical research, a reliable and fast parcellation technique is required.
Currently, only full manual parcellation using editing software is available; however, this technique is extremely time intensive. Previous work has shown successful application of heuristic rules into a semi-automatic platform1. We present here a semi-automatic algorithm which implements the rules currently used for manual parcellation of the striatum, but requires minimal user input and significantly reduces the time required for parcellation.
Keywords: Striatum, basal ganglia, parcellation, delineate, nucleus accumbens, putamen, caudate
1. INTRODUCTION
The striatum is the input component of the basal ganglia from the cerebral cortex. It includes the caudate, putamen, and nucleus accumbens. Thus, the striatum is an important component in limbic frontal-subcortical circuitry and is believed to be relevant both for reward-guided behaviors and for the expression of psychosis. The dorsal striatum is composed of the caudate and putamen, both of which are further subdivided into pre- and post-commissural components. The ventral striatum (VS) is primarily composed of the nucleus accumbens. The striatum can be functionally divided into three broad regions: 1) a limbic; 2) a cognitive and 3) a sensor-motor region. The approximate corresponding anatomic subregions for these 3 functional regions are: 1) the VS; 2) the pre/post-commissural caudate and the pre-commissural putamen and 3) the post-commissural putamen. There is significant interest in delineating the striatum into these five subregions of the striatum for more precise and descriptive analysis in clinical comparative studies. The three main subregions can be seen in Figure 1.
Fig. 1.
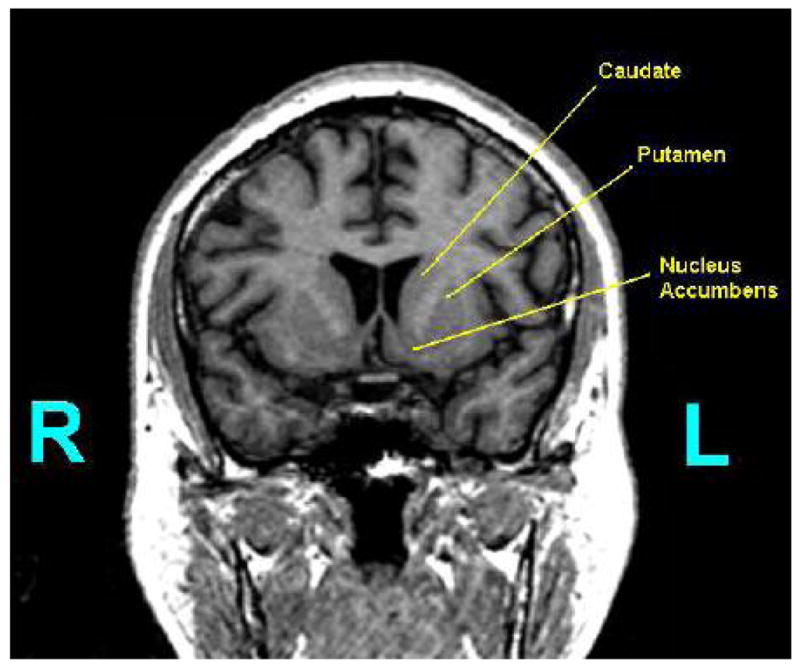
Shown is a coronal slice containing the striatum and the labeling of the following subregions: the caudate, putamen, and nucleus accumbens.
The caudate and putamen shown in Figure 1 can be further parcellated by the coronal position of the Anterior Commissure (AC). The AC is shown in Figure 2 from a coronal perspective and in Figure 3 from an axial perspective. The coronal location of the AC will provide the boundary between the pre- and post-commissural caudate and putamen. The section of the putamen and caudate which is anterior to the coronal location of the AC is considered pre-commissural. The remaining putamen and caudate, that which is at or posterior to the AC, is considered post-commissural. The axial location of the AC also plays an important role. This point will define the location of the Anterior Commissural/Posterior Commissural (AC/PC) plane. Assuming that the data has already been properly aligned to the AC/PC plane, then the AC/PC plane can be completely defined by simply the axial position of the AC.
Fig. 2.
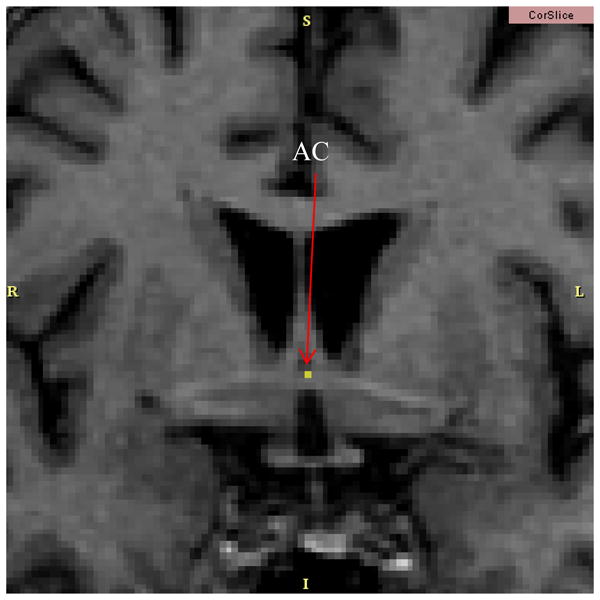
The Anterior Commissure (AC) is shown in a coronal slice. This coronal location of the AC is the point which parcellates the caudate and putamen into pre- and post-commissural sections.
Fig. 3.
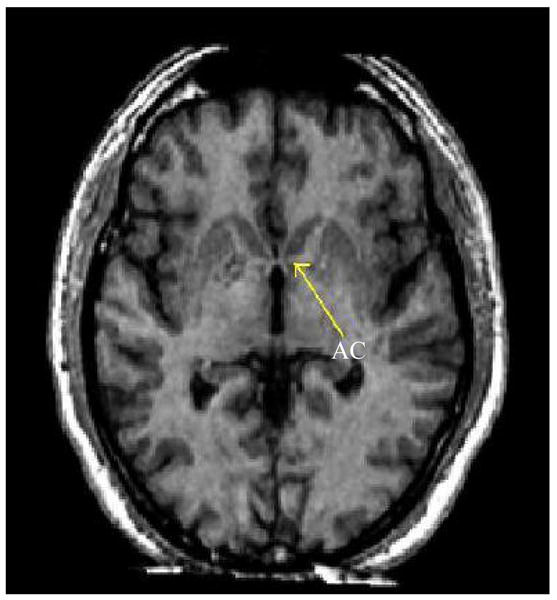
The Anterior Commissure (AC) is shown in an axial slice. This axial location of the AC will define the AC/PC plane, a crucial component in the separation of the caudate and putamen from the nucleus accumbens.
We believe assessing the aforementioned subregions, separately, in disorders with limbic and cognitive impairment such as schizophrenia may yield more informative group differences in comparison with normal controls than prior parcellation strategies of the striatum such as assessing the caudate and putamen. Since identification of these areas is important to clinical research, a reliable and fast parcellation technique is required.
2. METHOD
2.1 The Manual Neuoranatomical Rules
Here we will describe the specific rules that are used to parcellate the striatum into the aforementioned subregions using only manual techniques. The parcellation must be done manually for each coronal slice containing the striatum.
First, the AC/PC plane must be identified and drawn for reference (the pink line in Figure 4).
Next, a line must be drawn which is tangent to, or “skims,” the top of both the caudate and putamen. This can be visualized as placing a flat object against the striatum from the lateral, superior direction.
A line which is perpendicular to the first tangent line and is tangent to the putamen is drawn, forming an “X.” The intersection of this “X” defines the most lateral, superior point of the putamen.
-
A line is dropped directly vertical from this most lateral, superior point to the most inferior point on the putamen. These first four steps are shown in Figure 4.
If this most inferior point is inferior to the AC/PC plane, then continue to step 5.
If this most inferior point is superior to the AC/PC plane, then the AC/PC plane is used as the division between the caudate, putamen, and nucleus accumbens.
Using the AC/PC plane drawn on the figure, the most lateral and medial intersection between the striatum and the AC/PC plane must be determined. The midpoint of these points must then be calculated.
A line defined by the previous midpoint and the inferior point of the striatum calculated earlier is then drawn. This line provides the separation between the caudate, putamen and nucleus accumbens. These previous two steps are shown in Figure 5.
Fig. 4.
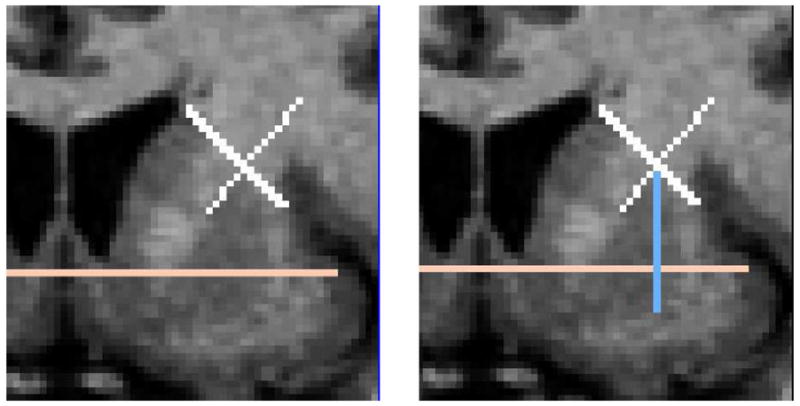
(Left) The AC/PC plane is seen as a pink line in a coronal slice. Also, the tangent line of the putamen and caudate and the corresponding perpendicular line are shown in white. (Right) The line dropped vertically from the intersection of the “X” to the inferior boundary of the striatum is shown in light blue.
Fig. 5.
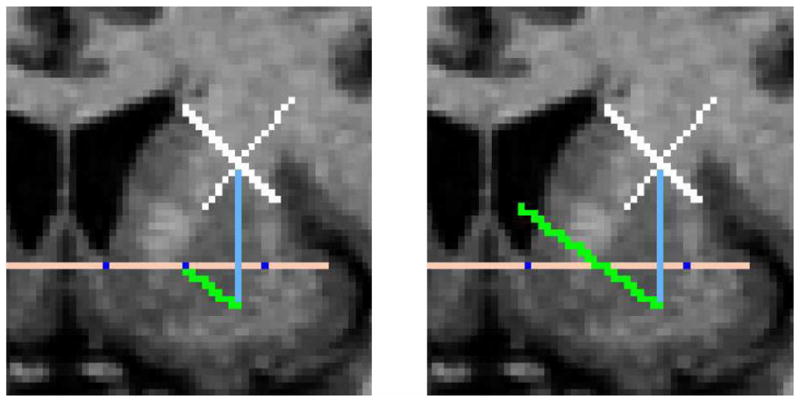
(Left) The most lateral and medial intersections between the striatum and the AC/PC plane are found and the midpoint is calculated (shown in dark blue). This midpoint is then connected to the inferior point of the putamen (shown in green). (Right) This green line is then extended to define the boundary between the desired subregions.
The time required to perform this manual parcellation is approximately 48 minutes. This time is unsatisfactory for processing data for use in clinical comparative studies and thus the need for implementation into a semi-automatic platform. We present in the next section a semi-automatic algorithm which implements the same rules currently used for manual parcellation of the striatum, but requires minimal user input and significantly reduces the time required for parcellation.
2.2 The Semi-Automatic Algorithm
While the above manual rules provide consistent results, the required time is unsatisfactory. Here we will discuss the semi-automatic algorithm developed and the implementation into a user-friendly software.
To begin parcellation of the fully segmented striatum into the five desired subregions using the semi-automatic algorithm, the user must first define the Anterior Commissure (AC) Point. This voxel defines three different boundaries in the semi-automatic algorithm: coronally, the most anterior slice of the post caudate/putamen; axially, the AC/PC plane; and sagittally, the division between the right and left hemisphere (subsequently, the division between the right and left striatum).
-
Next the user must identify the most lateral, superior point of the putamen on each slice with a single voxel. From here, the parcellation into the five desired sub regions is done automatically:
First, the most lateral and medial intersections of the ACPC plane with the striatum are found and the midpoint of these two intersections is calculated.
-
The algorithm drops a line from the most lateral, superior point of the putamen anteriorily until the inferior boundary of the striatum is intersected.
If this inferior point is inferior to the AC/PC plane, then this inferior boundary point of the striatum and the midpoint calculated earlier define the line that will provide the divisions between two sets of the desired subregions on this slice: the nucleus accumbens/putamen, and the nucleus accumbens/caudate.
However, if the inferior point of the striatum is superior to the AC/PC plane, then the AC/PC plane is used as the line that will divide the aforementioned sets of sub regions.
All voxels defined as the putamen that are anterior to the AC/PC point are identified as pre-putamen, and all others are identified as post-commissural putamen. The same general rule follows for identifying the post and pre-commissural caudate.
3. NEW WORK TO BE PRESENTED
In this work, we present the first semi-automatic parcellation of the corpus striatum into the five biologically significant sub regions using expert rules. The boundaries of these sub regions do not appear in MRI scans and thus geometric rules are necessary for its parcellation. By taking the rules that are used to manually parcellate the striatum and applying them into an algorithm with minimal user input, excellent results can be achieved while reducing the time required for parcellation from 48 minutes to 5 minutes.
4. RESULTS
We compare here the parcellation results from full manual parcellation by an expert and semi-automatic parcellation with the semi-automatic algorithm by the same expert. The corpus striatum of five cases was manually segmented and these labelmaps served as the starting point for the comparison since both techniques require this previous work. The time required to segment manually was kept by the expert, and the time required to segment semi-automatically was recorded as the time required supplying the necessary inputs for the algorithm and the algorithm run time. Figure 7 shows the markings produced while the algorithm is running. Note that this marked output is not the output of the module in 3D SLICER. Rather, this is the output from the MATLAB version that was used to visually confirm accurate parcellation. The actual output from the algorithm is shown in Figure 8.
Fig. 7.
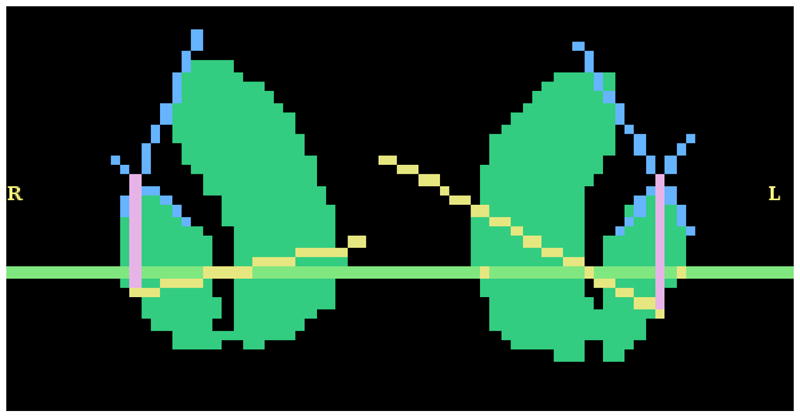
The markings that represent the algorithm’s steps during parcellation of the striatum are shown in this image. The intersection of the blue lines represents the most lateral, superior point of the putamen input by the user.
Fig. 8.
The labeled output of the semi-automatic algorithm is shown. Note that both the left and right striatum are shown in this figure, but only the right side is labeled.
The parcellation time was reduced from ~48 minutes to under 5 minutes (4 minutes input time + < 1 minute algorithm run time) using the semi-automatic algorithm. The expert manual parcellation was compared to the semi-automatic using the DICE coefficient for three separate cases2. The DICE coefficient equation, shown as Equation 1, compares the manual segmentation, or ground truth, (G) to the semi-automatic algorithm output (S). VX is the volume (i.e. the number of voxels) in the segmentation volume X. The nucleus accumbens (Mean DSC = 0.9064, σ2 = 0.0019) and dorsal striatum (Mean DSC = 0.9808, σ2 = 9*10−6) gave excellent parcellation results with low variability when compared to the manual parcellation [DSC values > 0.7 is good with regard to the literature2].
| (1) |
The algorithm above was first implemented and tested in MATLAB 6.5. However, the algorithm was then implemented into 3D SLICER for a more user-friendly environment. A screenshot of the module in 3D SLICER is shown in Figure 6.
Fig. 6.
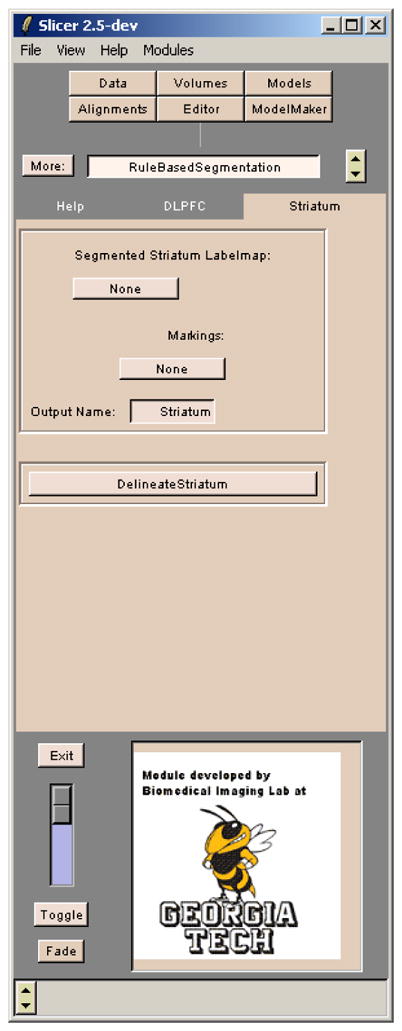
A screenshot of the module created in 3D SLICER using the semi-automatic algorithm described. The Editor tab of 3D SLICER is also used to create the required input markings on the striatum labelmap.
5. CONCLUSION
We have developed a fast and reliable technique for parcellating the striatum into the five biologically significant sub regions. Automatic geometric rule application along with minimal user input for semi-automatic parcellation was able to reduce the full parcellation time from ~48 minutes to 5 minutes while maintaining a very acceptable accuracy and reliability.
Acknowledgments
This work is part of the National Alliance for Medical Image Computing (NAMIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 EB005149. Information on the National Centers for Biomedical Computing can be obtained from http://nihroadmap.nih.gov/bioinformatics.
Also, special thanks to Laura Rosow for her participation in this collaborative effort and help in the development of figures used in this paper.
References
- 1.Al-Hakim Ramsey, Fallon James, Nain Delphine, Melonakos John, Tannenbaum Allen. A dorsolateral prefrontal cortex semi-automatic segmenter. SPIE Medical Imaging. 2006 [Google Scholar]
- 2.Zijdenbos A, Dawant B, Marjolin R. Morphometric analysis of white matter lesions in mr images: Methods and validation. IEEE TMI. 1994;13(4):716–724. doi: 10.1109/42.363096. [DOI] [PubMed] [Google Scholar]