Abstract
The cannabinoid receptor agonist delta9-tetrahydrocannabinol (THC) enhances the antinociceptive effects of mu opioid receptor agonists, raising the possibility of using a combination of THC and opioids for treating pain. This study examined the effects of noncontingent and contingent administration of THC on i.v. heroin self-administration in rhesus monkeys. Self-administration of different unit doses of heroin (0.0001–0.1 mg/kg/infusion) generated a typical inverted U-shaped dose-response curve. In one experiment (n=4), noncontingent THC (0.1–1.0 mg/kg) dose-dependently shifted the heroin dose-response curve downward in three monkeys and slightly leftward in one monkey. In a second experiment (n=4), monkeys could self-administer THC alone (0.0032–0.032 mg/kg/infusion), heroin alone, or a mixture of THC and heroin. THC alone did not maintain responding above that obtained with saline; however, increasing the THC dose with heroin dose-dependently decreased the number of infusions received and the rate of responding, as compared to data that were obtained with heroin alone. These results indicate that THC does not significantly enhance the positive reinforcing effects of heroin, further supporting the view that combining cannabinoid and opioid receptor agonists (e.g., for treating pain) does not increase, and might decrease, the abuse liability of the individual drugs.
Keywords: Heroin, THC, self-administration, rhesus monkey
Introduction
Chronic pain affects more Americans than cancer, heart disease, and diabetes combined (Institute of Medicine, 2011) and the effective treatment of pain remains a significant unmet public health problem. Mu opioid receptor agonists (e.g., oxycodone) remain the drugs of choice for treating moderate to severe pain, although the clinical use of opioids is limited both by unwanted effects (e.g., constipation, abuse) and by the ineffectiveness of opioids in some patients (Gutstein and Akil, 2005). Despite many years of research and drug development and growing concerns regarding the increased abuse of “prescription” opioids, few effective options are available for treating chronic pain.
The cannabinoid system is a target for the development of new and possibly more effective drugs for treating pain (Hosking and Zajicek 2008; Guindon and Hohmann 2009; Rahn and Hohmann, 2009; Welch, 2009). For example, the cannabinoid receptor agonist delta9-tetrahydrocannabinol (THC) has antinociceptive effects in several nonhuman species including mice (Welch and Stevens 1992), rats (Smith et al. 1998; Houser et al. 2000, De Vry et al. 2004), and rhesus monkeys (Vivian et al. 1998) and in various different models of nociception. The effectiveness of cannabinoid receptor agonists for treating pain in humans is less well established (see Kraft, 2012 for review). However, in addition to any therapeutic potential cannabinoid receptor agonists might exert when administered alone (e.g., Sativex®), it is possible that by combining a cannabinoid and an opioid, smaller doses of each drug could be administered while still achieving an adequate therapeutic effect. The need for smaller doses of each drug might also reduce the likelihood of adverse effects, as compared with what occurs when larger doses of each drug are administered alone. Several studies have examined the effects of cannabinoid and opioid receptor agonists administered in combination. For example, acute administration of THC enhances the antinociceptive effects of morphine in mice (Welch and Stevens 1992; Welch et al. 1995), rats (Finn et al. 2004; Smith et al. 2007), and rhesus monkeys (Li et al. 2008). However, because cannabinoids (i.e., marijuana) and opioids (e.g., heroin) are each widely abused, one potential concern is that combinations of cannabinoids and opioids might result in greater abuse and dependence liability than either drug alone.
Several studies have reported interactions between cannabinoid and opioid receptor agonists and antagonists, although the data are not entirely consistent across laboratories and species. For example, in humans, naltrexone increases (Haney et al. 2003; Cooper and Haney 2010), has no effect (Greenwald and Stitzer 2000; Wachtel and de Wit 2000), or decreases (Haney 2007) the subjective effects of THC, depending on the history of marijuana use. Neither morphine nor heroin alters the discriminative stimulus effects of THC in rhesus monkeys, although THC markedly attenuates the discriminative stimulus effects of morphine and the morphine-like discriminative stimulus effects of heroin (Li et al., 2008); these effects in monkeys are not consistent with studies showing that several effects of cannabinoid and opioid receptor agonists are mutually enhanced in rats (e.g., Solinas et al., 2005).
The current study examined potential interactions between THC and heroin using an intravenous (i.v.) self-administration procedure in rhesus monkeys. Most studies that have examined interactions between cannabinoid and opioid receptor agonists have administered one or both of the drugs noncontingently (i.e., by the experimenter), although the contingencies of drug administration can significantly affect the results obtained (Dworkin et al. 1995; Galici et al. 2000; Lecca et al. 2007). In this study, heroin self-administration was studied after a noncontingent injection of THC and also when THC and heroin were combined in the same solution with the drug mixture administered contingently.
Materials and methods
Subjects
Six rhesus monkeys (Macaca mulatta), 3 males (JA, AB, PE) and 3 females (MI, BE, AY), weighed between 6 and 10 kg and were housed individually in stainless-steel cages with free access to water. Monkeys received primate chow (High Protein Monkey Diet; Harlan Teklad, Madison, WI), fresh fruit, and peanuts daily in amounts sufficient to maintain normal age- and gender-appropriate body weights. Monkeys were maintained under a 14/10 h light/dark cycle with lights on at 0600 h. Animals used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and the 1996 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animals Resources on Life Sciences, National Research Council, National Academy of Sciences).
Surgery
Monkeys were anesthetized with 10 mg/kg of ketamine (s.c., Fort Dodge Laboratories, Fort Dodge, IA) prior to intubation, after which anesthesia was maintained by halothane (Butler Animal Health Supply, Grand Prairie, TX) and ventilation was sustained by the delivery of oxygen at a flow rate of 2 l/min. A polyurethane catheter (SIMS Deltec Inc., St. Paul, MN) was implanted in the jugular or femoral vein and connected to a s.c. vascular access port (Access Technologies, Skokie, IL; MA and TO) according to methods described elsewhere (Wojnicki et al. 1994). The catheter was exteriorized in the midscapular region for monkey JA who wore a jacket (Lomir Biomedical Inc., Malone, NY); the access port, connected to the catheter, was stored in a Velcro pocket in the jacket.
Apparatus
During experimental sessions, subjects were seated in commercially available chairs (Model R001; Primate Products, Miami, FL) that were placed in ventilated, sound-attenuating chambers. Response panels located in each chamber contained response levers and stimulus lights that could be illuminated red or green. Drugs were delivered i.v. by connecting vascular ports to a 185-cm (7.45-ml) extension set (Abbott Laboratories, Stone Mountain, GA) via 20-g Huber-point needles (Access Technologies, Skokie, IL). The opposing end of the extension set was connected to a 30-ml syringe that was mounted in a syringe driver (Razel Scientific Instruments, Inc., Stamford, CT) located outside the chambers. An interface (Med Associates, Inc., East Fairfield, VT) connected panels to a computer which controlled experimental events and recorded data.
Self-administration procedure
Monkeys were trained to self-administer heroin under a fixed ratio 30 schedule of i.v. drug administration. When the green light located above the active lever (right lever for BE and left lever for other monkeys) was illuminated, 30 responses on the active lever resulted in the illumination of red lights for 2 s and an infusion of drug or vehicle followed by a 180 s timeout period during which the chamber was dark and lever presses had no programmed consequence. Responses on the inactive lever were recorded but had no programmed consequence and sessions ended after 90 min.
The effects of noncontingent THC on heroin self-administration were evaluated in 4 monkeys by determining dose-effect curves for heroin alone and in combination with THC. A single dose of heroin (0.0001–0.1 mg/kg/infusion) was available in each daily session, and this dose remained the same until it was studied in combination with all doses of THC. Studies with a particular dose of heroin began with at least 3 consecutive sessions during which that dose was available in the absence of other treatment. When the number of infusions received per session did not exceed ± 20% of the average number of infusions received for the last 3 sessions, studies commenced with THC whereby monkeys received a noncontingent s.c. injection of THC (0.1, 0.32 or 1 mg/kg) 60 min before a single session. In subsequent sessions, the same dose of heroin was available for self-administration in the session until the same criteria (number of infusions received not exceeding ± 20% for 3 sessions) were satisfied and then a different dose of (noncontingent) THC was studied in a single session. After THC testing was completed, saline was available for self-administration (i.e., extinction) until the same criteria were satisfied. Next, a different dose of heroin was available for self-administration, first alone then in combination with noncontingent THC, according to the strategy described above. The order in which different doses of THC were studied was mixed among the 4 monkeys and for an individual monkey across two determinations for each dose. The same doses of THC were also studied when only saline was available for self-administration, using the same criteria. Two monkeys that participated in the study with noncontingent THC (AB and MI) and 2 other monkeys (BE and AY) participated in a second study examining the effects of THC administered contingently, either alone or with different doses of heroin. The approach (e.g., each dose of heroin was studied alone prior to being studied in combination with THC) and the criteria for this study were identical to the study with noncontingent THC, with the exception that different doses of THC (0.0032–0.032 mg/kg/infusion) were mixed in the same syringe with saline or heroin.
Data analyses
The mean (± SEM) number of infusions received per session and the mean (± SEM) rate of responding (i.e., responses/second on the active lever) were plotted as a function of the unit dose of heroin for individual monkeys. Heroin control dose-response curves and dose-response curves for heroin and THC administered together (contingently) represent the average number of infusions and the average rate of responding for three consecutive sessions during which self-administration was stable. For the study on noncontingent THC administration, each data point represents the average number of infusions and response rate from two determinations. The experiments were conducted using a single-subject design, replicated in several different subjects. This design was chosen because it allows detection of individual differences in sensitivity to drug effects (e.g., Maguire et al, 2012). Because the design was replicated for each subject, and because the order in which treatments were tested was nonsystematic, the results were analyzed for each subject by a two-way (i.e., heroin dose, THC dose) ANOVA (GraphPad Prism 5.0 for Windows; GraphPad Software Inc., San Diego, California, USA) followed by Dunnett’s test to compare each dose of THC with vehicle. Data obtained in a single-subject design replicated for a number of subjects can often be analyzed also by one repeated-measures ANOVA, to examine generality across subjects. Ideally, both an ANOVA for each subject and one repeated-measures ANOVA per group should be conducted (Pashler and Wixted, 2002). However, because of individual differences in sensitivity to the reinforcing effects of heroin, the dose range over which drug effects were examined was not the same for each subject, which precluded analysis by one repeated-measures ANOVA.
Drugs
The compounds studied were heroin hydrochloride and the levo enantiomer of delta9-tetrahydrocannabinol (THC; 100 mg/ml in absolute ethanol; The Research Technology Branch, National Institute on Drug Abuse, Rockville, MD, US). Heroin was dissolved with sterile 0.9% saline. THC was dissolved in a 1:1:18 mixture of absolute ethanol, Emulphor-620 (Rhone-Poulenc Inc., Princeton, NJ, USA), and 0.9% saline. Heroin was administered i.v. THC was administered s.c. for the noncontingent study and i.v. for the contingent study. For the self-administration study, a concentration of 5 mg/ml THC was diluted with 0.9% saline to the appropriate concentrations. Infusion volumes varied from 0.38 – 0.66 ml/infusion among the monkeys and were adjusted daily according to body weights.
Results
Heroin maintained self-administration responding that was greater than responding maintained by saline, as indicated by differences in the number of infusions received in the 90-min session (open circles, all panels, Figs 1a, 1b, 2a, and 2b). With increasing unit doses of heroin, responding (i.e., number of infusions received and rate of responding) increased then, with larger unit doses, decreased, resulting in an inverted U-shaped dose-response curve. The average number (range) of infusions received in 3 sessions during which saline was available was 2 (2–2) for monkey JA, 8.3 (7–10) for monkey AB, 7(6–8) for monkey MI, 1.3 (0–2) for monkey PE, 0.7 (0–1) for monkey BE, and 2 (1–3) for monkey AY. The average rate of responding for saline was 0.01 ± 0.01, 0.07 ± 0.01, 0.05 ± 0.01, 0.01 ± 0.01, 0.01 ± 0.01, and 0.02 ± 0 .01 responses per second for monkeys JA, AB, MI, PE, BE, and AY, respectively. In the absence of other treatment, the largest number of infusions of heroin received occurred at a unit dose of 0.0032 mg/kg/infusion in three monkeys (AB, PE and BE) and at a unit dose of 0.01 mg/kg/infusion in three other monkeys (JA, MI and AY), resulting in an average maximum of between 14.3 (monkey AY) and 27 (monkey JA) infusions of heroin per session. Maximum response rates for heroin among these six monkeys varied from 0.16 ± 0.01 responses per second for AY (0.01 mg/kg/infusion) to 2.69 ± 0.10 responses per second for JA (0.01 mg/kg/infusion).
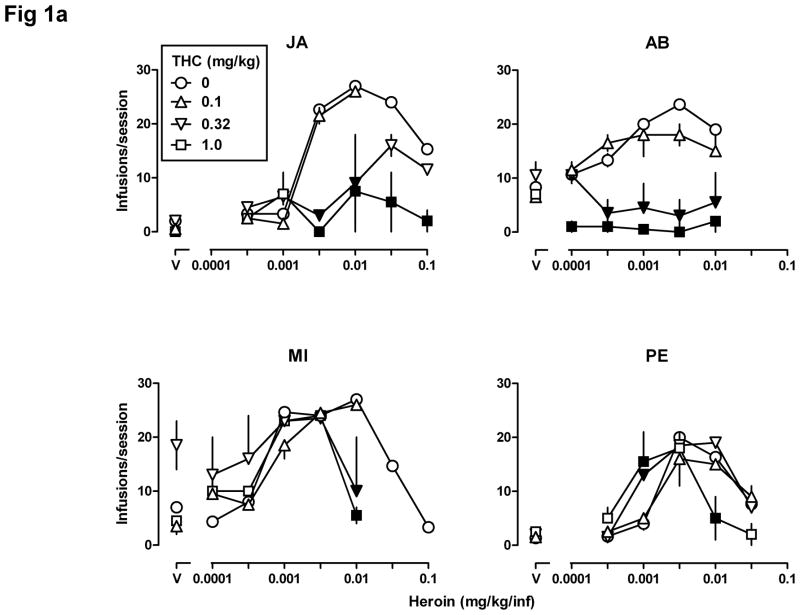
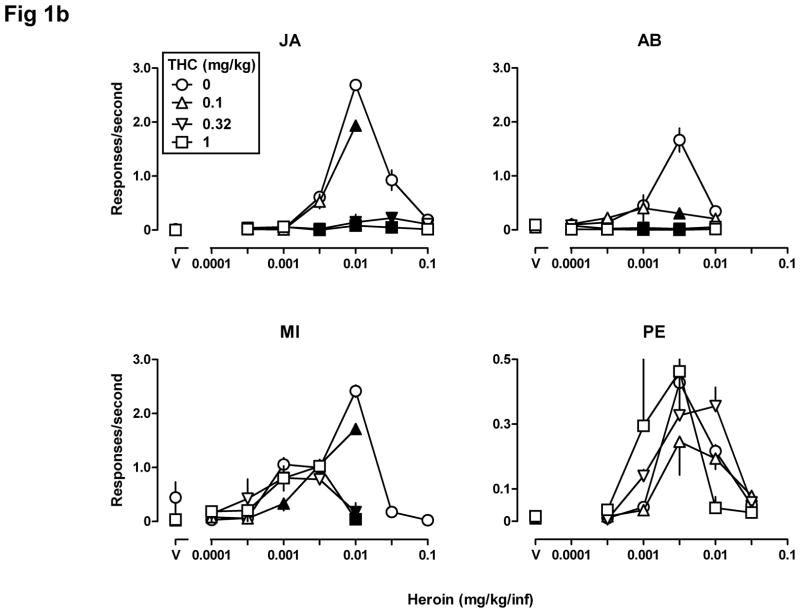
Fig 1.
Self-administration (n=4) of heroin alone (0.0001–0.1 mg/kg/infusion, i.v.) and after a noncontingent injection of THC (0.1, 0.32, and 1.0 mg/kg, s.c.) 60 min before the session. The number of infusions received ± SEM (Fig 1a) and the rate of responding in responses per second ± SEM (Fig 1b) in the 90-min session (ordinates) are plotted as a function of unit dose of drug (abscissae) expressed in mg/kg/infusion. Filled symbols indicate that the effect obtained after pretreatment with THC was significantly different (p<0.05) compared with the effect obtained with heroin alone. “V” = vehicle. Note the different ordinate scales in Fig 1b.
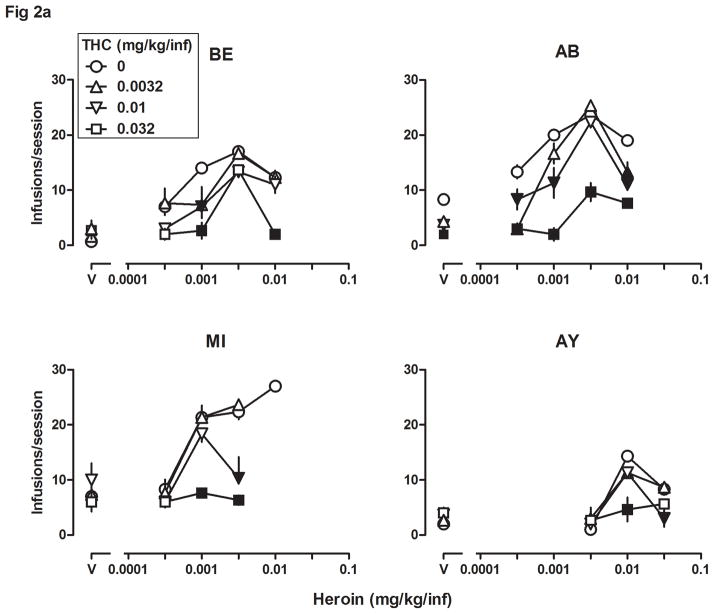
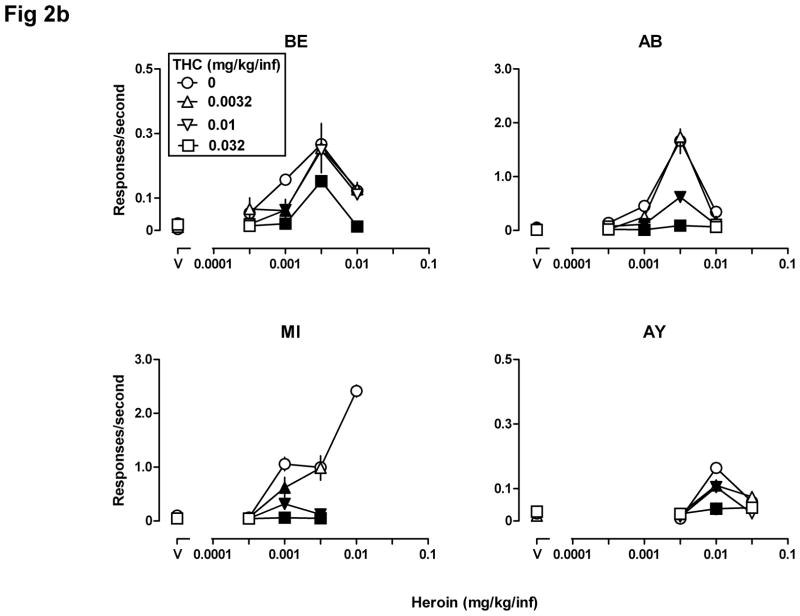
Fig 2.
Self-administration (n=4) of heroin alone (0.0032–0.032 mg/kg/infusion), THC alone (0.0032–0.032 mg/kg/infusion), and the combination of heroin and THC. See Fig 1 for other details. Note the different ordinate scales in Fig 2b.
Figs 1a and 1b show the number of infusions received per session and the average rate of responding (in the same sessions), respectively, when a noncontingent injection of THC was administered prior to a self-administration session. When THC was administered noncontingently as a pretreatment, the dose-effect curves for heroin self-administration were shifted downward in three monkeys (JA, AB, and MI) and appeared to be shifted modestly leftward (Fig 1a) in one monkey (PE). The number of infusions received (Fig 1a) and the rate of responding (Fig 1b) were significantly different (filled symbols) when THC was administered noncontingently as compared with results obtained with heroin alone. For all four monkeys, two-way ANOVA performed on the number of infusions received per session showed a significant heroin x THC interaction (JA: F[12,28]=6.65, p<0.0001; AB: F[15,30]=5.90, p<0.0001; MI: F[15,30]=2.95, p<0.01; PE: F[15,30]=3.70, p<0.01). Two-way ANOVA performed on the rate of responding showed a significant heroin x THC interaction for JA (F[12,28]=53.95, p<0.0001), for AB (F[15,30]=14.33, p<0.0001), and for MI (F[15,30]=18.15, p<0.0001), but not for PE (F[15,30]=1.10, p>0.20). PE showed a significant main effect of heroin (F[5,30]=11.33, p<0.0001) but not of THC (F[3,30]=0.50, p>0.20). Pretreatment with 0.32 or 1.0 mg/kg of THC either had no effect or decreased the number of infusions received and the rate of responding in monkeys JA, AB, and MI. The same doses of THC increased the number of infusions received of 0.001 mg/kg/infusion of heroin in monkey PE (Fig 1a, lower right panel); 1.0 mg/kg of THC also decreased the number of infusions received of 0.01 mg/kg/infusion heroin, resulting in a leftward shift in both limbs of the heroin dose-response curve in this monkey. THC pretreatment also tended to increase the number of saline infusions for monkey MI, although this increase did not reach statistical significance (inverted triangle above “V”, Fig 1a, lower left panel).
Figs 2a and 2b show the number of infusions received per session and the average rate of responding (in the same sessions), respectively, when THC was administered contingently, either alone or in combination with different unit doses of heroin. THC alone did not maintain responding that was significantly different from responding maintained by saline (triangles, squares, and inverted triangles above “V”, Figs 2a and 2b, all panels). Responding for the combination of THC and heroin was consistently lower than responding for heroin alone for all four monkeys. The number of infusions received (Fig 2a) and the rate of responding (Fig 2b) were significantly lower (filled symbols) for some dose combinations of THC and heroin and there were no conditions under which responding for THC and heroin was greater than responding for the same unit dose of heroin alone. Two-way ANOVA showed a significant heroin x THC interaction for all four monkeys when performed on the number of infusions received (BE: F[12,40]=2.36, p<0.05; AB: F[12,40]=7.31, p<0.0001; MI: F[9,32]=5.91, p<0.0001; AY: F[9,32]=5.06, p<0.001), and when performed on the rate of responding for all monkeys (AB: F[12,40]=13.42, p<0.0001; MI: F[9,32]=11.12, p<0.0001; AY: F[9,32]=8.96, p<0.0001) except BE (F[12,40]=1.43, p>0.1), which showed significant main effects of heroin (F[4,40]=38.20, p<0.0001) and THC (F[3,40]=7.43, p<0.001).
Discussion
Converging lines of evidence, from cellular (Rios et al. 2006) to neurochemical (Schoffelmeer et al. 2006) to behavioral (Haney 2007; Li et al. 2008) measures, suggest a significant interaction between cannabinoid and (mu) opioid systems. Although the precise mechanism of such interaction(s) is not established, both cannabinoid and opioid systems modulate some of the same underlying neurochemical systems, including dopamine neurotransmission (Gardner and Vorel 1998; Tanda and Goldberg 2003).
Cannabinoid receptor agonists, such as THC, exert antinociceptive effects in a variety of nonhuman species and under a broad range of conditions and those effects have been shown to be mediated by cannabinoid CB1 receptors (see Pertwee 2001; Iversen and Chapman 2002; and Welch 2009 for review). The effectiveness of cannabinoid receptor ligands in preclinical studies on pain combined with the ineffectiveness of currently available drugs (e.g., opioid receptor agonists) for treating pain in many human patients, continues to stimulate the consideration of cannabinoid receptor ligands for treating certain types of pain, administered either alone or in combination with other drugs (Cichewicz 2004; Welch 2009). Indeed, THC enhances the antinociceptive effects of morphine in several different species (Welch and Stevens 1992; Welch et al. 1995; Finn et al. 2004; Smith et al. 2007; Li et al. 2008). However, results of studies with combinations of cannabinoids and opioids in humans have not unanimously provided confirmation of preclinical studies with the same drug combinations (Kraft, 2012). For example, THC failed to enhance analgesic effects of opioid receptor agonists in humans subjected to experimental pain or suffering from postoperative or cancer pain (Naef et al. 2003; Roberts et al. 2006; Seeling et al. 2006). In contrast, other studies indicate that cannabinoid and opioid receptor agonists attenuate some components (e.g., affective) of pain in a greater-than-additive matter (Roberts et al., 2006) and a recent study suggests that combinations of cannabinoids (e.g., THC and cannabidiol) might be more effective than THC alone for treating cancer-related pain (Johnson et al. 2010). Thus, the effectiveness of cannabinoid receptor agonists, alone or in combination with other drugs, which is evident in the preclinical laboratory, has yet to be fully realized in the clinic.
A concern regarding the use of cannabinoids and opioids in combination is the possibility that such a drug combination will have a high liability for abuse, given that cannabinoids (e.g., marijuana) and opioids (e.g., oxycodone) are abused individually. A number of procedures in the preclinical laboratory are predictive of abuse liability and in those procedures cannabinoids and opioids interact in a manner that appears to vary among species and laboratories. For example, heroin and morphine enhance the discriminative stimulus effects of THC in rats (Solinas and Goldberg 2005) but not in rhesus monkeys (Li et al., 2008); however, THC attenuates the discriminative stimulus effects of morphine in rhesus monkeys (Li et al., 2008). The opioid receptor antagonist naltrexone decreases THC induced conditioned place preference in rats (Braida et al. 2004), the discriminative stimulus effects of THC in rats (Solinas and Goldberg 2005), and THC self-administration in squirrel monkeys (Justinova et al. 2004). The cannabinoid CB1 receptor antagonist SR 141716A decreases heroin self-administration in rats responding under a progressive ratio schedule and either decreases (Navarro et al. 2001) or has no effect (Solinas et al. 2003) on heroin self-administration in rats responding under a continuous reinforcement schedule.
When THC was administered noncontingently to rhesus monkeys in the current study, the heroin self-administration dose-response curve was shifted downward in 3 monkeys and shifted slightly leftward in a fourth monkey. Thus, noncontingent administration of THC did not enhance the positive reinforcing effects of heroin (i.e., did not shift the heroin dose-response curve leftward) in 3 of 4 monkeys studied. It is not clear whether decreased responding for heroin after noncontingent administration of THC reflects decreased reinforcing effectiveness of heroin. The doses of THC that shift the heroin self-administration dose-response curve downward do not alter rates of responding in monkeys discriminating morphine and responding under a schedule of stimulus shock termination (Li et al., 2008) although doses of THC larger than 0.32 mg/kg decrease responding that is maintained by the presentation of food (McMahon et al., 2005; Li, unpublished observation). Thus, decreased self-administration of heroin after noncontingent injection of THC might reflect a nonselective suppression of responding.
The contingency of drug administration can impact the behavioral effects of some drugs (Dworkin et al. 1995; Galici et al. 2000; Lecca et al. 2007). THC itself maintains self-administration responding under a relatively limited range of conditions (Justinova et al. 2004; Takahashi and Singer, 1070; Tanda et al., 2000); in the current study, THC alone failed to maintain responding at rates greater than what was obtained with saline. Thus, THC alone had no apparent positive reinforcing effects under these experimental conditions. Moreover, when THC was available in a drug mixture with different unit doses of heroin, monkeys received either the same (i.e., with small unit doses of THC) or significantly fewer (i.e., with larger unit doses of THC) injections as compared to the number of injections received with the same unit dose of heroin alone. Thus, combinations of THC and heroin resulted in an overall decrease in the number of injections (and in rate of responding) of heroin with no evidence of a shift leftward in the heroin dose-response curve that might be expected if THC enhanced the positive reinforcing effects of heroin. Reinforcing effects of drugs can vary across different experimental conditions (e.g., Solinas et al, 2005) and it is possible that the nature of interaction between THC and mu opioid receptor agonists would be different under conditions where reinforcing effects are examined independently of rate measures (e.g., choice).
In summary, this study examined the impact of contingent and noncontingent administration of the cannabinoid receptor agonist THC on the positive reinforcing effects of i.v. heroin in rhesus monkeys. With the exception of one unit dose of heroin in one monkey, noncontingent administration of THC did not enhance, but rather diminished, heroin self administration. When administered contingently, THC itself did not maintain self-administration responding and in combination with heroin there was evidence only for decreased self-administration of the drug mixture. That is, there was no combination of THC and heroin doses that resulted in a significantly greater number of infusions as compared with what was obtained when monkeys self-administered heroin alone. Enhancement of the antinociceptive effects of opioids by cannabinoid receptor agonists is well established in nonhuman species although the translation of those findings to the clinic is not fully evident. Nevertheless, to the extent that combinations of opioid and cannabinoid receptor agonists are found to provide improved therapeutic value (e.g., pain relief), results of this study suggest that those drug combinations will not be associated with a greater liability for abuse.
Acknowledgments
The authors thank Christopher Cruz, Toni Andrew, and Victoria Hill for excellent technical assistance.
Source of Funding:
The research was supported by United States Public Health Service Grant R01DA05018; CPF is supported by a Senior Scientist Award (K05DA17918)
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Committee on Advancing Pain Research, Care, and Education, Board on Health Sciences Policy, Institute of Medicine of the National Academies; Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Braida D, Iosuè S, Pegorini S, Sala M. Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol. 2004;506:63–69. doi: 10.1016/j.ejphar.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2004;74:1317–1324. doi: 10.1016/j.lfs.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Welch SP. Modulation or oral morphine antinociceptive tolerance and naloxone-precipitated withdrawal signs by oral delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2003;305:812–817. doi: 10.1124/jpet.102.046870. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Opioid antagonism enhances marijuana's effects in heavy marijuana smokers. Psychopharmacology. 2010;211:141–148. doi: 10.1007/s00213-010-1875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vry J, Kuhl E, Franken-Kunkel P, Eckel G. Pharmacological characterization of the chronic constriction injury model of neuropathic pain. Eur J Pharmacol. 2004;491:137–148. doi: 10.1016/j.ejphar.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology. 1995;117:262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Finn DP, Beckett SR, Roe CH, Madjd A, Fone KC, Kendall DA, Marsden CA, Chapman V. Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. Eur J Neurosci. 2004;19:678–686. doi: 10.1111/j.0953-816x.2004.03177.x. [DOI] [PubMed] [Google Scholar]
- Galici R, Pechnick RN, Poland RE, France CP. Comparison of noncontingent versus contingent cocaine administration on plasma corticosterone levels in rats. Eur J Pharmacol. 2000;387:59–62. doi: 10.1016/s0014-2999(99)00780-3. [DOI] [PubMed] [Google Scholar]
- Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiol Dis. 1998;5:502–533. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend. 2000;59:261–275. doi: 10.1016/s0376-8716(99)00128-3. [DOI] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2009;8:403–421. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein H, Akil H. Opioid analgesics. In: Brunton L, Lazo J, Parker K, editors. The Pharmacological Basis of Therapeutics. Vol. 11. New York: McGraw-Hill; 2005. pp. 547–590. [Google Scholar]
- Haney M, Bisaga A, Foltin RW. Interaction between naltrexone and oral THC in heavy marijuana smokers. Psychopharmacology. 2003;166:77–85. doi: 10.1007/s00213-002-1279-8. [DOI] [PubMed] [Google Scholar]
- Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–1403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- Hosking RD, Zajicek JP. Therapeutic potential of cannabis in pain medicine. Br J Anaesth. 2008;101:59–68. doi: 10.1093/bja/aen119. [DOI] [PubMed] [Google Scholar]
- Houser SJ, Eads M, Embrey JP, Welch SP. Dynorphin B and spinal analgesia: induction of antinociception by the cannabinoids CP55,940, Delta(9)-THC and anandamide. Brain Res. 2000;857:337–342. doi: 10.1016/s0006-8993(00)01981-8. [DOI] [PubMed] [Google Scholar]
- Iversen L, Chapman V. Cannabinoids: a real prospect for pain relief? Curr Opin Pharmacol. 2002;2:50–55. doi: 10.1016/s1471-4892(01)00120-5. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduced the reinforcing effects of delta9-tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology. 2004;173:186–194. doi: 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010;39:167–179. doi: 10.1016/j.jpainsymman.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Kraft B. Is there any clinically relevant cannabinoid-induced analgesia? Pharmacology. 2012;89:237–246. doi: 10.1159/000337376. [DOI] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology. 2007;191:653–667. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- Li JX, McMahon LR, Gerak LR, Becker GL, France CP. Interactions between delta(9)-tetrahydrocannabinol and mu opioid receptor agonists in rhesus monkeys: discrimination and antinociception. Psychopharmacology. 2008;199:199–208. doi: 10.1007/s00213-008-1157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Li J-X, Koek W, France CP. Effects of 1-(2,3-dimethyl-4-methylphenyl)-2-aminopropane (DOM) and quipazine on heroin self-administration in rhesus monkeys. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2803-0. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Amin MR, France CP. SR 141716A differentially attenuates the behavioral effects of delta9-THC in rhesus monkeys. Behav Pharmacol. 2005;16:363–372. doi: 10.1097/00008877-200509000-00008. [DOI] [PubMed] [Google Scholar]
- Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R. The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain. 2003;105:79–88. doi: 10.1016/s0304-3959(03)00163-5. [DOI] [PubMed] [Google Scholar]
- Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, Chowen JA, Gomez R, del Arco I, Villanua MA, Maldonado R, Koob GF, Rodriguez de Fonseca F. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H, Wixted JT, editors. Methodology in Experimental Psychology. 3. Vol. 4. New York: Wiley; 2002. Stevens' Handbook of Experimental Psychology. [Google Scholar]
- Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;6:713–737. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JD, Gennings C, Shih M. Synergistic affective analgesic interaction between delta-9-tetrahydrocannabinol and morphine. Eur J Pharmacol. 2006;530:54–58. doi: 10.1016/j.ejphar.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, Hogenboom F, Wardeh G, De Vries TJ. Interactions between CB1 cannabinoid and mu opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacology. 2006;51:773–781. doi: 10.1016/j.neuropharm.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Seeling W, Kneer L, Büchele B, Gschwend JE, Maier L, Nett C, Simmet T, Steffen P, Schneider M, Rockemann M. Delta(9)-tetrahydrocannabinol and the opioid receptor agonist piritramide do not act synergistically in postoperative pain. Anaesthesist. 2006;55:391–400. doi: 10.1007/s00101-005-0963-6. [DOI] [PubMed] [Google Scholar]
- Smith FL, Fujimori K, Lowe J, Welch SP. Characterization of delta9-tetrahydrocannabinol and anandamide antinociception in nonarthritic and arthritic rats. Pharmacol Biochem Behav. 1998;60:183–191. doi: 10.1016/s0091-3057(97)00583-2. [DOI] [PubMed] [Google Scholar]
- Smith PA, Selley DE, Sim-Selley LJ, Welch SP. Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur J Pharmacol. 2007;571:129–137. doi: 10.1016/j.ejphar.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl) -4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther. 2003;306:93–102. doi: 10.1124/jpet.102.047928. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Involvement of mu-, delta- and kappa-opioid receptor subtypes in the discriminative-stimulus effects of delta-9-tetrahydrocannabinol (THC) in rats. Psychopharmacology. 2005;179:804–812. doi: 10.1007/s00213-004-2118-x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Tanda G, Makriyannis A, Matthews SA, Goldberg SR. Cannabinoid agonists but not inhibitors of endogenous cannabinoid transport or metabolism enhance the reinforcing efficacy of heroin in rats. Neuropsychopharmacology. 2005;30:2046–2057. doi: 10.1038/sj.npp.1300754. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Singer G. Self-administration of Δ9-tetrahydrocannabinol by rats. Pharmacol Biochem Behav. 1979;11:737–740. doi: 10.1016/0091-3057(79)90274-0. [DOI] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms--a review of recent preclinical data. Psychopharmacology. 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Kishioka S, Butelman ER, Broadbear J, Lee KO, Woods JH. Analgesic, respiratory and heart rate effects of cannabinoid and opioid agonists in rhesus monkeys: antagonist effects of SR 141716A. J Pharmacol Exp Ther. 1998;286:697–703. [PubMed] [Google Scholar]
- Wachtel SR, de Wit H. Naltrexone does not block the subjective effects of oral Delta(9)- tetrahydrocannabinol in humans. Drug Alcohol Depend. 2000;59:251–260. doi: 10.1016/s0376-8716(99)00127-1. [DOI] [PubMed] [Google Scholar]
- Welch SP. Interaction of the cannabinoid and opioid systems in the modulation of nociception. Int Rev Psychiatry. 2009;21:143–151. doi: 10.1080/09540260902782794. [DOI] [PubMed] [Google Scholar]
- Welch SP, Stevens DL. Antinociceptive activity of intrathecally administered cannabinoids alone, and in combination with morphine, in mice. J Pharmacol Exp Ther. 1992;262:10–18. [PubMed] [Google Scholar]
- Welch SP, Thomas C, Patrick GS. Modulation of cannabinoid-induced antinociception after intracerebroventricular versus intrathecal administration to mice: possible mechanisms for interaction with morphine. J Pharmacol Exp Ther. 1995;272:310–321. [PubMed] [Google Scholar]
- Wojnicki FH, Bacher JD, Glowa JR. Use of subcutaneous vascular access ports in rhesus monkeys. Lab Anim Sci. 1994;44:491–494. [PubMed] [Google Scholar]