Abstract
Evidence from in vivo and epidemiological studies suggests that organophosphorus insecticides (OPs) are developmental neurotoxicants, but possible underlying mechanisms are still unclear. Astrocytes are increasingly recognized for their active role in normal neuronal development. This study sought to investigate whether the widely-used OP diazinon (DZ), and its oxygen metabolite diazoxon (DZO), would affect glial-neuronal interactions as a potential mechanism of developmental neurotoxicity. Specifically, we investigated the effects of DZ and DZO on the ability of astrocytes to foster neurite outgrowth in primary hippocampal neurons. The results show that both DZ and DZO adversely affect astrocyte function, resulting in inhibited neurite outgrowth in hippocampal neurons. This effect appears to be mediated by oxidative stress, as indicated by OP-induced increased reactive oxygen species production in astrocytes and prevention of neurite outgrowth inhibition by antioxidants. The concentrations of OPs were devoid of cytotoxicity, and cause limited acetylcholinesterase inhibition in astrocytes (18 and 25% for DZ and DZO, respectively). Among astrocytic neuritogenic factors, a most important one is the extracellular matrix protein fibronectin. DZ and DZO decreased levels of fibronectin in astrocytes, and this effect was also attenuated by antioxidants. Underscoring the importance of fibronectin in this context, adding exogenous fibronectin to the co-culture system successfully prevented inhibition of neurite outgrowth caused by DZ and DZO. These results indicate that DZ and DZO increase oxidative stress in astrocytes, and this in turn modulates astrocytic fibronectin, leading to impaired neurite outgrowth in hippocampal neurons.
Keywords: Organophosphorus insecticides, diazinon, diazoxon, neurite outgrowth, glial-neuronal interactions, oxidative stress
Introduction
There is increasing concern in the public and regulatory spheres over exposure to organophosphorus insecticides (OPs) and their ability to adversely affect neurodevelopment (Costa, 2006; Eaton et al., 2008; Eskenazi et al., 2008). The cause for concern resides in the fact that there is widespread exposure of children to OPs, both in rural and urban environments (Barr et al., 2004; Beamer et al., 2008; Eskenazi et al., 2007; Fenske et al., 2002; Lu et al., 2000; Valcke et al., 2006). Additionally, evidence from animal studies indicates that the developing nervous system may be more susceptible to the neurotoxicity of OPs than the mature nervous system (Moser et al., 1998; Moser and Padilla, 1998; Pope and Liu, 1997; Won et al., 2001). This is compounded by epidemiological studies that link early exposure to OPs and various neurobehavioral deficits in children, such as increased incidence of attention deficit hyperactivity disorder and lowered I.Q. (Bouchard et al., 2010; Eskenazi et al., 2007; Rauh et al., 2011; Rohlman et al., 2011).
While acute toxicity to OPs primarily occurs as a result of acetylcholinesterase (AChE) inhibition, the mechanisms of lower-level, chronic exposures on neurodevelopment remain unclear. As reviewed by Lukaszewicz-Hussain (2010), several studies support the ability of various OPs to induce oxidative stress in humans (Ranjbar et al., 2005; Vidyasagar et al., 2004), in animal models (Jafari et al., 2012; Slotkin et al., 2005; Yilmaz et al., 2012), and in various in vitro models (Crumpton et al., 2000; Giordano et al., 2007; Lee et al., 2012; Slotkin and Seidler, 2009). These effects manifest in the form of altered levels and activity of different antioxidant factors, as well as increases in various markers of oxidative stress, including increased lipid peroxidation and levels of reactive oxygen species (ROS). ROS, including O2-, ·OH, and hydrogen peroxide, are produced by a variety of enzymatic and chemical processes, many of which are an integral part of normal physiological functioning and cell signaling (Dickinson and Chang, 2011). In contrast, the overabundance and mismanagement of ROS leads to oxidative stress, which is more recently implicated in the progression of various neurodegenerative diseases, including Alzheimer's disease and Parkinson's disease, as well as Frederick's ataxia and Amyotrophic lateral sclerosis (Barnham et al., 2004; Potashkin and Meredith, 2006). Additionally, oxidative damage and related mechanisms have been more recently implicated in other instances of neurodevelopmental dysfunction, such as autism spectrum disorders and schizophrenia (Do et al., 2009; Frustaci et al., 2012; Tang et al., 2013).
Underscoring the link between OP-induced oxidative stress and the susceptibility of the developing brain to these exposures, Samarawickrema and colleagues (2008) provide evidence of increased lipid peroxidation in fetal cord blood samples obtained from pregnant women living in a rural farming community that were exposed to OPs during crop-spraying season. These increases correlated with significantly inhibited fetal butyrylcholinesterase activity (Samarawickrema et al., 2008). The brain, and specifically the developing brain, is particularly susceptible to oxidative damage. This is due to its high oxygen consumption, high lipid content, and a relatively low amount of endogenous antioxidants (Lukaszewicz-Hussain, 2010; Matés, 2000). While these findings suggest that oxidative stress may play a role in the developmental neurotoxic mechanisms of OPs, possible consequences of such oxidative stress are for the most part unknown.
The OP diazinon (DZ) is widely used in agriculture in the U.S. and abroad, though it has been banned for residential use in the U.S. in 2004 (EPA, 2011). Those living in close proximity to crops sprayed with DZ are at risk for increased exposure and subsequent adverse health effects (ATSDR, 2008). The literature suggests that DZ and its oxygen-metabolite diazoxon (DZO), may be developmental neurotoxicants, but the mechanisms by which they exert these effects are unclear. Developmental effects are evident in studies of long-term effects of late gestational and neonatal exposures to DZ: early exposure to diazinon affected learning and memory (Levin et al., 2008; Roegge et al., 2006; Timofeeva et al., 2008), as well as neural cell development and synaptic function (Slotkin et al., 2008) in adolescent rodents. Most studies attempting to explain mechanisms of DZ and DZO neurotoxicity have been completed in cell lines (Axelrad et al., 2003; Flaskos et al., 2007; Sidiropoulou et al., 2009), primarily focused on direct damage to neurons. The present study highlights the effects of DZ and DZO on astrocyte function and their ability to foster neuronal development, using primary cell cultures of cortical astrocytes and hippocampal neurons to explore a novel mechanism of OP developmental neurotoxicity.
Previous work in our laboratory had shown that manganese and oxidants (hydrogen peroxide (H2O2) and 2,3-dimethoxy-1,4-naphthoquinone (DMNQ)) affected astrocyte-neuronal interactions leading to impaired neuritogenesis (Giordano et al., 2009). Astrocytes are increasingly recognized as having essential roles in the function and development of the brain (Benarroch, 2005; Guizzetti et al., 2008; He and Sun, 2007). Astrocytes tend to be more resistant to oxidative stress than other neural cell types, likely due to the fact that they contain higher levels of endogenous antioxidants, such as glutathione, than neuronal cells (Giordano et al., 2006; Thorburne and Juurlink, 1996). However, oxidative stress in astrocytes, while not leading to decreases in cell viability, may alter astrocyte functions. In the present study, we investigated the potential for DZ and DZO to impair the ability of cortical astrocytes to foster neurite outgrowth in primary hippocampal neurons, by causing oxidative stress in astrocytes and negatively modulating expression of the pro-neuritogenic extracellular matrix protein fibronectin.
Materials and Methods
Materials
Neurobasal-A medium, DMEM medium, fetal bovine serum (FBS), Hanks' balanced salt solution (HBSS), GlutaMAX, anti-mouse Alexa fluor-488 secondary antibody, Hoechst 33342, 2,7′-dichlorofluorescin diacetate (H2DCF-DA), SuperSignal West Pico Chemiluminescent Substrate (Pierce), papain, and gentamicin were from Invitrogen (Carlsbad, CA). Diazinon (DZ; 99.4%) and diazinon-O-analog (diazoxon; DZO; 98%) were from Chem-Service (West Chester, PA). Poly-D-lysine, antibodies: peroxidase-conjugated anti-mouse IgG, mouse anti-beta-actin, horseradish peroxidase-conjugated anti-rabbit IgG, rabbit anti-fibronectin, rabbit anti-map-2, mouse anti-tau, goat serum, dimethyl sulfoxide (DMSO), hydrogen peroxide (H2O2), 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT), and N-t-butyl-alpha-phenylnitrone (PBN) were from Sigma-Aldrich (St. Louis, MO). Protease inhibitors were from Roche Diagnostics (Indianapolis, IN). Mouse β-III-tubulin antibody was from Millipore (Billerica, MA). Melatonin was from EMD Chemicals (Rockland, MA). Purified human fibronectin (FN) was from BD Biosciences (Bedford, MA).
Preparation of fetal rat cortical astrocytes
Primary cultures of cortical astrocytes were prepared from E21Sprague-Dawley rat fetuses, as previously described (Guizzetti and Costa, 1996). Cultures were randomly checked for their purity (>95%) by immunofluorescence using an antibody against glial fibrillary acidic protein (GFAP). After at least 12 days in culture, astrocytes were plated in 24-well plates for astrocyte–neuron co-culture experiments (2×106 cells/well), on glass coverslips (2.5×105 cells/coverslip) for immunocytochemistry, or in 100 mm dishes for Western blot analysis (2×106 cells/dish).
Astrocyte treatments
Four days after plating, astrocytes were rinsed twice with PBS and placed in serum-free media (DMEM-BSA (0.1%)) for an additional 24 h, after which they were treated for 24 h with either DZ or DZO (both dissolved in DMSO), with H2O2, or with vehicle control (DMSO). DMSO concentrations in the treatment solutions never exceed 0.1%. In some experiments, astrocytes were pre-treated with the antioxidants melatonin (200 μM) or N-t-butyl-alpha-phenylnitrone (PBN; 100 μM) for 3 h. After two washes with PBS, astrocytes were treated with either DZ or DZO for 24 h.
Measurement of cell viability
Astrocyte viability was measured by the MTT assay, where 50 μL of MTT reagent (5 mg/mL) was added to each well after 24 h treatment with DZ or DZO. After 15 min. at 37 °C, the medium was removed and the formazan reaction product was dissolved in 250 μL DMSO. Absorbance was read at 562 nm and results were expressed as mean percentage of viable cells relative to untreated controls.
Preparation of fetal rat hippocampal neurons
Hippocampal neurons from E21 rat fetuses were prepared as previously described (Brewer et al., 1993; Guizzetti et al., 2008; VandeMark et al., 2009). For quantitative analysis of neurite outgrowth, neurons were plated on glass coverslips (2×104 cells/coverslip), previously coated overnight with 100 μg/mL poly-D-lysine at 37 °C.
Astrocyte-neuron co-cultures
Hippocampal neurons were prepared as described, and plated on glass coverslips to which 4 paraffin beads were previously affixed. After 1 h incubation in Neurobasal A/FBS (10%) medium to allow neurons to attach, the glass coverslips were inverted onto 24-well plates containing astrocytes, as described by Viviani et al. (1998). Rat cortical astrocytes were previously treated with vehicle control, DZ, DZO or H2O2 for 24 h, followed by wash-out and replacement of the medium with fresh “serum-free” medium (DMEM-BSA (0.1%)). Freshly isolated hippocampal neurons were co-cultured with these astrocytes for 48 h. This “sandwich” co-culture system prevents direct contact between neurons and astrocytes while allowing them to share the same medium. After 48 h of co-culture, the coverslips with the attached hippocampal neurons were removed from the plates containing the astrocytes, flipped over, and washed twice with warmed HBSS. The neurons were then fixed for 15 min. at 37 °C with 4% paraformaldehyde (PFA) for quantitative morphological analysis. For experiments evaluating the effect of exogenous FN on DZ and DZO-induced inhibition of neurite outgrowth, 10 μg/mL FN was added to the co-culture system when the neurons were placed with the astrocytes for the 48 h incubation. Stock solutions of FN were prepared as per manufacturer's recommendations and stored in 1 mg/mL aliquots at -20 °C.
Quantitative morphological analysis of neurite outgrowth
After 48 of co-culture with astrocytes, neurons were fixed in 4% PFA in HBSS and permeabilized in 0.1% Triton X-100. Neurons were labeled with an anti-β-III-tubulin isoform antibody followed by a fluorescein-conjugated secondary antibody (Alexa-488); the nuclei were stained with Hoechst 33342. Glass coverslips were then mounted on microscope slides. Only pyramidal neurons (which represent more than 90% of neurons in the cultures (Giordano et al. 2009)) with three or more neurites, not touching any other cells/neurites were included for quantitative analysis. Neurons whose processes were intermingled with those of neighboring cells were excluded from the analysis. For each cell, the following parameters were measured: axon length (including the length of any branches originating from the axon); length of minor neurites; total number of neurites/cell. Neurite length was measured from the point of emergence at the cell body to the tip of each segment. The identity of the longest neurite as the axon, and of minor neuritis as dendrites was verified by staining with the specific markers Tau and MAP-2 (microtubule-associated protein-2), as previously described (Guizzetti et al., 2008; Giordano et al., 2009). Quantification of the morphological parameters was carried out using MetaMorph 6.1 analysis software. At least 30 randomly chosen cells per treatment were analyzed for each experiment, and the analysis was carried out blind.
Measurement of reactive oxygen species
Reactive oxygen species (ROS) formation was determined by fluorescence using 2,7′-dichlorofluorescin diacetate (DCFH2-DA). Upon entering cells DCFH2-DA is de-esterified to DCFH2, which is then oxidized by ROS to form the fluorescent 2,7′-dichlorofluorescein (DCF). Astrocytes were incubated for 30 min. with 10 μM DCFH2-DA in Locke's solution. Afterwards, the probe was washed out and cells were treated with the appropriate chemical for the desired time point(s). After treatments (at 37 °C), the test solution was removed, and 0.1 M KH2PO4 0.5% Triton X-100 (pH 7.2) was added. Cells were then scraped from the dish and the extract centrifuged (10 min at 12,000 rpm). The supernatant was collected and the fluorescence was immediately read using a Perkin-Elmer spectrofluorimeter (excitation 488 nm, emission 525 nm).
Western blot analysis
Measurements of fibronectin protein levels in astrocyte lysate and in medium by Western blot were carried out as previously described (Guizzetti et al., 2008). Cells were scraped in 200 μL of lysis buffer (0.2% SDS, 1mM PMSF, 1 mL 10x Cell Signaling lysis buffer (Cell Signaling Technology, Inc., Beverly, MA), and a protease inhibitor EDTA-free mixture), sonicated, and stored at -20 °C. Proteins were quantified by the BCA method, separated by electrophoresis, and transferred to PVDF membranes. Membranes were blocked for 2-3 hours at room temperature in 5% milk/Tris-buffered saline plus 0.1% Tween-20 (TBST) buffer. Membranes were then incubated with a rabbit anti-fibronectin (1:1000) antibody in 3% BSA/TBST for 1 h at room temperature. Membranes were then washed in TBST and incubated for 1 h at room temperature with anti-rabbit horse radish peroxidase (HRP)-labeled antibody (1:1000) in 3% BSA/TBST. Membranes from cell lysate proteins were re-probed for beta-actin as a loading control: mouse anti-β-actin (1:10,000) in 3% BSA/TBST for 30 min., followed by anti-mouse HRP-linked antibody (1:1000) in 3% BSA/TBST for 30 min., all at room temperature. After being washed with TBST, membranes are visualized using SuperSignal West Pico Chemiluminescent Substrate (Pierce). Optical density of each band was quantified using ImageJ software (National Institutes of Health).
Confocal microscopy analysis of fibronectin levels
Astrocytes were cultured and treated as in all other experiments, then washed and fixed in 4% paraformaldehyde for 5 min. at room temperature. Surface expression of fibronectin on astrocytes was assessed as previously described (Guizzetti et al., 2008). Briefly, the cells were blocked in 10% goat serum in PBS for 1 h at room temperature. Astrocytes were labeled with an anti-fibronectin antibody (1:50) in 3% BSA/PBS, followed by fluorescein-conjugated secondary antibody (Alexa-594; 1:75 in 3% BSA/PBS). Nuclei were stained with Hoechst 33342. Extracellular fibronectin levels on astrocytes were assessed by taking images of 10 random fields per treatment per experiment (n=3) with an Olympus Fluoview-1000 confocal microscope. Each image consists of 21 z-directional slices with a 0.5 μm step-size. Integrated fluorescence intensity was measured using MetaMorph software integrated morphometry analysis tools. The sum of integrated optical density over all planes for each image was taken; integrated intensity was normalized by cell number. A total of 10 random images per treatment were taken and analyzed for each experiment where treatments were completed in triplicate wells. Extracellular fibronectin levels are expressed as mean integrated intensity relative to untreated control.
Acetylcholinesterase (AChE) activity measurements
AChE activity in astrocytes were measured as previously described (Li et al., 2000) in a microtiter plate assay based on the Ellman method (Ellman et al., 1961). Cell lysates were collected in a 0.1 M sodium phosphate buffer (pH 8.0). For duplicate assays, 50 μL of cell lysates was combined with 150 μL of the assay buffer containing 0.1 M sodium phosphate and 0.1 mM of DTNB (5,5′-dithiobis-2-nitrobenzoic acid). The kinetic assay was initiated by addition of acetylthiocholine (final concentration: 1 mM) and the reaction was continuously monitored for 10 min. at room temperature. Absorbance was read at 412 nm in a Beckman DU-70 spectrophotometer. The amount of 5-thio-2-nitrobenzoate formed was calculated using an extinction coefficient of 13 600 M-1/cm. AChE activity was expressed as nmol/min/mg protein.
Statistical Analysis
For neurite outgrowth measurements, the following parameters were measured for each cell using Metamorph software: longest neurite length (including the length of any branches originating from the neurite); length of minor neurites; and total number of neurites/cell. At least 30 cells per treatment were analyzed in each experiment. Final data summaries are displayed as the mean (± SEM) for 90-120 cells per treatment derived from at least 3 independent experiments, unless otherwise indicated. For Western blot analysis, ROS measurements, and extracellular fibronectin levels, at least 3 separate experiments were performed where all samples were completed in duplicate or triplicate. Results are reported as the mean percent of the control (± SEM) of results from at least 3 experiments. Statistical significance for all analyses (unless otherwise indicated) was evaluated by one-way analysis of variance (ANOVA), followed by Bonferroni's multiple comparison post-hoc test. Western blot data were analyzed using the non-parametric analysis of variance Kruskal-Wallis test followed Dunn's post-test. P < 0.05 was considered statistically significant. All data were analyzed using GraphPad Prism software.
Results
DZ and DZO impair the ability of astrocytes to promote neurite outgrowth in hippocampal neurons
Astrocytes previously treated with DZ or DZO for 24 h, followed by wash-out, were co-cultured with primary hippocampal neurons for 48 h, after which neurons were fixed, and several parameters of neurite outgrowth were measured (length of the longest neurite, length of minor neurites, and number of neurites/cell). There was a highly significant, 50% decrease in the length of the longest neurite in hippocampal neurons cultured with astrocytes previously treated with 10 μM DZ (Fig. 1A). Results also indicate a small, but significant, decrease in minor neurite length in neurons incubated with astrocytes previously treated with concentrations as low as 0.1 μM DZ (Fig. 1B), while no differences in the number of neurites per cell were observed for any DZ concentration (Fig. 1C). The oxidant H2O2 (20 μM) was used as a positive control (Giordano et al., 2009), and caused effects similar to DZ and DZO (Fig. 1). Neurons cultured with astrocytes previously treated with either 1 or 10 μM DZO showed a 35% and 60% decrease in longest neurite length, respectively (Fig. 2A). Of interest is that the effect of DZO appeared to be biphasic, with the lowest concentration (0.1 μM) causing an increase in the length of the major neurite. This possible “hormetic-like” effect warrants further investigations. Similar to the results with DZ, astrocytes treated with 0.1-10 μM DZO resulted in a decrease in minor neurite length (Fig. 2B). For both DZ and DZO, the changes in minor neurite length were much smaller in magnitude than those observed for the longest neurite length. No differences in number of neurites per cell were seen as a result of astrocytes treated with DZO (Fig. 2C).
Figure 1. DZ inhibits the ability of astrocytes to foster neurite outgrowth in hippocampal neurons.
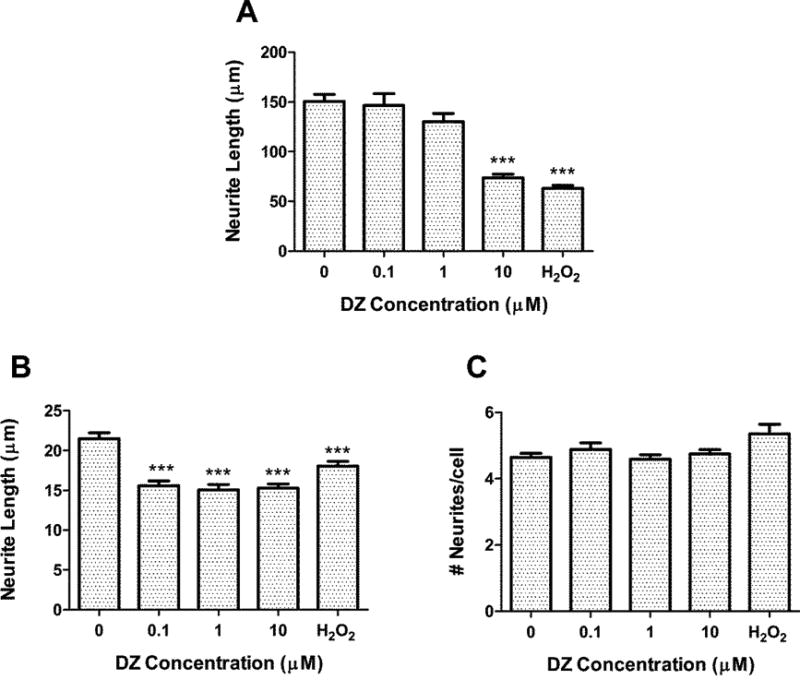
Rat hippocampal neurons were plated on glass coverslips and inverted on top of astrocytes (previously untreated or treated with DZ for 24 h) for 48 h. Neurons were then fixed and stained with a neuron-specific tubulin antibody, and analyzed for (A) longest neurite length, (B) minor neurite length, and (C) number of neurites per cell. Results represent the mean (± SEM) of 90-120 cells derived from at least three separate experiments. Concentration of H2O2 was 20 μM. *** Significantly different from untreated control, p<0.001.
Figure 2. DZO inhibits the ability of astrocytes to foster neurite outgrowth in hippocampal neurons.
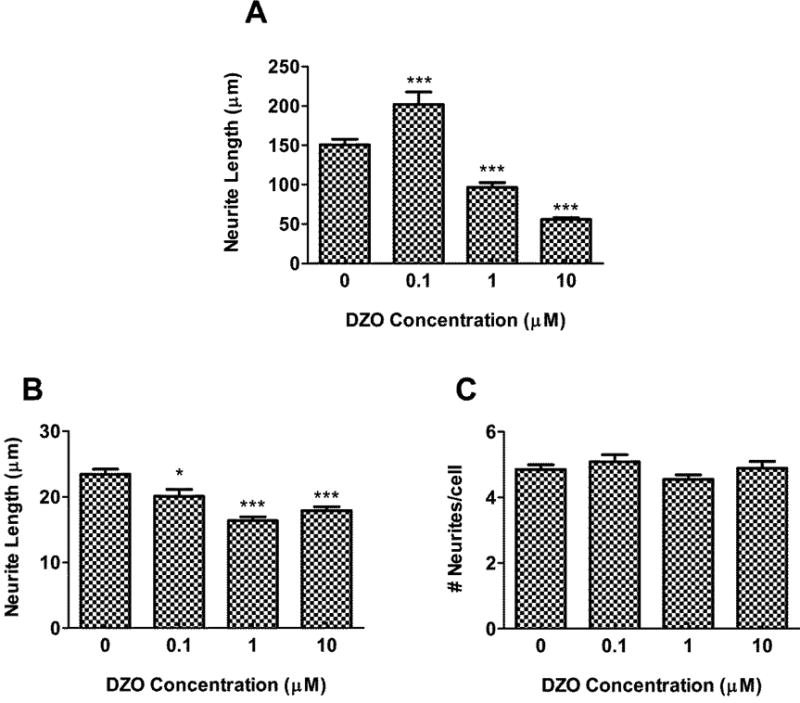
Rat hippocampal neurons were plated on glass coverslips and inverted on top of astrocytes (previously untreated or treated with DZ for 24 h) for 48 h. Neurons were then fixed and stained with a neuron-specific tubulin antibody, and analyzed for (A) longest neurite length, (B) minor neurite length, and (C) number of neurites per cell. Results represent the mean (± SEM) of 90-120 cells derived from at least three separate experiments. Significantly different from untreated control, *p<0.05, ***p<0.001.
Representative neurons in Fig. 3 further illustrate the effect of DZ and DZO on neurite outgrowth: neurons cultured with astrocytes previously treated with either 10 μM DZ or DZO display starkly stunted neurite lengths compared to the untreated control (Fig. 3C and D). This effect is comparable to that of neurons cultured with astrocytes that were previously treated with H2O2 (20 μM; Fig. 3B). No cytotoxicity was observed in astrocytes at any concentration of either DZ or DZO (Table 1).
Figure 3. DZ- and DZO-treated astrocytes have decreased ability to foster neurite outgrowth in hippocampal neurons.
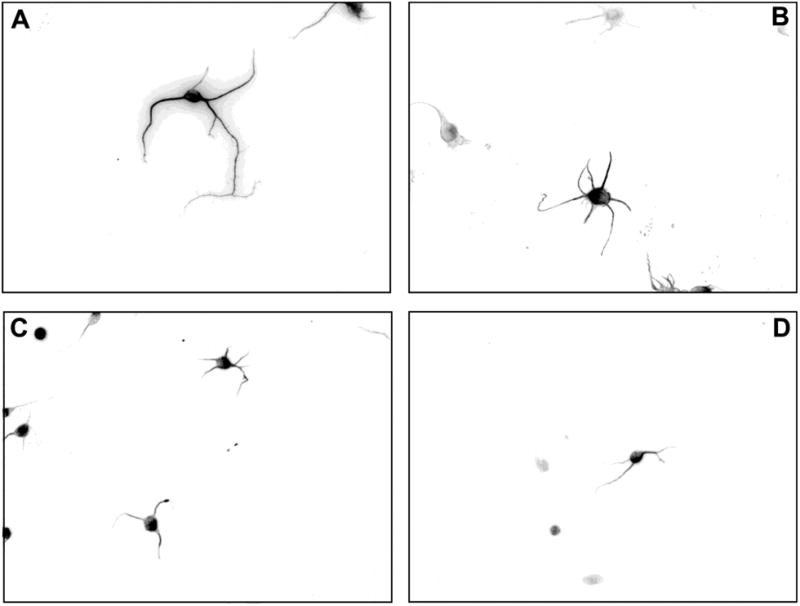
Astrocytes were treated with 24 h with DZ, DZO, or the positive control H2O2, then after wash-out, co-cultured for 48 h with hippocampal neurons. Neurons were then fixed and stained as described in Methods. Images show representative neurons co-incubated with astrocytes previously treated with (A) Control, (B) 20 μM H2O2, (C) 10 μM DZ, or (D) 10 μM DZO.
Table 1. Viability of DZ or DZO-treated astrocytes.
| Viability (% ± SEM) | ||
|---|---|---|
| Concentration (μM) |
DZ | DZO |
| 0 | 100 | 100 |
| 1 | 101.2 ± 7.9 | 100.8 ± 6.5 |
| 5 | 104.3 ± 5.1 | 97.5 ± 1.8 |
| 10 | 106.0 ± 8.0 | 91.4 ± 2.2 |
| 25 | 104.3 ± 6.8 | 98.2 ± 3.2 |
| 50 | 114.5 ± 8.1 | 99.1 ± 2.1 |
Astrocytes were treated with varying concentrations of either DZ or DZO for 24 h and cell viability was assessed by the MTT method. Results are expressed as percent control (mean ± SEM) and are from three separate experiments where astrocytes where treated in triplicate.
The effects of DZ and DZO on neurite outgrowth are modulated by oxidative stress in astrocytes
As we had previously found that oxidative stress in astrocytes impairs their ability to foster neurite outgrowth (Giordano et al., 2009) and that various OPs, including DZ and DZO cause oxidative stress in neuronal cells (Giordano et al., 2007), we sought to investigate whether oxidative stress may be involved in the inhibitory effect of DZ and DZO on neurite outgrowth. Reactive oxygen species (ROS) formation was measured in astrocytes treated with various concentrations of DZ or DZO (0.1, 1, and 10 μM). As shown in Fig. 4, DZ and DZO caused a concentration-dependent increase in ROS levels in astrocytes, comparable at the highest concentration (10 μM) to the effect of H2O2 (Fig. 4). To establish an involvement of oxidative stress in astrocytes in DZ- and DZO-induced inhibition of neuritogenesis, experiments were carried out in which astrocytes were pre-treated with the antioxidants melatonin (200 μM) or N-t-butyl-alpha-phenylnitrone (PBN; 100 μM) for 3 h. After 3 h, the antioxidants were washed-out and the astrocytes were treated with either 10 μM DZ or DZO for 24 h, followed by wash-out and 48 h of co-culture with hippocampal neurons. The results, shown in Table 2, indicate that pre-treatment with either antioxidant completely prevented the decrease in longest neurite length caused by DZ or DZO. In contrast, neither antioxidant prevented the decrease of minor neurite length (Table 2), suggesting perhaps different underlying mechanisms.
Figure 4. DZ and DZO increase oxidative stress in astrocytes.
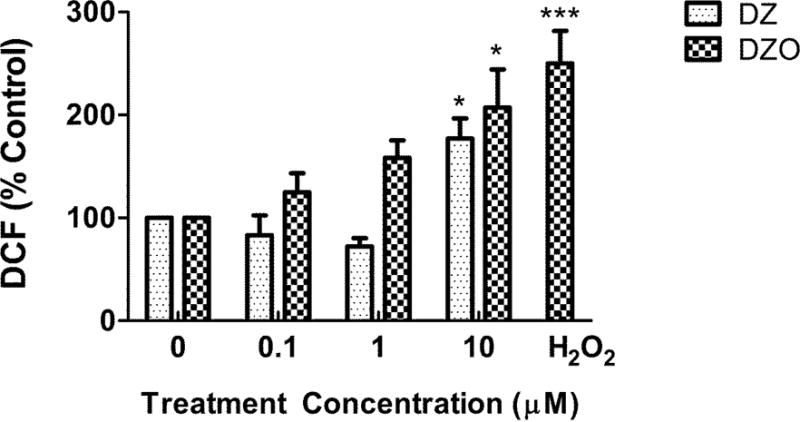
Intracellular ROS production in DZ- and DZO-treated astrocytes over 1 h period. Concentration of the positive control H2O2 was 20 μM. Results are expressed as percent of untreated astrocytes, and bars represent the mean (± SEM) of three separate experiments done in duplicate. Significantly different from the respective untreated controls, *p<0.05, ***p<0.001.
Table 2. Effects of antioxidants on the inhibitory effects of DZ and DZO on neurite outgrowth.
| Treatment | Longest neurite length (μm) |
Minor neurite length (μm) |
No. neurites/cell |
|---|---|---|---|
| Control | 140.3 ± 6.8 | 22.0 ± 0.7 | 4.7 ± 0.1 |
| PBN | 172.4 ± 15.4 | 13.5 ± 0.7# | 5.0 ± 0.2 |
| Mel | 149.7 ± 15.5 | 21.0 ± 1.4 | 4.1 ± 0.2 |
| DZ | 64.8 ± 3.0# | 15.5 ± 0.4# | 4.6 ± 0.1 |
| DZ + PBN | 210.5 ± 20.4# | 15.7 ± 0.9# | 5.5 ± 0.4 |
| DZ + Mel | 175.3 ± 15.6 | 16.2 ± 0.8# | 4.6 ± 0.2 |
| DZO | 51.8 ± 2.3# | 15.6 ± 0.5# | 4.6 ± 0.2 |
| DZO + PBN | 188.1 ± 21.8* | 20.3 ± 1.1* | 5.4 ± 0.3 |
| DZO + Mel | 138.2 ± 12.2 | 16.8 ± 1.2# | 4.7 ± 0.2 |
Astrocytes were pre-treated with 200 μM melatonin (Mel) or 100 μM PBN for 3 h prior to washout and treatment with 10 μM DZ or DZO for 24 h. After further wash-out, astrocytes were co-cultured with hippocampal neurons for 48 h, as described in Methods. Results represent the mean (± SEM) of at least 60 cells per treatment group. Significantly different from untreated control,
, p<0.05;
, p<0.001.
DZ and DZO decrease fibronectin levels in astrocytes
In efforts to explain how DZ and DZO would affect astrocyte function, and subsequently neuritogenesis, alterations to the production and secretion the neuritogenic extracellular matrix (ECM) protein fibronectin (FN) in astrocytes was investigated. Western blot analysis was used to quantify relative intracellular FN in astrocytes treated with or without 10 μM DZ or DZO (Fig. 5A). Quantification of these data (Fig. 5B) shows that DZ and DZO caused a 26% and 33% decrease in FN levels, respectively. Such decrease could be ascribed to OP-induced oxidative stress, as pre-treatment of astrocytes with the antioxidants melatonin or PBN prevented these decreases in FN (Fig. 5B).
Figure 5. DZ and DZO decrease fibronectin lysate levels in astrocytes: reversal by antioxidants.
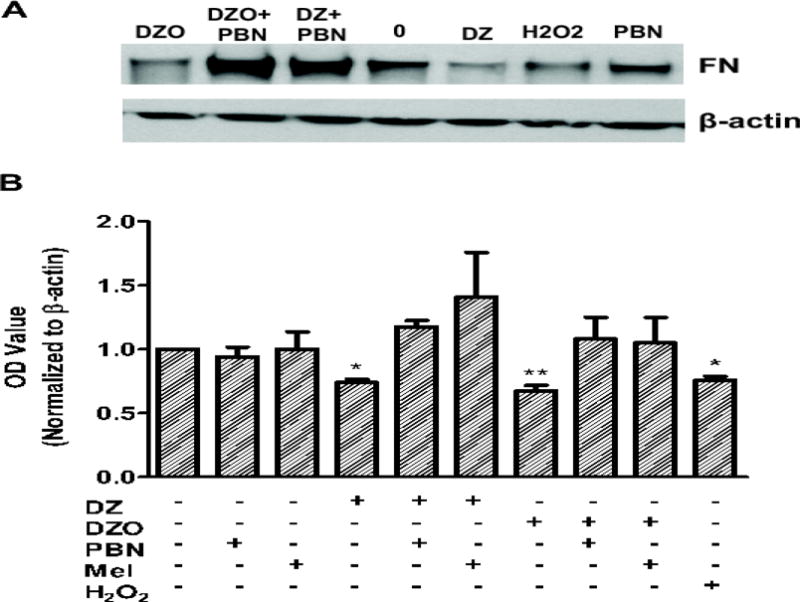
Astrocytes were treated for 24 h with 10 μM DZ or DZO followed by wash-out. In some groups, astrocytes were pre-treated for 3 h with the antioxidants melatonin (200 μM) or PBN (100 μM) before wash-out and OP treatment. Concentration of the positive control H2O2 was 20 μM. (A) Representative Western blot demonstrating a decrease in FN levels compared to untreated control, and the effect of PBN. (B) Quantification of all Western blot data. Results represent the mean (± SEM) of at least three independent experiments in which astrocytes were treated in duplicate. **Significantly different from control, p<0.01.
Confocal microscopy and immunocytochemistry were used to confirm that DZ and DZO decrease FN protein levels in astrocytes. Relative integrated intensity measurements demonstrate a marked decrease in FN bound to the extracellular surface of the astrocytes as a result of 10 μM DZ or DZO exposure, as compared to an untreated control (Fig. 6A). Similar to the effects on lysate FN levels, DZO (10 μM) decreases extracellular FN levels on astrocytes by 40%, as compared to untreated control astrocytes (p<0.01), while 10 μM DZ appears to cause a 30% decrease in extracellular FN levels (p=0.069; Fig. 6B).
Figure 6. DZ and DZO decrease extracellular expression of fibronectin in astrocytes.
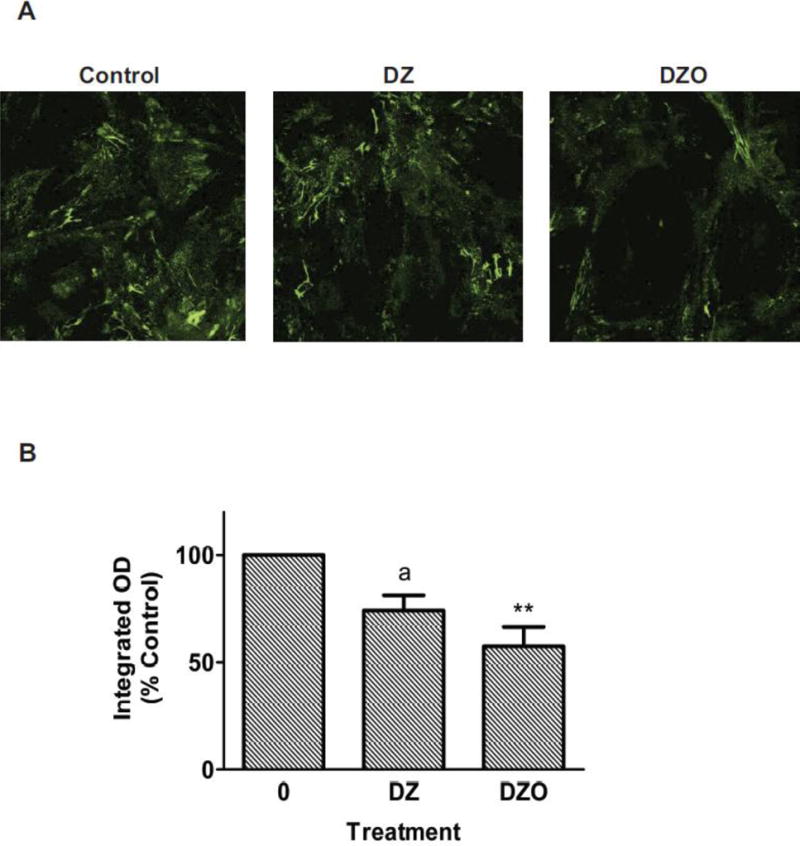
Extracellular-bound FN on astrocytes was imaged by confocal microscopy. Integrated intensity was measured and normalized by cell number. DZ and DZO (both at 10 μM) decrease extracellular expression of FN in astrocytes. (A) Representative confocal microscopy images of extracellular FN expression on astrocytes; (B) Quantification of the integrated fluorescence intensity relative to control. Results are expressed as percent mean integrated fluorescence of untreated control (± SEM) and derive from three independent experiments. **Significantly different from control, p<0.01. The effect of DZ approached significance (ap<0.069).
Fibronectin antagonizes the effects of DZ and DZO on astrocyte-mediated neuritogenesis
Exogenous FN was added to the co-culture system in the presence of 10 μM DZ or DZO to confirm the involvement of FN on the effect of these compounds on astrocytes' ability to foster neurite growth. Purified FN (final concentration: 10 μg/mL) was added to the astrocyte-neuron culture system at the time when the newly prepared neurons were added to the plates containing the astrocytes. The astrocytes and neurons were then co-cultured for 48 h, as in the previous experiments. Exogenous FN completely prevented the inhibition of the longest neurite length normally observed in neurons cultured with astrocytes previously treated with DZ or DZO (Fig. 7A). No protective effect of exogenous FN was seen for the decreases in minor neurite length (Fig. 7B).
Figure 7. Fibronectin prevents inhibition of neurite outgrowth caused by DZ and DZO.
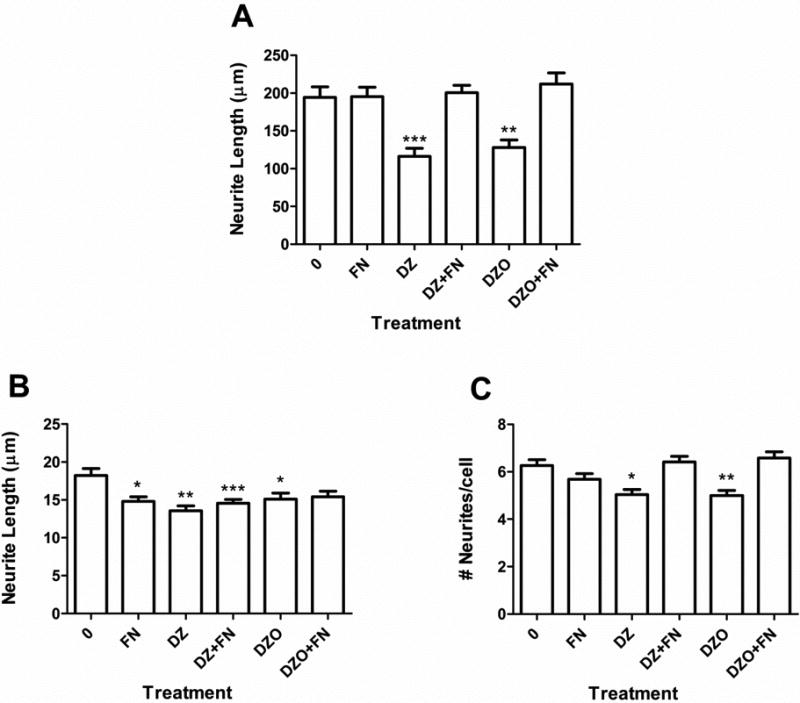
Purified FN (final concentration: 10 μg/mL) was added to the medium of the astrocyte-neuronal co-culture system when newly-prepared neurons were placed on top of previously-treated astrocytes. Results are from 90-100 cells derived from at least three independent experiments and are expressed as means (± SEM). Parameters measured were: (A) Longest neurite length; (B) minor neurite length; and (C) number of neurites per cell. Significantly different from control, *p<0.05, **p<0.01, ***p<0.001.
Effects of DZ and DZO on acetylcholinesterase (AChE) activity in astrocytes
Acetylcholinesterase (AChE; EC 3.1.1.7) activity in astrocytes was measured after 24 h treatment with 1 or 10 μM DZ or DZO. DZO caused greater inhibition of AChE activity than DZ, as expected for the oxon form of the parent compound. Astrocytes treated with 1 μM DZ exhibited AChE activity levels similar to untreated controls (less than 4% inhibition), whereas 10 μM DZ decreased AChE activity by 18%, compared to untreated astrocytes. AChE activity in astrocytes was decreased about 25% relative to control levels after 24 h exposure to either 1 or 10 μM DZO with no significant difference between the two concentrations of DZO used (Fig. 8).
Figure 8. AChE Activity in Astrocytes.
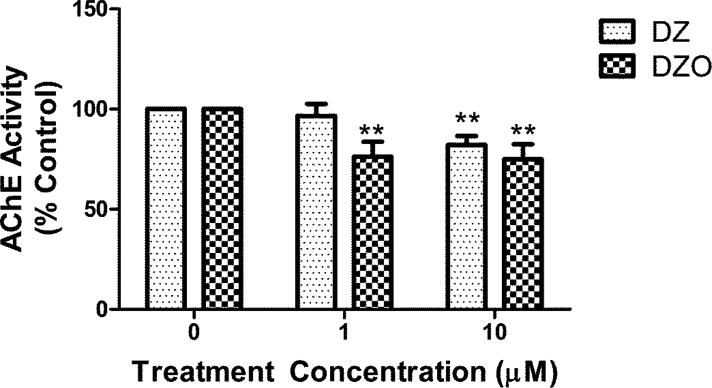
Astrocytes were treated with 1 or 10 μM DZ or DZO for 24 h, after which lysates were collected and AChE activity was measured using a modified Ellman assay. Mean AChE activity (± SEM) in untreated control astrocytes was 3.09 ± 0.33 nmol/min/mg protein. Results are expressed as mean percent control of untreated astrocytes (± SEM) from three independent experiments. Significantly different than untreated control, **p<0.01.
Discussion
The main finding in this study is the ability of the OP insecticide DZ and of its oxygen metabolite, DZO, to interfere with a particular aspect of glial-neuronal interactions, i.e. the ability of astrocytes to promote neuritogenesis in developing hippocampal neurons. Astrocytes are increasingly recognized as important contributors to central nervous system function and development. In addition to playing important roles in maintaining ion homeostasis, forming the blood brain barrier, and regulating glutamate recycling from the synapse (He and Sun, 2007; Nedergaard et al., 2003), astrocytes have more recently been shown to have integral roles in neuronal differentiation, including neuritogenesis (Giordano et al., 2011; Guizzetti et al., 2008; 2010) and synaptogenesis (Christopherson et al., 2005). Our laboratory has previously shown that certain chemicals, including manganese and ethanol, can affect astrocyte functions and indirectly inhibit neurite outgrowth (Giordano et al., 2009; Guizzetti et al., 2010; VanDeMark et al., 2008). In particular, manganese was shown to cause oxidative stress in astrocytes, thereby impairing their ability to promote hippocampal neuron neuritogenesis (Giordano et al., 2009). While work by others has previously demonstrated that DZ and DZO exert direct effects on neurons, including effects on neuronal differentiation (Flaskos et al., 2007; Sidiropoulou et al., 2009; Slotkin et al., 2008), the present study focused on novel aspects of astrocyte-neuronal interactions.
Exposure of rat cortical astrocytes to either DZ or DZO resulted in a significant reduction in neurite lengths of the longest and minor neurites of hippocampal neurons upon co-culture. Neither DZ nor DZO significantly affect astrocyte viability (Table 1). In a previous study (Guizzetti et al. 2005) we had found that the highest concentration of DZ and DZO tested in the present experiments (10 μM) inhibited proliferation of astrocytes by a non-significant 5-12%. It should be noted, however, that in those experiments astrocytes were kept in “proliferating conditions” with 10% serum, while in the present experiments astrocytes were treated with the OPs in the absence of serum, a condition that does not favor astrocyte proliferation (Guizzetti et al. 2005). We thus feel that the possible contribution of an OP-induced decreased number of astrocytes to the observed effects on neurite outgrowth would be minimal, if present at all. The effects of DZ and DZO appear to be due to their ability to alter astrocyte function (specifically fibronectin expression) by mechanisms involving oxidative stress. Indeed, DZ and DZO caused a concentration-dependent increase in ROS production in astrocytes, and two different antioxidants, melatonin and PBN, completely prevented inhibition of the longest neurite outgrowth. Additionally, the inhibitory effects of DZ and DZO were comparable to that of a known oxidant, H2O2, which similarly inhibited astrocytes' ability to foster neurite outgrowth in hippocampal neurons. Interestingly the inhibitory effects of DZ and DZO on minor neurite length were not prevented by antioxidants, potentially suggesting diverse mechanisms of inhibition between axons and dendrites. Reports of differing effects of another OP, chlorpyrifos, on axonal and dendritic growth are documented in the literature (Howard et al., 2005). It should be noted, however, that the effects on minor neurite outgrowth were less dramatic and less consistent than those on longest neurite length. Further studies would be required to determine if these divergent effects are truly a feature of DZ and DZO neurotoxicity.
A number of OPs, including chlorpyrifos and DZ, have been shown to increase oxidative stress in vitro in PC12 neuronal cells (Crumpton et al., 2000; Lee et al., 2012; Slotkin and Seidler, 2009) and primary cerebellar granule cells (Giordano et al., 2007). Importantly, there is also evidence demonstrating that OPs cause oxidative stress in brain in vivo: for example, lactational exposure to malathion caused oxidative stress in the brain, plasma, and erythrocytes of rat pups (Selmi et al., 2012), and DZ increased lipid peroxidation and other markers of oxidative stress in the brain, heart, and spleen of Wistar and Norway rats (Jafari et al., 2012; Yilmaz et al., 2012), and in the brains of a freshwater fish, Oreochromis niloticus (Uner et al., 2006). As with all other OPs, future work on the specific sources of DZ and DZO-induced ROS is needed. Evidence exists demonstrating the ability for OPs to impair various aspects of mitochondrial function, including mitochondrial respiration and energy production (Karami-Mohajeri and Abdollahi, 2013; Massicotte et al., 2005). Mitochondria are both producers and targets of ROS and reactive nitrogen species (RNS); damage to the respiratory chain uncouples the processes that utilize free radicals, which results in even further free radical generation and a detrimental cycle for cellular health and survival (Cardinali et al., 2013; Genova et al., 2004; Raha and Robinson, 2000). For these reasons, exploring mitochondrial integrity and function in astrocytes exposed to DZ and DZO may further explain how these compounds increase oxidative stress, which ultimately results in astrocyte-mediated neurite outgrowth inhibition in hippocampal neurons.
Astrocytes primarily mediate their effects on neuritogenesis and neuronal development by secreting various factors, including ECM proteins, growth factors, and “glio-transmitters” (e.g. D-serine, ATP, and glutamate) (Volterra and Meldolesi, 2005). Previous work by Moore et al. (2009) used a proteomic approach to identify various factors secreted by astrocytes stimulated with the “pro-neuritogenic” cholinergic agonist carbachol (Guizzetti et al., 2008). Several ECM proteins involved in promoting neuronal differentiation were identified, including FN and laminin (Moore et al., 2009). FN in particular plays a primary role in cell adhesion, cell migration, and neurite outgrowth (Kiryushko et al., 2004; Matthiessen et al., 1989; Tom et al., 2004). Highlighting its role in neuronal development, inhibition of FN by function-blocking antibodies strongly inhibits neurite outgrowth, as shown in primary neurons (Guizzetti et al., 2008), as well as in hippocampal slices (Giordano et al., 2011). In the present study we showed that DZ and DZO decrease intracellular and extracellular-bound protein levels of FN in astrocytes. Interestingly, the same antioxidants (melatonin and PBN) that successfully prevented the inhibition of neurite outgrowth in hippocampal neurons also prevented the decreases in FN levels in astrocytes caused by DZ and DZO. The decreases in FN lysate levels are comparable to those found in our prior work with manganese (Giordano, et al., 2009). It is important to note that a known oxidant, H2O2, also caused similar decreases in intracellular protein levels of FN in astrocytes. Additionally, we found that the addition of purified FN to the media of the astrocyte-neuronal co-culture system completely attenuated the effects of DZ and DZO on astrocytes' ability to foster neurite outgrowth in hippocampal neurons. Taken together, these findings implicate astrocyte-derived FN in the observed effects of DZ and DZO on neurite outgrowth.
The modulation of FN by oxidative stress is unclear. FN appears to be modulated by a variety of endogenous and exogenous factors. Ethanol in rat C6 glioma cells (Ren et al., 2000), kainic acid in hippocampal astrocytes (Mahler et al., 1997), interferon-gamma in primary cortical astrocytes (DiProspero et al., 1997), as well as ascorbate (vitamin C) in human skin fibroblasts (Peterszegi et al., 2002) were found to decrease levels of FN. Ethanol and kainic acid, in particular, have been shown to elicit oxidative stress (Giordano et al., 2006; Montoliu et al., 1995), and may potentially modulate FN in this way as well. In addition to oxidative stress, DZ and DZO may be indirectly modulating FN by affecting various other growth factors or cytokines that have been shown to alter levels of the protein. For example, factors such as epidermal growth factor, transforming growth factor-β (TGF- β) 1 and 2, platelet derived growth factor, basic fibroblast growth factor, and interleukin-6 (IL-6) have been shown to alter FN in the brains or neuronal cell types of rodents (Gris et al., 2007; Mahler et al., 1997; Martinez and Gomes, 2002; Pasinetti et al., 1993). Evidence in the literature also suggests the potential for other neurotoxic OPs (e.g. chlorpyrifos, malathion) to induce neuro-inflammation, in some cases specifically up-regulating levels of aforementioned inflammatory agents, including interferon-gamma and IL-6 (Banks and Lein, 2012; Mense et al., 2006; Rodgers and Xiong, 1997). The role of inflammation in the neurotoxicity of DZ/DZO remains unexplored and may be involved in the mechanism of impaired neurite outgrowth by modulation of FN in astrocytes.
Matrix metalloproteinase (MMP) enzymes are another possible modulator of FN under conditions of elevated oxidative stress. MMPs comprise a family of zinc-dependent enzymes that play an important role in ECM turnover and remodeling, as well as in other physiologic processes in the CNS, including tissue morphogenesis, wound-healing, neurite outgrowth, and neuro-inflammation (Yong et al., 2001). MMP-2 and -9, in particular, can bind and degrade FN (Wang and Lai, 2013; Watanabe et al., 2000; Woessner, 1991). These same MMPs are also up-regulated in response to oxidative stress in the brain (Lin et al., 2012; Skowronska et al., 2012). Lin and colleagues (2012) specifically demonstrate that ROS increase MMP-9 in astrocytes and rat brain tissue. DZ and DZO may increase members of the MMP family by increasing oxidative stress, and subsequently modulate FN in this manner; further study is needed to explore this possible mechanism.
The finding that the parent compound, DZ, elicits oxidative stress and inhibits astrocyte-mediated neurite outgrowth indicates that it can cause neurotoxic effects on its own. It is important to note that neurite outgrowth was inhibited as a result of co-incubation with astrocytes treated with an order of magnitude lower concentration of DZO. There is a possibility, then, that contamination of the parent compound by small amounts of the oxon, or biotransformation of DZ to DZO in the astrocytes could have contributed to the effect of DZ on ROS formation and inhibition of neurite outgrowth. Using AChE inhibition as a proxy for DZ biotransformation to DZO in astrocytes, however, may suggest otherwise. Unlike the oxon form of OPs, the parent compound does not affect AChE activity directly; thus, inhibition of AChE activity in the astrocytes is thought to occur by the presence of DZO, either by biotransformation or oxon contamination of the parent compound. Astrocytes treated with DZ exhibited a slight decrease in AChE activity, suggesting that there may be a small amount of oxon found in the DZ-treated astrocytes. DZO exposure caused slightly greater AChE inhibition in astrocytes at the concentrations tested, though no clear concentration-response was observed (Fig. 8). The reason for this apparent “saturation” of AChE inhibition is not clear. Nevertheless, AChE inhibition is not likely to be the key mediator in the process of OP-induced inhibition of neurite outgrowth, for two reasons: first, the effects of DZ and DZO on astrocytes-mediated inhibition of neurite outgrowth are completely prevented by antioxidants. Seeing as antioxidants should have no bearing on AChE activity, the effects of these compounds on neurite outgrowth would likely not have been prevented by antioxidants if these mechanisms were governed by AChE inhibition. Second, the parent compound increases ROS production in astrocytes and causes astrocyte-mediated inhibition of neurite outgrowth in a similar manner to the oxon, without the same extent of AChE inhibition.
In summary, the findings of this study indicate that through a mechanism of oxidative stress, DZ and DZO are able to inhibit neurite outgrowth, in part by decreasing FN levels in astrocytes. Given the ubiquitous use of OPs in conjunction with the suspected involvement of oxidative stress in various diseases, these findings support further investigations into the role of OP-induced oxidative stress in the neurotoxic mechanisms of these compounds.
Highlights.
Diazinon (DZ) and diazoxon (DZO) inhibit astrocyte-mediated neurite outgrowth in rat hippocampal neurons
Oxidative stress is involved in inhibition of neuritogenesis by DZ and DZO
DZ and DZO decrease expression of the neuritogenic factor fibronectin in astrocytes
Exogenous fibronectin antagonizes the effect of DZ and DZO on neuritogenesis
Acknowledgments
This study was supported in part by the Center for Child Environmental Health Risk Research (P01ES009601), the Center for Ecogenetics and Environmental Health (P30ES007033), and the Environmental Toxicology and Pathology (EP/T) Training Grant (T32 ES007032-35). We thank Dr. Judit Marsillach-Lopez for her assistance with the AChE measurements, and Dr. Gennaro Giordano for his overall guidance in the co-culture experiments.
Footnotes
Conflict of Interest: The authors have no competing interest and financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATSDR (Agency of Toxic Substances and Disease Registry) Toxicological profile for Diazinon. Atlanta, GA: 2008. [Google Scholar]
- Axelrad JC, Howard CV, McLean WG. The effects of acute pesticide exposure on neuroblastoma cells chronically exposed to diazinon. Toxicology. 2003;185:67–78. doi: 10.1016/s0300-483x(02)00592-9. [DOI] [PubMed] [Google Scholar]
- Banks CN, Lein PJ. A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology. 2012;33:575–584. doi: 10.1016/j.neuro.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Barr DB, Bravo R, Weerasekera G, Caltabiano LM, Whitehead RD, Olsson AO, Caudill SA, Schober SE, Pirkle JL, Sampson EJ, Jackson RJ, Needham LL. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the US population. Environ Health Perspect. 2004;112:186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer P, Canales RA, Ferguson AC, Eskenazi B, Leckie JO, Bradman A. Organophosphate Pesticide and Exposure Route Contribution to Aggregate and Cumulative Dose for Young Farmworker Children. Epidemiology. 2008;19:S264–S264. doi: 10.3390/ijerph9010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80:1326–1338. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. Attention-Deficit/Hyperactivity Disorder and Urinary Metabolites of Organophosphate Pesticides. Pediatrics. 2010;125:E1270–E1277. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized Survival of Hippocampal-Neurons in B27-Supplemented Neurobasal™, a New Serum-Free Medium Combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Cardinali DP, Pagano ES, Bernasconi PAS, Reynoso R, Scacchi P. Melatonin and mitochondrial dysfunction in the central nervous system. Horm Behav. 2013;63:322–330. doi: 10.1016/j.yhbeh.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CCA, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ, Slotkin TA. Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Dev Brain Res. 2000;121:189–195. doi: 10.1016/s0165-3806(00)00045-6. [DOI] [PubMed] [Google Scholar]
- Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011;7:504–511. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiProspero NA, Meiners S, Geller HM. Inflammatory cytokines interact to modulate extracellular matrix and astrocytic support of neurite outgrowth. Exp Neurol. 1997;148:628–639. doi: 10.1006/exnr.1997.6700. [DOI] [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, Coyle J, McKhann G, Mobley WC, Nadel L, Neubert D, Schulte-Hermann R, Spencer PS. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol. 2008;38:1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- EPA (U.S. Environmental Protection Agency) Pesticides Industry Sales and Usage: 2006-2007 Market Estimates. Washington, D.C.: 2011. [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NA. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Persp. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Rosas LG, Marks AR, Bradman A, Harley K, Holland N, Johnson C, Fenster L, Barr DB. Pesticide toxicity and the developing brain. Basic Clin Pharmacol. 2008;102:228–236. doi: 10.1111/j.1742-7843.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- Fenske RA, Kedan G, Lu C, Fisker-Andersen JA, Curl CL. Assessment of organophosphorous pesticide exposures in the diets of preschool children in Washington State. J Expo Sci Environ Epidemiol. 2002;12:21. doi: 10.1038/sj.jea.7500197. [DOI] [PubMed] [Google Scholar]
- Flaskos J, Harris W, Sachana M, Munoz D, Tack J, Hargreaves AJ. The effects of diazinon and cypermethrin on the differentiation of neuronal and glial cell lines. Toxicol Appl Pharmacol. 2007;219:172–180. doi: 10.1016/j.taap.2006.10.033. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, Dalla Bernardina B, Bonassi S. Oxidative stress-related biomarkers in autism: Systematic review and meta-analyses. Free Radic Biol Med. 2012;52:2128–2141. doi: 10.1016/j.freeradbiomed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Genova ML, Pich MM, Bernacchia A, Bianchi C, Biondi A, Bovina C, Falasca AI, Formiggini G, Castelli GP, Lenaz G. The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann N Y Acad Sci. 2004;1011:86–100. doi: 10.1007/978-3-662-41088-2_10. [DOI] [PubMed] [Google Scholar]
- Giordano G, Afsharinejad Z, Guizzetti M, Vitalone A, Kavanagh TJ, Costa LG. Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency. Toxicol Appl Pharmacol. 2007;219:181–189. doi: 10.1016/j.taap.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Giordano G, Guizzetti M, Dao K, Mattison HA, Costa LG. Ethanol impairs muscarinic receptor-induced neuritogenesis in rat hippocampal slices: Role of astrocytes and extracellular matrix proteins. Biochem Pharmacol. 2011;82:1792–1799. doi: 10.1016/j.bcp.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G, Pizzurro D, VanDeMark K, Guizzetti M, Costa LG. Manganese inhibits the ability of astrocytes to promote neuronal differentiation. Toxicol Appl Pharm. 2009;240:226–235. doi: 10.1016/j.taap.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Giordano G, White CC, McConnachie LA, Fernandez C, Kavanagh TJ, Costa LG. Neurotoxicity of domoic acid in cerebellar granule neurons in a genetic model of glutathione deficiency. Mol Pharmacol. 2006;70:2116–2126. doi: 10.1124/mol.106.027748. [DOI] [PubMed] [Google Scholar]
- Gris P, Tighe A, Levin D, Sharma R, Brown A. Transcriptional regulation of scar gene expression in primary astrocytes. Glia. 2007;55:1145–1155. doi: 10.1002/glia.20537. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Inhibition of muscarinic receptor-stimulated glial cell proliferation by ethanol. J Neurochem. 1996;67:2236–2245. doi: 10.1046/j.1471-4159.1996.67062236.x. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Pathak S, Giordano G, Costa LG. Effect of organophosphates and their metabolites on astroglial cell proliferation. Toxicology. 2005;215:182–190. doi: 10.1016/j.tox.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Moore NH, Giordano G, Costa LG. Modulation of Neuritogenesis by Astrocyte Muscarinic Receptors. J Biol Chem. 2008;283:31884–31897. doi: 10.1074/jbc.M801316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizzetti M, Moore NH, Giordano G, VanDeMark KL, Costa LG. Ethanol Inhibits Neuritogenesis Induced by Astrocyte Muscarinic Receptors. Glia. 2010;58:1395–1406. doi: 10.1002/glia.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Sun YE. Glial cells more than support cells? Int J Biochem Cell Biol. 2007;39:661–665. doi: 10.1016/j.biocel.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Howard AS, Bucelli R, Jett DA, Bruun D, Yang DR, Lein PJ. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol Appl Pharmacol. 2005;207:112–124. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Jafari M, Salehi M, Ahmadi S, Asgari A, Abasnezhad M, Hajigholamali M. The role of oxidative stress in diazinon-induced tissues toxicity in Wistar and Norway rats. Toxicol Mech Methods. 2012;22:638–647. doi: 10.3109/15376516.2012.716090. [DOI] [PubMed] [Google Scholar]
- Karami-Mohajeri S, Abdollahi M. Mitochondrial dysfunction and organophosphorus compounds. Toxicol Appl Pharmacol. 2013;270:39–44. doi: 10.1016/j.taap.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Kiryushko D, Berezin V, Bock E. Regulators of neurite outgrowth: role of cell adhesion molecules. Ann N Y Acad Sci. 2004;1014:140–154. doi: 10.1196/annals.1294.015. [DOI] [PubMed] [Google Scholar]
- Lee JE, Park JH, Shin IC, Koh HC. Reactive oxygen species regulated mitochondria-mediated apoptosis in PC12 cells exposed to chlorpyrifos. Toxicol Appl Pharmacol. 2012;263:148–162. doi: 10.1016/j.taap.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Levin E, Timofeeva O, Seidler F, Slotkin T. Long-term cognitive effects of low-level developmental organophosphate pesticide exposure: Divergent effects of chlorpyrifos, diazinon and parathion. Neurotoxicol Teratol. 2008;30:251–251. [Google Scholar]
- Li WF, Costa LG, Richter RJ, Hagen T, Shih DM, Tward A, Lusis AJ, Furlong CE. Catalytic efficiency determines the in-vivo efficacy of PON1 for detoxifying organophosphorus compounds. Pharmacogenetics. 2000;10:767–779. doi: 10.1097/00008571-200012000-00002. [DOI] [PubMed] [Google Scholar]
- Lin CC, Hsieh HL, Shih RH, Chi PL, Cheng SE, Chen JC, Yang CM. NADPH oxidase 2-derived reactive oxygen species signal contributes to bradykinin-induced matrix metalloproteinase-9 expression and cell migration in brain astrocytes. Cell Commun Signal. 2012;10 doi: 10.1186/1478-811X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Fenske RA, Simcox NJ, Kalman D. Pesticide Exposure of Children in an Agricultural Community: Evidence of Household Proximity to Farmland and Take Home Exposure Pathways. Environ Res. 2000;84:290–302. doi: 10.1006/enrs.2000.4076. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz-Hussain A. Role of oxidative stress in organophosphate insecticide toxicity -Short review. Pestic Biochem Physiol. 2010;98:145–150. [Google Scholar]
- Mahler M, Ben-Ari Y, Represa A. Differential expression of fibronectin, tenascin-C and NCAMs in cultured hippocampal astrocytes activated by kainate, bacterial lipopolysaccharide or basic fibroblast growth factor. Brain Res. 1997;775:63–73. doi: 10.1016/s0006-8993(97)00901-3. [DOI] [PubMed] [Google Scholar]
- Martinez R, Gomes FCA. Neuritogenesis induced by thyroid hormone-treated astrocytes is mediated by epidermal growth factor/mitogen-activated protein kinase-phosphatidylinositol 3-kinase pathways and involves modulation of extracellular matrix proteins. J Biol Chem. 2002;277:49311–49318. doi: 10.1074/jbc.M209284200. [DOI] [PubMed] [Google Scholar]
- Massicotte C, Knight K, Van Der Schyf CJ, Jortner BS, Ehrich M. Effects of organophosphorus compounds on ATP production and mitochondrial integrity in cultured cells. Neurotox Res. 2005;7:203–217. doi: 10.1007/BF03036450. [DOI] [PubMed] [Google Scholar]
- Matés JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- Matthiessen HP, Schmalenbach C, Muller HW. Astroglia-released neurite growth-inducing activity for embryonic hippocampal neurons is associated with laminin bound in a sulfated complex and free fibronectin. Glia. 1989;2:177–188. doi: 10.1002/glia.440020307. [DOI] [PubMed] [Google Scholar]
- Mense SM, Sengupta A, Lan CG, Zhou M, Bentsman G, Volsky DJ, Whyatt RM, Perera FP, Zhang L. The common insecticides cyfluthrin and chlorpyrifos alter the expression of a subset of genes with diverse functions in primary human astrocytes. Toxicol Sci. 2006;93:125–135. doi: 10.1093/toxsci/kfl046. [DOI] [PubMed] [Google Scholar]
- Montoliu C, Sanchotello M, Azorin I, Burgal M, Valles S, Renaupiqueras J, Guerri C. Ethanol increases cytochrome P4502E1 and induces oxidative stress in astrocytes. J Neurochem. 1995;65:2561–2570. doi: 10.1046/j.1471-4159.1995.65062561.x. [DOI] [PubMed] [Google Scholar]
- Moore NH, Costa LG, Shaffer SA, Goodlett DR, Guizzetti M. Shotgun proteomics implicates extracellular matrix proteins and protease systems in neuronal development induced by astrocyte cholinergic stimulation. J Neurochem. 2009;108:891–908. doi: 10.1111/j.1471-4159.2008.05836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser VC, Chanda SM, Mortensen SR, Padilla S. Age- and gender-related differences in sensitivity to chlorpyrifos in the rat reflect developmental profiles of esterase activities. Toxicol Sci. 1998;46:211–222. doi: 10.1006/toxs.1998.2526. [DOI] [PubMed] [Google Scholar]
- Moser VC, Padilla S. Age- and gender-related differences in the time course of behavioral and biochemical effects produced by oral chlorpyrifos in rats. Toxicol Appl Pharmacol. 1998;149:107–119. doi: 10.1006/taap.1997.8354. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, Nichols NR, Tocco G, Morgan T, Laping N, Finch CE. Transforming Growth-Factor-Beta-1 and Fibronectin Messenger-Rna in Rat-Brain -Responses to Injury and Cell-Type Localization. Neuroscience. 1993;54:893–907. doi: 10.1016/0306-4522(93)90583-2. [DOI] [PubMed] [Google Scholar]
- Peterszegi G, Dagonet FB, Labat-Robert J, Robert L. Inhibition of cell proliferation and fibronectin biosynthesis by Na ascorbate. Eur J Clin Invest. 2002;32:372–380. doi: 10.1046/j.1365-2362.2002.00992.x. [DOI] [PubMed] [Google Scholar]
- Pope CN, Liu J. Age-related differences in sensitivity to organophosphorus pesticides. Environ Toxicol Pharmacol. 1997;4:309–314. doi: 10.1016/s1382-6689(97)10029-1. [DOI] [PubMed] [Google Scholar]
- Potashkin JA, Meredith GE. The role of oxidative stress in the dysregulation of gene expression and protein metabolism in neurodegenerative disease. Antioxid Redox Signal. 2006;8:144–151. doi: 10.1089/ars.2006.8.144. [DOI] [PubMed] [Google Scholar]
- Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- Ranjbar A, Solhi H, Mashayekhi FJ, Susanabdi A, Rezaie A, Abdollahi M. Oxidative stress in acute human poisoning with organophosphorus insecticides; a case control study. Environ Toxicol Pharmacol. 2005;20:88–91. doi: 10.1016/j.etap.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R. 7-Year Neurodevelopmental Scores and Prenatal Exposure to Chlorpyrifos, a Common Agricultural Pesticide. Environ Health Perspect. 2011;119:1196–1201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren LQ, Garrett DK, Syapin M, Syapin PJ. Differential fibronectin expression in activated C6 glial cells treated with ethanol. Mol Pharmacol. 2000;58:1303–1309. doi: 10.1124/mol.58.6.1303. [DOI] [PubMed] [Google Scholar]
- Rodgers K, Xiong SQ. Effect of administration of malathion for 90 days on macrophage function and mast cell, degranulation. Toxicol Lett. 1997;93:73–82. doi: 10.1016/s0378-4274(97)00069-6. [DOI] [PubMed] [Google Scholar]
- Roegge C, Timofeva O, Seidler F, Slotkin TA, Levin ED. Persisting effects of early postnatal diazinon exposure on emotional reactivity in rats. Neurotoxicol Teratol. 2006;28:709–709. [Google Scholar]
- Rohlman DS, Anger WK, Lein PJ. Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. Neurotoxicology. 2011;32:268–276. doi: 10.1016/j.neuro.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarawickrema N, Pathmeswaran A, Wickremasinghe R, Peiris-John R, Karunaratna M, Buckley N, Dawson A, de Silva J. Fetal effects of environmental exposure of pregnant women to organophosphorus compounds in a rural farming community in Sri Lanka. Clin Toxicol (Phila) 2008;46:489–495. doi: 10.1080/15563650701837030. [DOI] [PubMed] [Google Scholar]
- Selmi S, El-Fazaa S, Gharbi N. Oxidative stress and cholinesterase inhibition in plasma, erythrocyte and brain of rats' pups following lactational exposure to malathion. Environ Toxicol Pharmacol. 2012;34:753–760. doi: 10.1016/j.etap.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Sidiropoulou E, Sachana M, Flaskos J, Harris W, Hargreaves AJ, Woldehiwet Z. Diazinon oxon affects the differentiation of mouse N2a neuroblastoma cells. Arch Toxicol. 2009;83:373–380. doi: 10.1007/s00204-008-0339-1. [DOI] [PubMed] [Google Scholar]
- Skowronska M, Zielinska M, Wojcik-Stanaszek L, Ruszkiewicz J, Milatovic D, Aschner M, Albrecht J. Ammonia increases paracellular permeability of rat brain endothelial cells by a mechanism encompassing oxidative/nitrosative stress and activation of matrix metalloproteinases. J Neurochem. 2012;121:125–134. doi: 10.1111/j.1471-4159.2012.07669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Levin ED, Seidler FJ. Neonatal exposure to low doses of diazinon: Long-term effects on neural cell development and acetylcholine systems. Environ Health Perspect. 2008;116:340–348. doi: 10.1289/ehp.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Oliver CA, Seidler FJ. Critical periods for the role of oxidative stress in the developmental neurotoxicity of chlorpyrifos and terbutaline, alone or in combination. Dev Brain Res. 2005;157:172–180. doi: 10.1016/j.devbrainres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Oxidative and Excitatory Mechanisms of Developmental Neurotoxicity: Transcriptional Profiles for Chlorpyrifos, Diazinon, Dieldrin, and Divalent Nickel in PC12 Cells. Environ Health Perspect. 2009;117:587–596. doi: 10.1289/ehp.0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang GM, Rios PG, Kuo SH, Akman HO, Rosoklija G, Tanji K, Dwork A, Schon EA, DiMauro S, Goldman J, Sulzer D. Mitochondrial abnormalities in temporal lobe of autistic brain. Neurobiol Dis. 2013;54:349–361. doi: 10.1016/j.nbd.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburne SK, Juurlink BH. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem. 1996;67:1014–1022. doi: 10.1046/j.1471-4159.1996.67031014.x. [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol Teratol. 2008;30:38–45. doi: 10.1016/j.ntt.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Doller CM, Malouf AT, Silver J. Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J Neurosci. 2004;24:9282–9290. doi: 10.1523/JNEUROSCI.2120-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uner N, Oruc EO, Sevgiler Y, Sahin N, Durmaz H, Usta D. Effects of diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus. Environ Toxicol Pharmacol. 2006;21:241–245. doi: 10.1016/j.etap.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Valcke M, Samuel O, Bouchard M, Dumas P, Belleville D, Tremblay C. Biological monitoring of exposure to organophosphate pesticides in children living in peri-urban areas of the Province of Quebec, Canada. Int Arch Occup Environ Health. 2006;79:568–577. doi: 10.1007/s00420-006-0085-8. [DOI] [PubMed] [Google Scholar]
- VanDeMark KL, Guizzetti M, Giordano G, Costa LG. Ethanol inhibits muscarinic receptor-induced axonal growth in rat hippocampal neurons. Alcohol Clin Exp Res. 2009;33:1945–1955. doi: 10.1111/j.1530-0277.2009.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidyasagar J, Karunakar N, Reddy MS, Rajnarayana K, Surender T, Krishna DR. Oxidative stress and antioxidant status in acute organophosphorous insecticide poisoning. Indian J Pharmacol. 2004;36:76–79. [Google Scholar]
- Viviani B, Corsini E, Galli CL, Marinovich M. Glia increases degeneration of hippocampal neurons through release of tumor necrosis factor-alpha. Toxicol Appl Pharmacol. 1998;150:271–276. doi: 10.1006/taap.1998.8406. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Wang MF, Lai SC. Fibronectin degradation by MMP-2/MMP-9 in the serum of pregnant women and umbilical cord with Toxoplasma gondii infection. J Obstet Gynaecol. 2013;33:370–374. doi: 10.3109/01443615.2013.769501. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Takahashi H, Habu Y, Kamiya-Kubushiro N, Kamiya S, Nakamura H, Yajima H, Ishii T, Katayama T, Miyazaki K, Fukai F. Interaction with Heparin and Matrix Metalloproteinase 2 Cleavage Expose a Cryptic Anti-adhesive Site of Fibronectin. Biochemistry. 2000;39:7138–7144. doi: 10.1021/bi992670r. [DOI] [PubMed] [Google Scholar]
- Woessner JF. Matrix metalloproteinases and their inhibitors in connective-tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- Won YK, Liu J, Olivier K, Zheng Q, Pope CN. Age-related effects of chlorpyrifos on acetylcholine release in rat brain. Neurotoxicology. 2001;22:39–48. doi: 10.1016/s0161-813x(00)00009-7. [DOI] [PubMed] [Google Scholar]
- Yilmaz N, Yilmaz M, Altuntas I. Diazinon-induced brain toxicity and protection by vitamins E plus C. Toxicol Ind Health. 2012;28:51–57. doi: 10.1177/0748233711404035. [DOI] [PubMed] [Google Scholar]
- Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]


