Abstract
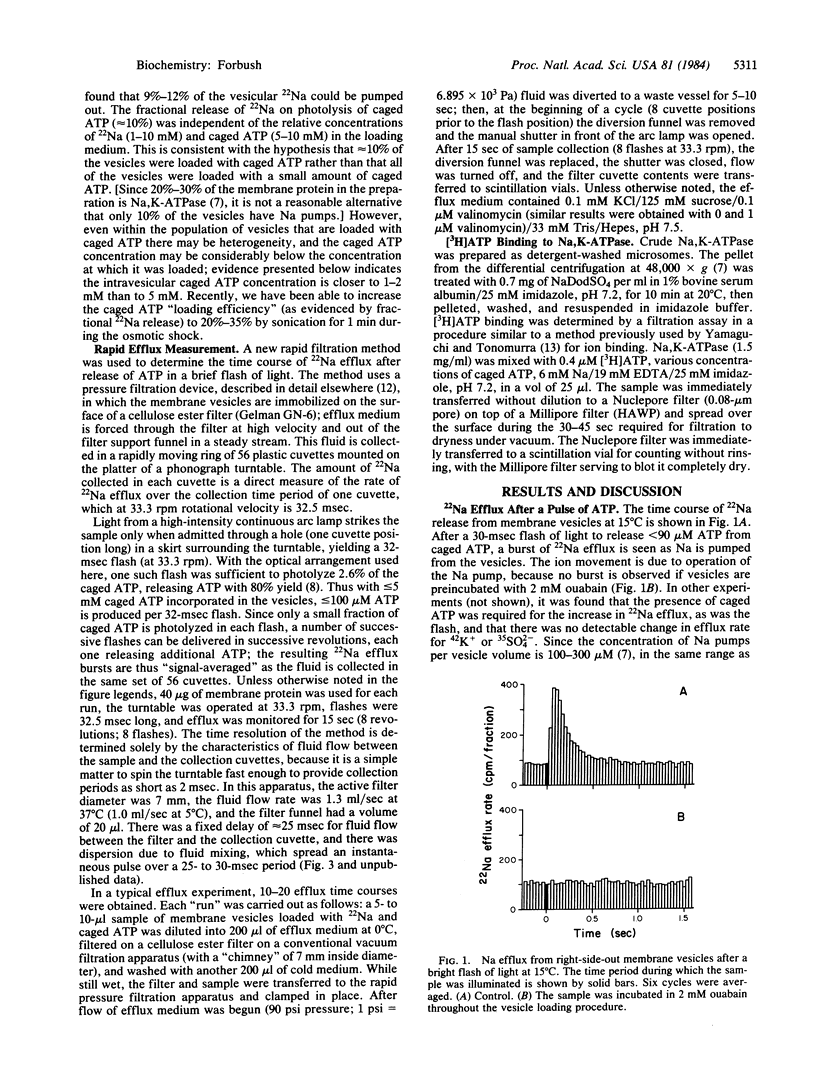
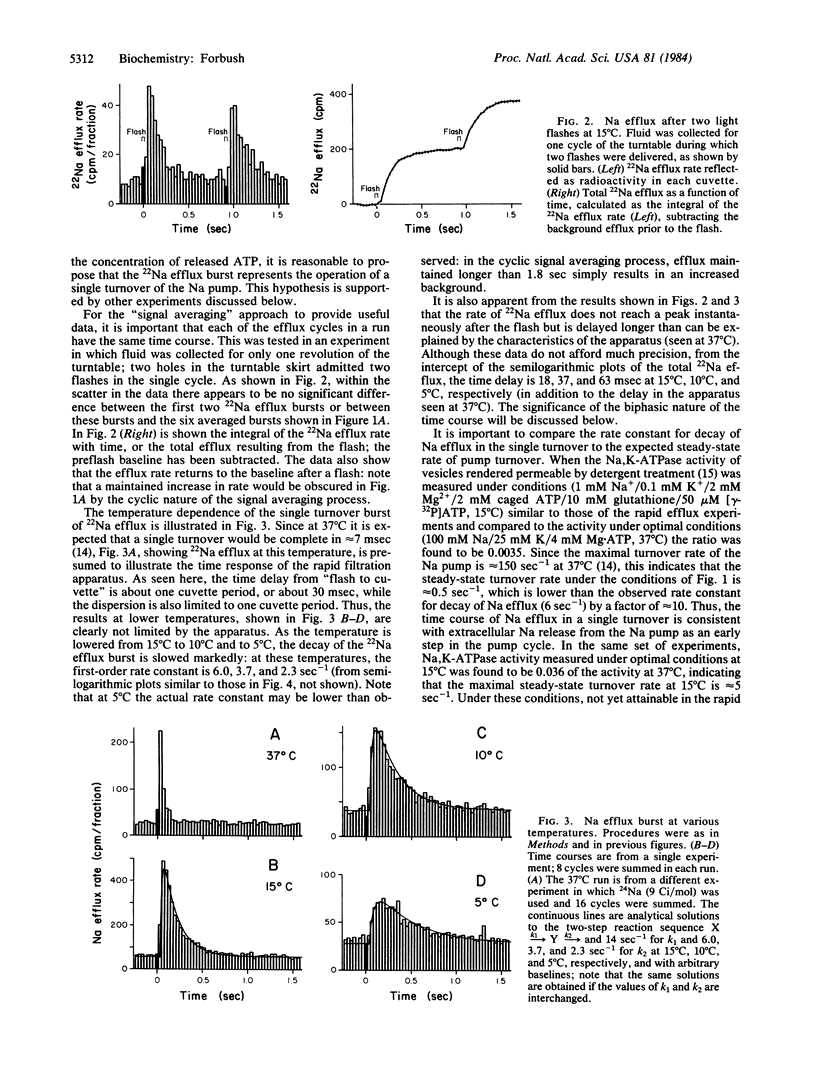
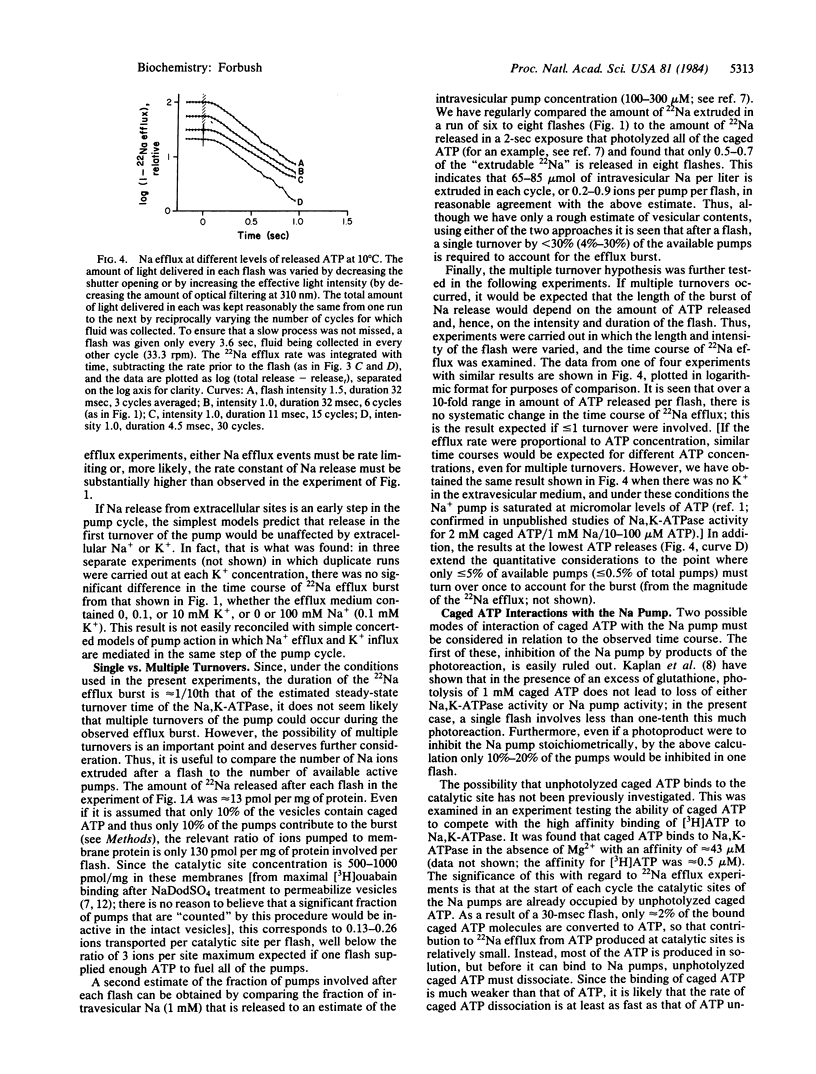
Ouabain-sensitive 22Na efflux from right-side-out membrane vesicles prepared from dog kidney has been examined with a time resolution of 30 msec. The vesicles are preloaded with 22Na and caged ATP [P3-1-(2-nitro)phenylethyl adenosine triphosphate], so that transport by the Na pump can be initiated by light. After a brief illumination, which releases less ATP than the number of catalytic sites, a burst of 22Na extrusion is observed corresponding to a single turnover of the Na pump. By the use of a rapid filtration apparatus, with which a continuous record of the rate of efflux is obtained, it has been possible to resolve the efflux burst in the time range of 20-1500 msec. The rate of efflux rises rapidly, but not instantaneously, to a peak and then decays, with a time constant of approximately equal to 6 sec-1 at 15 degrees C. The time course of Na efflux is unaffected by extracellular K+, as predicted by models of the Na pump in which Na is released early in the cycle. Unphotolyzed caged ATP is found to bind to the catalytic site of Na,K-ATPase, in competition with ATP that is produced in the flash, and the possibility has not been excluded that dissociation of unphotolyzed caged ATP and binding of ATP are involved in the Na efflux time course. It seems most likely that binding of ATP and translocation of 22Na are involved in the increase in the 22Na efflux rate in the single turnover and that the release of transported 22Na from extracellular pump sites limits the slow decay.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cash D. J., Hess G. P. Quenched flow technique with plasma membrane vesicles: acetylcholine receptor-mediated transmembrane ion flux. Anal Biochem. 1981 Mar 15;112(1):39–51. doi: 10.1016/0003-2697(81)90257-8. [DOI] [PubMed] [Google Scholar]
- Forbush B., 3rd An apparatus for rapid kinetic analysis of isotopic efflux from membrane vesicles and of ligand dissociation from membrane proteins. Anal Biochem. 1984 Aug 1;140(2):495–505. doi: 10.1016/0003-2697(84)90200-8. [DOI] [PubMed] [Google Scholar]
- Forbush B., 3rd Assay of Na,K-ATPase in plasma membrane preparations: increasing the permeability of membrane vesicles using sodium dodecyl sulfate buffered with bovine serum albumin. Anal Biochem. 1983 Jan;128(1):159–163. doi: 10.1016/0003-2697(83)90356-1. [DOI] [PubMed] [Google Scholar]
- Forbush B., 3rd Characterization of right-side-out membrane vesicles rich in (Na,K)-ATPase and isolated from dog kidney outer medulla. J Biol Chem. 1982 Nov 10;257(21):12678–12684. [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. The sodium pump. Annu Rev Physiol. 1975;37:13–55. doi: 10.1146/annurev.ph.37.030175.000305. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Isolation and characterization of the components of the sodium pump. Q Rev Biophys. 1974 May;7(2):239–274. doi: 10.1017/s0033583500001426. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H., Forbush B., 3rd, Hoffman J. F. Rapid photolytic release of adenosine 5'-triphosphate from a protected analogue: utilization by the Na:K pump of human red blood cell ghosts. Biochemistry. 1978 May 16;17(10):1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- Karlish S. J., Yates D. W., Glynn I. M. Elementary steps of the (Na+ + K+)-ATPase mechanism, studied with formycin nucleotides. Biochim Biophys Acta. 1978 Jul 7;525(1):230–251. doi: 10.1016/0005-2744(78)90218-8. [DOI] [PubMed] [Google Scholar]
- Märdh S., Post R. L. Phosphorylation from adenosine triphosphate of sodium- and potassium-activated adenosine triphosphatase. Comparison of enzyme-ligand complexes as precursors to the phosphoenzyme. J Biol Chem. 1977 Jan 25;252(2):633–638. [PubMed] [Google Scholar]
- Will H., Blanck J., Smettan G., Wollenberger A. A quench-flow kinetic investigation of calcium ion accumulation by isolated cardiac sarcoplasmic reticulum. Dependence of initial velocity on free calcium ion concentration and influence of preincubation with a protein kinase, MgATP, and cyclic AMP. Biochim Biophys Acta. 1976 Nov 9;449(2):295–303. doi: 10.1016/0005-2728(76)90141-9. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Tonomura Y. Simultaneous binding of three Na+ and two K+ ions to Na+,K+-dependent ATPase and changes in its affinities for the ions induced by the formation of a phosphorylated intermediate. J Biochem. 1979 Aug;86(2):509–523. doi: 10.1093/oxfordjournals.jbchem.a132551. [DOI] [PubMed] [Google Scholar]


