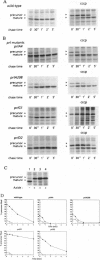
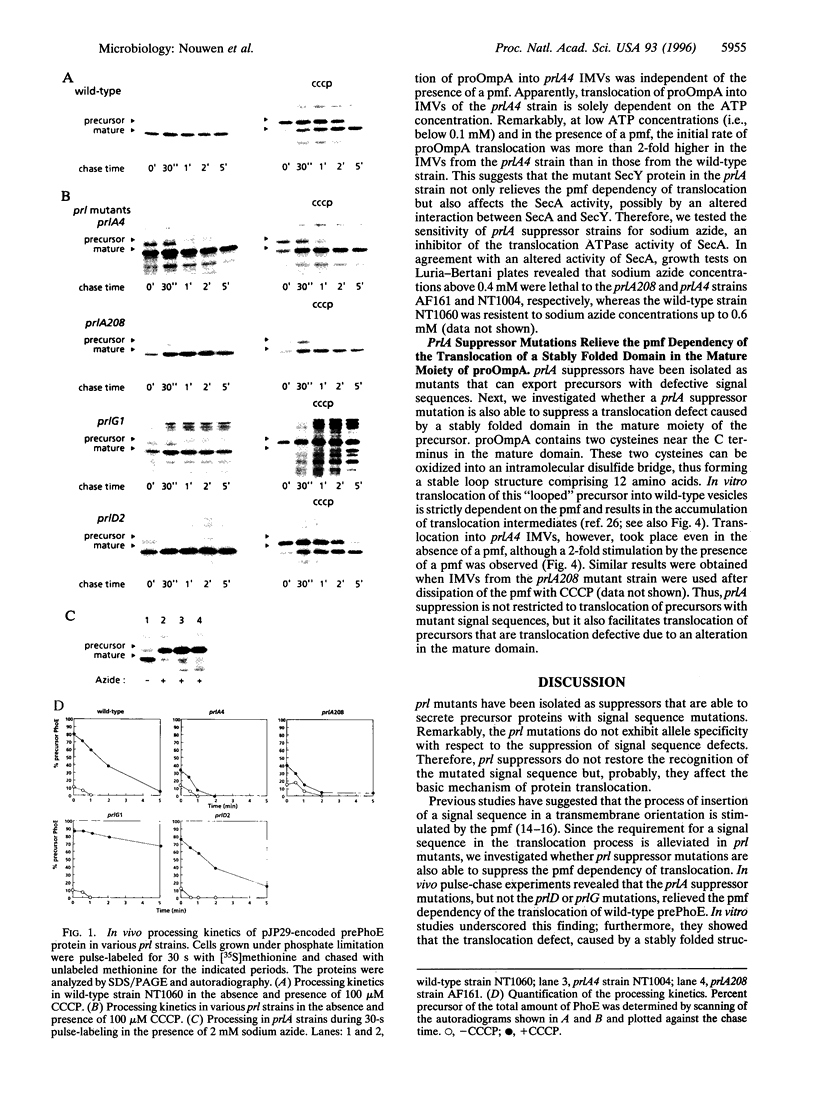
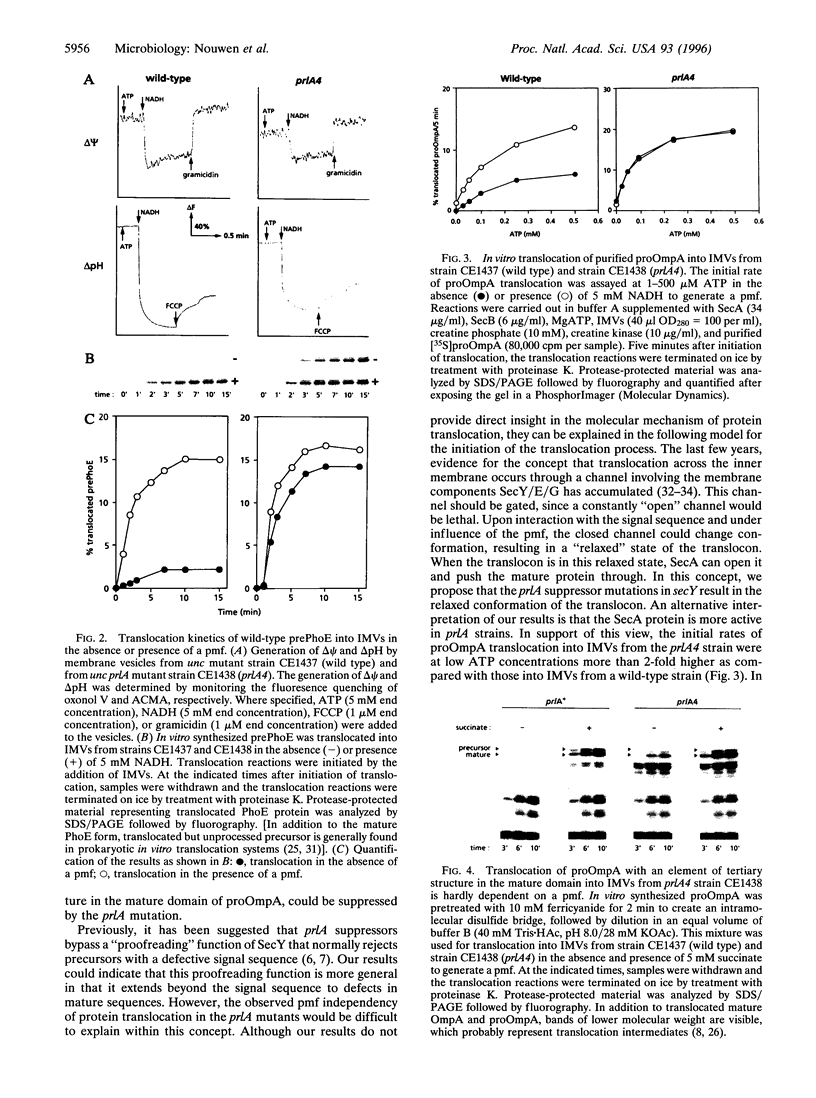
Abstract
The SecY protein of Escherichia coli is an integral membrane component of the protein export apparatus. Suppressor mutations in the secY gene (prlA alleles) have been isolated that restore the secretion of precursor proteins with defective signal sequences. These mutations have never been shown to affect the translocation of wild-type precursor proteins. Here, we report that prlA suppressor mutations relieve the proton-motive force (pmf) dependency of the translocation of wild-type precursors, both in vivo and in vitro. Furthermore, the proton-motive force dependency of the translocation of a precursor with a stably folded domain in the mature region was suppressed by prlA mutations in vitro. These data show that prlA mutations cause a general relaxation of the export apparatus rather than a specific change that results in bypassing of the recognition of the signal sequence. In addition, these results are indicative for a mechanism in which the proton-motive force stimulates translocation by altering the conformation of the translocon.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson H., von Heijne G. Membrane protein topology: effects of delta mu H+ on the translocation of charged residues explain the 'positive inside' rule. EMBO J. 1994 May 15;13(10):2267–2272. doi: 10.1002/j.1460-2075.1994.tb06508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieker K. L., Phillips G. J., Silhavy T. J. The sec and prl genes of Escherichia coli. J Bioenerg Biomembr. 1990 Jun;22(3):291–310. doi: 10.1007/BF00763169. [DOI] [PubMed] [Google Scholar]
- Bosch D., Leunissen J., Verbakel J., de Jong M., van Erp H., Tommassen J. Periplasmic accumulation of truncated forms of outer-membrane PhoE protein of Escherichia coli K-12. J Mol Biol. 1986 Jun 5;189(3):449–455. doi: 10.1016/0022-2836(86)90316-5. [DOI] [PubMed] [Google Scholar]
- Breukink E., Demel R. A., de Korte-Kool G., de Kruijff B. SecA insertion into phospholipids is stimulated by negatively charged lipids and inhibited by ATP: a monolayer study. Biochemistry. 1992 Feb 4;31(4):1119–1124. doi: 10.1021/bi00119a021. [DOI] [PubMed] [Google Scholar]
- Cao G., Kuhn A., Dalbey R. E. The translocation of negatively charged residues across the membrane is driven by the electrochemical potential: evidence for an electrophoresis-like membrane transfer mechanism. EMBO J. 1995 Mar 1;14(5):866–875. doi: 10.1002/j.1460-2075.1995.tb07068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke E., Guthrie B., Lecker S., Lill R., Wickner W. ProOmpA is stabilized for membrane translocation by either purified E. coli trigger factor or canine signal recognition particle. Cell. 1988 Sep 23;54(7):1003–1011. doi: 10.1016/0092-8674(88)90115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. J., Bole D. G., Quay S. C., Oxender D. L. Role for membrane potential in the secretion of protein into the periplasm of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5396–5400. doi: 10.1073/pnas.78.9.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vrije T., Tommassen J., De Kruijff B. Optimal posttranslational translocation of the precursor of PhoE protein across Escherichia coli membrane vesicles requires both ATP and the protonmotive force. Biochim Biophys Acta. 1987 Jun 12;900(1):63–72. doi: 10.1016/0005-2736(87)90278-1. [DOI] [PubMed] [Google Scholar]
- Derman A. I., Puziss J. W., Bassford P. J., Jr, Beckwith J. A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J. 1993 Mar;12(3):879–888. doi: 10.1002/j.1460-2075.1993.tb05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J. Precursor protein translocation by the Escherichia coli translocase is directed by the protonmotive force. EMBO J. 1992 Mar;11(3):847–853. doi: 10.1002/j.1460-2075.1992.tb05122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Wickner W. Proton transfer is rate-limiting for translocation of precursor proteins by the Escherichia coli translocase. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2471–2475. doi: 10.1073/pnas.88.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou A., Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994 Sep 9;78(5):835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- Economou A., Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994 Sep 9;78(5):835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Hanley-Way S., Silhavy T. J. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell. 1981 Jan;23(1):79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Ernst F., Hoffschulte H. K., Thome-Kromer B., Swidersky U. E., Werner P. K., Müller M. Precursor-specific requirements for SecA, SecB, and delta muH+ during protein export of Escherichia coli. J Biol Chem. 1994 Apr 29;269(17):12840–12845. [PubMed] [Google Scholar]
- Flower A. M., Doebele R. C., Silhavy T. J. PrlA and PrlG suppressors reduce the requirement for signal sequence recognition. J Bacteriol. 1994 Sep;176(18):5607–5614. doi: 10.1128/jb.176.18.5607-5614.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francetić O., Hanson M. P., Kumamoto C. A. prlA suppression of defective export of maltose-binding protein in secB mutants of Escherichia coli. J Bacteriol. 1993 Jul;175(13):4036–4044. doi: 10.1128/jb.175.13.4036-4044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Geller B., Zhu H. Y., Cheng S., Kuhn A., Dalbey R. E. Charged residues render pro-OmpA potential dependent for initiation of membrane translocation. J Biol Chem. 1993 May 5;268(13):9442–9447. [PubMed] [Google Scholar]
- Kawasaki S., Mizushima S., Tokuda H. Membrane vesicles containing overproduced SecY and SecE exhibit high translocation ATPase activity and countermovement of protons in a SecA- and presecretory protein-dependent manner. J Biol Chem. 1993 Apr 15;268(11):8193–8198. [PubMed] [Google Scholar]
- Kusters R., de Vrije T., Breukink E., de Kruijff B. SecB protein stabilizes a translocation-competent state of purified prePhoE protein. J Biol Chem. 1989 Dec 15;264(35):20827–20830. [PubMed] [Google Scholar]
- Lecker S., Lill R., Ziegelhoffer T., Georgopoulos C., Bassford P. J., Jr, Kumamoto C. A., Wickner W. Three pure chaperone proteins of Escherichia coli--SecB, trigger factor and GroEL--form soluble complexes with precursor proteins in vitro. EMBO J. 1989 Sep;8(9):2703–2709. doi: 10.1002/j.1460-2075.1989.tb08411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. M., Yamada H., Mizushima S. A proline residue near the amino terminus of the mature domain of secretory proteins lowers the level of the proton motive force required for translocation. J Biol Chem. 1991 May 25;266(15):9977–9982. [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Peters R., Bernheimer H., Berendsen W. Influence of cultural conditions and mutations on the composition of the outer membrane proteins of Escherichia coli. Mol Gen Genet. 1976 Sep 23;147(3):251–262. doi: 10.1007/BF00582876. [DOI] [PubMed] [Google Scholar]
- Müller M., Blobel G. In vitro translocation of bacterial proteins across the plasma membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7421–7425. doi: 10.1073/pnas.81.23.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama K., Mizushima S., Tokuda H. A novel membrane protein involved in protein translocation across the cytoplasmic membrane of Escherichia coli. EMBO J. 1993 Sep;12(9):3409–3415. doi: 10.1002/j.1460-2075.1993.tb06015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouwen N., de Kruijff B., Tommassen J. Delta mu H+ dependency of in vitro protein translocation into Escherichia coli inner-membrane vesicles varies with the signal-sequence core-region composition. Mol Microbiol. 1996 Mar;19(6):1205–1214. doi: 10.1111/j.1365-2958.1996.tb02466.x. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Cabelli R. J., Dolan K. M., Jarosik G. P. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne R. S., Silhavy T. J. PrlA suppressor mutations cluster in regions corresponding to three distinct topological domains. EMBO J. 1993 Sep;12(9):3391–3398. doi: 10.1002/j.1460-2075.1993.tb06013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Beckwith J. Genetic analysis of protein export in Escherichia coli. Annu Rev Genet. 1990;24:215–248. doi: 10.1146/annurev.ge.24.120190.001243. [DOI] [PubMed] [Google Scholar]
- Schiebel E., Driessen A. J., Hartl F. U., Wickner W. Delta mu H+ and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell. 1991 Mar 8;64(5):927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
- Schiebel E., Wickner W. Preprotein translocation creates a halide anion permeability in the Escherichia coli plasma membrane. J Biol Chem. 1992 Apr 15;267(11):7505–7510. [PubMed] [Google Scholar]
- Shiozuka K., Tani K., Mizushima S., Tokuda H. The proton motive force lowers the level of ATP required for the in vitro translocation of a secretory protein in Escherichia coli. J Biol Chem. 1990 Nov 5;265(31):18843–18847. [PubMed] [Google Scholar]
- Simon S. M., Blobel G. Signal peptides open protein-conducting channels in E. coli. Cell. 1992 May 15;69(4):677–684. doi: 10.1016/0092-8674(92)90231-z. [DOI] [PubMed] [Google Scholar]
- Tani K., Tokuda H., Mizushima S. Translocation of ProOmpA possessing an intramolecular disulfide bridge into membrane vesicles of Escherichia coli. Effect of membrane energization. J Biol Chem. 1990 Oct 5;265(28):17341–17347. [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Outer membrane protein e of Escherichia coli K-12 is co-regulated with alkaline phosphatase. J Bacteriol. 1980 Jul;143(1):151–157. doi: 10.1128/jb.143.1.151-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Tokuda H., Mizushima S. Proton motive force-dependent and -independent protein translocation revealed by an efficient in vitro assay system of Escherichia coli. J Biol Chem. 1989 Jan 25;264(3):1723–1728. [PubMed] [Google Scholar]
- Yamane K., Ichihara S., Mizushima S. In vitro translocation of protein across Escherichia coli membrane vesicles requires both the proton motive force and ATP. J Biol Chem. 1987 Feb 15;262(5):2358–2362. [PubMed] [Google Scholar]