Abstract
Introduction
Increased sedentary behavior predicts greater cardiovascular morbidity and mortality, and does so independently of physical activity (PA). This association is only partially explained by BMI and overall body fat, suggesting mechanisms besides general increased adiposity. The purpose of this study was to explore associations of self-reported leisure PA and sitting time with regional fat depositions and abdominal muscle among community-dwelling older adults.
Methods
Participants were 539 diverse adults (mean age 65) who completed a study visit in 2001-2002. Areas of pericardial, intra-thoracic, subcutaneous, visceral, and intermuscular fat, as well as abdominal muscle, were measured using computed tomography. Leisure PA and sitting hours were entered simultaneously into multivariate regression models to determine associations with muscle and fat areas.
Results
After adjusting for demographics, smoking, diabetes, hypertension, triglycerides, and cholesterol, greater PA was associated with less intra-thoracic, visceral, subcutaneous, and intermuscular fat (for all p < .05), while greater sedentary time was associated with greater pericardial and intra-thoracic fat (for both p < .05). After further adjusting for BMI, each hour of weekly PA was associated with 1.85 cm2 less visceral fat (p < .01), but was not associated with other fat depositions. Conversely, each hour of daily sitting was associated with 2.39cm2 more pericardial fat (p < .05), but was not associated with any other fat depositions. There were no associations with abdominal muscle area. Adjusting for common inflammatory markers had little effect. Associations between fat and PA were stronger for men.
Conclusions
Sitting and physical activity have distinct associations with regional fat deposition in older adults. The association between sitting and pericardial fat could partially explain the link between sitting and coronary heart disease.
Keywords: sitting, body composition, visceral fat, pericardial fat, cardiovascular disease
Introduction
The health benefits of physical activity are well established, and include reduced risk for a variety of chronic diseases(42), particularly cardiovascular disease(3,39). Recent studies, however, suggest that sedentary behavior is itself an independent risk factor for cardiovascular disease(10,20,29). Even when controlling for physical activity levels, increased sedentary time, particularly time spent sitting in leisure activities such as television watching, has been associated with greater risk for hypertension(4), diabetes(18), metabolic syndrome(35), coronary heart disease(12) and mortality, especially due to cardiovascular disease(20). The mechanisms behind these relationships, however, are incompletely understood.
While greater sedentary behavior is associated with the development of obesity(18), studies show that the relationship between sedentary time and cardiovascular risk factors hold even when adjusting for BMI(1,4,35,40), as well as total and central body fat(34). The effects of sedentary behavior, then, appear to extend beyond general increased adiposity, and may lie in the location of fat deposition. In some studies, the location of fat has been more strongly associated with cardiovascular morbidity and mortality than weight or BMI(13). For instance, pericardial fat is strongly associated with cardiovascular disease(9,23), even when adjusting for both BMI and waist-hip ratio(32).
There is some work suggesting that physical activity may alter body composition without concomitant changes in BMI by preferentially reducing visceral and/or subcutaneous fat(38,41). Little is known, however, about sedentary behavior and regional body composition, which could help elucidate the link between sedentary time and CVD. Only one recent study has examined this relationship, and found no association between sedentary behavior and regional body composition(25). However, the study used a relatively small sample (N=126) of only inactive overweight and obese individuals, and regional measures were limited to visceral and subcutaneous abdominal fat, thus excluding other potentially important regional deposits such as in the pericardium and intra-thoracic cavity. Associations between behavior and body composition could also greatly vary by ethnic group, as there are marked ethnic differences in regional fat deposits(2,6), yet this association has not been explored in a multi-ethnic sample. Consequently, there are many questions still to be answered about sedentary behavior, physical activity, and regional body composition.
Therefore, the purpose of the current study was to examine the independent associations between physical activity and sedentary behavior, specifically time spent in seated leisure activities, with the distribution of adipose tissue in the abdomen, pericardium and intra-thoracic cavity measured by CT among a multi-ethnic population derived from the Rancho Bernardo Study, the UCSD Filipino Women’s Health Study, and the Health Assessment Study of African-American Women. Also, while the majority of research on body composition and CVD risk has focused on fat, differences in BMI and body composition related to physical activity or sedentary behavior may also be due to differences in muscle area. Therefore, we also assessed associations between physical activity and leisure sitting time with abdominal muscle area.
Methods
Study Participants
The Rancho Bernardo Study (RBS) is a prospective cohort study of community-dwelling adults, primarily of Caucasian descent, established between 1972 and 1974, when 82% of the adult residents of a San Diego, California suburb first participated in a survey of heart disease risk factors. In 2001-2002, as part of the research clinic examination, participants ≥ 55 years old who were free of overt cardiovascular disease had electron beam computed tomography (CT) scans performed on the chest and mid-abdominal regions. Participants at this visit also filled out questionnaires on physical activity and daily sitting time.
Filipina women and African-American women from the UCSD Filipino Women’s Health Study and the Health Assessment Study of African-American Women (HASAAW) cohort were recruited as ethnic comparison groups to the Rancho Bernardo study in 1994-1999 using the same research protocol, staff, and diagnostic laboratories. Those free of known cardiovascular disease in 2001-2002 were invited to have CT scans performed at this time, which included the chest and abdominal regions.
Data on pericardial, intra-thoracic, visceral, subcutaneous and intermuscular fat, as well as abdominal lean muscle and relevant covariates, and sitting time and physical activity were available for a total of 539 participants: 135 Caucasian men, 168 post-menopausal Caucasian women, 104 post-menopausal Filipina women and 132 post-menopausal African-American women. For our studies, post-menopausal was defined as having no menses for the past year. The Rancho Bernardo, Filipino Women’s Health and HASAAW studies were approved by the Institutional Review Board of the University of California, San Diego; participants gave written informed consent.
Measurement of Adiposity
More detailed descriptions of adiposity measurement and quantification have been published previously (see Wassel et al.(43)). CT was used to measure five different fat deposits in the chest and abdomen: perdicardial fat, visceral fat, subcutaneous fat, intra-thoracic fat, and intermuscular fat. Intra-thoracic and pericardial adipose tissue areas were measured from chest CT scans obtained in 2001-02 using semi-automated muscle segmentation software (MIPAV 4.1.2, National Institutes of Health). Fifteen CT slices of 3mm thickness were selected originating from the right coronary artery, 4 slices above the right coronary artery and 10 slices below.
Using abdominal CT scans also from 2001-02 and the same MIPAV software for analysis, a transverse cross section slice of 6 mm thickness was selected at the umbilicus for analysis of subcutaneous, visceral and intermuscular fat. The abdomen was defined as the region within the epidermal layer. Within the abdomen, selections of subcutaneous, visceral, and 4 muscle group areas were defined and selected. Subcutaneous area was defined as the area superficial to the abdominal wall musculature. Visceral fat was selected using the inner most border of the abdominal muscle wall following the parietal peritoneum. Fat was discerned from other tissue types by the voxel count that fell within the threshold of −190 and −30 Hounsfield units. The area of fat tissue was determined by voxel count and reported in cm2. The entire subcutaneous area was classified as fat tissue to reduce error due to scan artifacting.
Measurement of Abdominal Muscle
Muscle segmentation was performed on 4 separate muscle groups in the abdomen using the 6mm slices described above: psoas, paraspinals, obliques, and rectus. Lean area was discerned by the voxel count that fell within the threshold of 0 to 100 HU. Each selection followed the muscle fascia line and was performed on both the left and right side of the abdomen. The psoas group consisted of the psoas major and psoas minor and was traced around its fascia line. The paraspinal group consisting spinalis, longissimus, illiocostalis erector spinae, multifidus, and quadratus lombrum was selected along the thoracolumbar fascia and quadratus lomborum fascia. The oblique selection consisted of the internal oblique, external oblique, and the transverse abdominus. The rectus selection consisted of the rectus abdominus. Only the largest abdominal muscle (psoas) was analyzed separately, using the average of the left and right psoas muscle. Total abdominal muscle was obtained by summing over the left and right muscle for the psoas, oblique, paraspinal and rectus abdominus groups.
Measurement of Covariates and Adipocytokines
Information on age, sex, smoking status, alcohol use, medical history, and medication use was obtained via standard self-administered or interviewer-administered questionnaires. Trained technicians measured weight and height with participants wearing light clothing and no shoes, and this was used to compute body mass index (BMI). Systolic and diastolic blood pressures were measured twice in seated subjects at rest for at least 5 minutes using the Hypertension Detection and Follow-up Program protocol; the average of the 2 readings was used in analyses.
Morning fasting blood samples were obtained by venipuncture after a requested 12-hour fast. Fasting total cholesterol and triglycerides were measured by enzymatic methods using the ABA-200 biochromatic analyzer (Abbott Laboratories, Abbott Park, IL). High-density cholesterol (HDL) was determined by precipitation analysis using a protocol from the Lipid Research Clinic. Low-density cholesterol (LDL) was calculated with the Friedewald equation(14).
Comorbidities included type 2 diabetes and hypertension. Diabetes was defined as self-report of physician diagnosis, anti-diabetes medication use, fasting glucose ≥ 126 mg/dL, or 2-h post-challenge glucose ≥ 200 mg/dl. Hypertension was defined as self-report of physician diagnosis, anti-hypertensive medication use, systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg.
Inflammatory markers (adiponectin, leptin, IL-6, and TNF-α) were measured from fasting plasma samples obtained at the visit between 1992-1996 for Caucasians and 1994-1999 visit for Filipina and African-American women, and stored at −70°C; see Wassel et al.(43) for a detailed description of measurement protocol.
Sitting Time
Leisure sitting time was measured by a single self-report item on the health questionnaire at the 2000-2001 visit. Participants indicated the average time they spent on a typical weekday, rounded to the nearest quarter-hour, in leisure time seated activities such as television watching, reading, or playing cards. This single item measure does not capture global sedentary behavior; however, domain-specific measures such as this may be more valid that self-report measures of global sitting(16), and past studies show that leisure time sitting in particular shows strong negative associations with cardiovascular health(10,18). Such single-item measures of leisure time sitting have shown significant associations with health outcomes in past studies(1,5).
Leisure Time Physical Activity
Leisure physical activity was measured at the 2000-2001 visit by self-report using a modified version of the Paffenbarger Questionnaire, a validated self-report measure(30). This modified measure has shown strong correlations in previous studies with physiological measures related to PA, such as blood pressure, pulse rate and HDL (26,31). Participants were given a list of physical activities, and indicated the number of times they participated in each within the past two weeks and the duration in minutes of each bout of activity. The majority of participants (73%) regularly engaged in walking for exercise. Many also reported calisthenics (22.4%), swimming (11.1%), and bicycling (8.7%), while activities such as handball and hiking were more rare (<5%). Total amount of activity was determined by adding the total minutes spent in all activities of at least moderate aerobic activity over the past two weeks, and dividing by two to produce a measure of average weekly physical activity. Several activities included in the questionnaire were not included in the total measure of activity because they were not at least moderate intensity aerobic activities (e.g., bowling, golf).
Statistical Analysis
Analyses were performed using SPSS 20 software. To examine the univariate associations of baseline characteristics with tertiles of sitting time in hours per day, chi-square tests, Kruskal-Wallis tests, and ANOVA were used as appropriate. Multivariate linear regression was used to examine the association of physical activity time and sitting time with regional adiposity and total abdominal and psoas muscle measures. Physical activity and sitting time were modeled as continuous variables in number of hours per week (activity) and hours per day (sitting). As pericardial, intra-thoracic, visceral, subcutaneous, and intermuscular fat, as well as psoas and total abdominal muscle, were approximately normally distributed, no transformations were performed. Multivariate linear regression models were performed in stages to examine the effect of potential confounders and mediators. Physical activity and sedentary hours were entered simultaneously into all models in order to determine their independent effects. In the first stage of analysis, physical activity and sitting time were both in the models, followed by the addition of demographics (age, sex and race/ethnicity). Next, cardiovascular risk factors were added (hypertension, diabetes, smoking, HDL and LDL cholesterol, and triglycerides). In order to examine whether the associations of physical activity and sitting time with regional adipose and muscle areas were independent of overall adiposity, we additionally adjusted for BMI in the third stage (we also performed this analysis using total abdominal muscle and height instead of BMI, and found no marked differences in the results). In the final stage, we additionally adjusted for common inflammatory markers (leptin, IL-6, TNF-α, and adiponectin) in order to examine any potential mediation effects of these biomarkers, which have been associated with sitting time, PA, and adiposity and muscle.
To assess whether the associations of sitting time and physical activity with regional adiposity and muscle measures were modified by sex/ethnic groups, we examined interactions of sex/ethnic group and physical activity and sitting time on an additive scale in the multivariate linear regression models. Across models, sample size was restricted to participants with complete data for all covariates.
Results
Table 1 shows the unadjusted characteristics of the study cohort, both overall and by tertiles of leisure sitting hours. The sample was roughly equally comprised of Caucasian men, Caucasian women, African American women, and Filipina women. Caucasian women were more likely to be in the higher sitting tertile, while Filipinas were more likely to be in the lowest. Age was significantly higher across sitting time tertiles, as was the prevalence of smoking.
Table 1. Participant characteristics by hours of leisure sitting time in tertiles.
| Daily Leisure Sitting Hour Tertiles |
|||||
|---|---|---|---|---|---|
| Overall | ≤ 2.5 Hours Sitting | > 2.5 ≤ 4.0 Hours Sitting | > 4 Hours Sitting | p-value | |
| N | 539 | 182 | 221 | 136 | |
| Age, years | 64.68 (7.48) | 63.14 (6.61) | 65.033 (7.87) | 66.48 (7.59) | .001* |
| Group (%) | |||||
| Caucasian Male | 24.9% | 21.4% | 24.9% | 29.6% | <.001* |
| Caucasian Female | 31.2% | 21.4% | 32.6% | 42.2% | |
| African American Female | 24.5% | 23.6% | 28.1% | 20.0% | |
| Filipina | 19.3% | 33.5% | 14.5% | 8.1% | |
| Body Mass Index, kg/m2 | 26.93 (4.76) | 26.45 (4.42) | 27.31 (4.60) | 26.99 (5.42) | .198 |
| Inflammatory Markers | |||||
| TNF-α, pg/mL | 1.24 (0.94) | 1.23 (1.14) | 1.28 (0.87) | 1.19 (0.76) | .692 |
| Adiponectin, μg/mL | 10.42 (6.00) | 9.44 (4.98) | 10.89 (6.65) | 10.80 (5.95) | .032* |
| Leptin, ng/mL | 14.35 (9.70) | 13.17 (8.71) | 14.92 (10.58) | 14.78 (9.35) | .159 |
| IL-6, pg/mL | 2.05 (2.10) | 2.19 (2.93) | 1.93 (1.63) | 2.10 (1.31) | .452 |
| Lipids | |||||
| HDL cholesterol, mg/dL | 59.30 (15.88) | 57.47 (14.54) | 60.28 (16.17) | 60.33 (16.96) | .155 |
| LDL cholesterol, mg/dL | 125.27 (32.66) | 124.50 (35.31) | 127.28 (30.47) | 122.72 (32.44) | .415 |
| Triglycerides, mg/dL | 122.99 (67.72) | 114.03 (59.96) | 128.28 (65.71) | 126.22 (79.49) | .083 |
| Diabetes (%) | 13.4% | 14.8% | 12.7% | 12.6% | .778 |
| Hypertension (%) | 53.9% | 48.9% | 56.1% | 57.0% | .247 |
| Ever Smoker (%) | 45.3% | 37.4% | 47.1% | 53.3% | .015* |
| Physical activity hours/week | 2.56 (2.97) | 2.79 (2.87) | 2.49 (2.91) | 2.37 (3.25) | .477 |
| Pericardial fat, cm2 | 170.41 (76.16) | 168.09 (75.96) | 169.63 (72.97) | 175.16 (81.53) | .311 |
| Intra-thoracic fat, cm2 | 71.86 (64.18) | 61.20 (50.54) | 75.88 (70.94) | 80.08 (66.10) | .018* |
| Visceral fat, cm2 | 120.03 (59.45) | 114.75 (57.07) | 124.65 (62.46) | 122.82 (58.53) | .237 |
| Intermuscular fat, cm2 | 21.43 (11.07) | 19.41 (8.88) | 23.50 (12.14) | 21.42 (11.32) | .001* |
| Subcutaneous fat, cm2 | 253.86 (122.73) | 243.43 (106.32) | 273.21 (131.48) | 246.65 (126.23) | .034* |
| Total abdominal muscle, cm2 | 91.87 (26.59) | 92.25 (26.74) | 90.85 (25.80) | 93.07 (27.80) | .001* |
| Average Psoas muscle, cm2 | 9.03 (8.78, 9.27) | 8.99 (8.57, 9.40) | 9.04 (8.67, 9.42) | 9.05 (8.56, 9.53) | .977 |
Continuous variables are presented as mean (SD)
p < .05
Mean physical activity time was 2.56 hours per week, which did not differ by sitting time tertile. Median sitting time was 3.25 hours per day. There were no differences between sitting time tertiles in measures of lipids or inflammatory markers except for adiponectin, which was lower in the lowest sitting group. Intra-thoracic fat appeared to increase linearly with sitting time, and subcutaneous fat, intermuscular fat and total abdominal muscle differed between groups but not in a linear pattern.
Table 2 shows unadjusted variables across tertiles of leisure activity time. Caucasian men and women were more likely to be in the highest activity tertile, while African American women and Filipinas were more likely to be less active. BMI decreased linearly across tertiles, as did leptin, IL-6, LDL cholesterol, triglycerides, diabetes prevalence, and hypertension, while HDL cholesterol and adiponectin increased. Significant differences were also seen across tertiles for subcutaneous, pericardial, visceral, and intermuscular fat, with all but intermuscular fat showing linear decreases across tertiles. Because this was a multi-ethnic sample, we also examined participant characteristics across cohorts (Table 3). Significant differences were found in nearly all variables. Leisure sedentary time was markedly lower in Filipino women, while leisure PA was much lower in African American women.
Table 2. Participant characteristics by hours of leisure physical activity in tertiles.
| Weekly Physical Activity Hour Tertiles |
||||
|---|---|---|---|---|
| < 0.75 Hours PA | ≥ 0.75 < 3 Hours PA | ≥ 3 Hours PA | p-value | |
| N | 174 | 181 | 184 | |
| Age, years | 64.31 (7.07) | 65.02 (7.79) | 65.13 (7.32) | .533 |
| Group (%) | ||||
| Caucasian Male | 21.8% | 26.0% | 26.6% | .033* |
| Caucasian Female | 28.2% | 27.6% | 37.5% | |
| African American Female | 32.2% | 22.7% | 19.6% | |
| Filipina | 17.8% | 23.8% | 16.3% | |
| Body Mass Index, kg/m2 | 27.97 (4.98) | 27.23 (4.04) | 25.65 (3.18) | <.001* |
| Inflammatory Markers | ||||
| TNF-α, pg/mL | 1.26 (1.07) | 1.25 (0.85) | 1.20 (0.85) | .823 |
| Adiponectin, μg/mL | 9.83 (5.70) | 9.69 (5.55) | 11.65 (6.43) | .002* |
| Leptin, ng/mL | 15.86 (10.91) | 14.71 (9.47) | 12.42 (8.07) | .002* |
| IL-6, pg/mL | 2.27 (2.38) | 2.17 (2.03) | 1.75 (1.86) | .048* |
| Lipids | ||||
| HDL cholesterol, mg/dL | 58.16 (16.40) | 58.33 (14.97) | 61.52 (16.64) | .081 |
| LDL cholesterol, mg/dL | 129.90 (30.59) | 124.42 (35.42) | 121.42 (31.21) | .045* |
| Triglycerides, mg/dL | 132.78 (67.92) | 127.93 (77.20) | 108.35 (50.60) | .001* |
| Diabetes (%) | 18.4% | 13.8% | 8.2% | .017* |
| Hypertension (%) | 59.8% | 55.2% | 46.7% | .042* |
| Ever Smoker (%) | 46.6% | 44.8% | 44.6% | .918 |
| Sitting hours/day | 3.84 (2.51) | 3.53 (2.25) | 3.63 (2.34) | .449 |
| Pericardial fat, cm2 | 179.02 (86.06) | 170.99 (66.92) | 157.46 (73.58) | .032* |
| Intra-thoracic fat, cm2 | 78.79 (72.24) | 69.17 (56.19) | 63.99 (62.93) | .102 |
| Visceral fat, cm2 | 127.61 (63.65) | 130.11 (59.22) | 105.02 (55.04) | <.001* |
| Intermuscular fat, cm2 | 24.11 (13.10) | 21.07 (9.53) | 19.68 (9.92) | .001* |
| Subcutaneous fat, cm2 | 274.43 (117.70) | 267.50 (139.73) | 229.40 (105.51) | .001* |
| Total abdominal muscle, cm2 | 88.42 (26.55) | 91.42 (26.04) | 95.13 (26.76) | .056 |
| Average Psoas muscle, cm2 | 8.81 (2.76) | 8.94 (2.74) | 9.26 (2.59) | .266 |
Continuous variables are presented as mean (SD)
p < .05
Table 3. Participant Characteristics by Sex/Racial Group*.
| White Men n=134 |
White Women n=168 |
A-A women n=133 |
Filipina Women n=104 |
p-value | |
|---|---|---|---|---|---|
| Age, years | 68 ± 8 | 66 ± 6 | 60 ± 7 | 64 ± 6 | <0.001 |
| Ever smoker, n(%) | 72 (54%) | 92 (55%) | 67 (50%) | 13 (13%) | <0.001 |
| Physical activity, hours/day† | 2.0 (0.5, 4.0) | 2.2 (0.5, 4.1) | 1.1 (0, 3.0) | 1.8 (0.5, 3.4) | <0.001 |
| Sedentary time, hours/day† | 3.5 (2.0, 5.0) | 4.0 (3.0, 5.0) | 3.0 (2.0, 4.0) | 1.0 (1.0, 3.5) | <0.001 |
| Body Mass Index, kg/m2 | 27 ± 4 | 25 ± 4 | 30 ± 6 | 26 ± 3 | <0.001 |
| Visceral Fat, cm2 | 157 ± 64 | 107 ± 56 | 97 ± 47 | 126 ± 56 | <0.001 |
| Subcutaneous Fat, cm2† | 177 (138, 236) | 227 (159, 298) | 352 (261, 436) | 250 (197, 297) | <0.001 |
| Intermuscular Fat, cm2 | 24 ± 13 | 23 ± 11 | 21 ± 11 | 18 ± 7 | <0.001 |
| Pericardial Fat, cm2† | 184 (135, 244) | 144 (105, 186) | 123 (91, 160) | 191 (150, 236) | <0.001 |
| Intra-thoracic Fat, cm2† | 114 (63, 177) | 47 (26, 74) | 29 (15, 44) | 46 (31, 74) | <0.001 |
| Total Abdominal Muscle area, cm2 | 119 ± 25 | 84 ± 17 | 85 ± 23 | 78 ± 19 | <0.001 |
| Psoas Muscle area, cm2 | 12 ± 3 | 8 ± 2 | 8 ± 2 | 7 ± 1 | <0.001 |
| LDL cholesterol, mg/dL | 122 ± 30 | 121 ± 29 | 125 ± 37 | 135 ± 35 | 0.004 |
| HDL cholesterol, mg/dL | 50 ± 11 | 67 ± 17 | 63 ± 16 | 53 ± 12 | <0.001 |
| Triglycerides, mg/dL† | 108 (82, 155) | 115 (82, 156) | 78 (58, 112) | 138 (99, 189) | <0.001 |
| Hypertension, n(%) | 75 (56%) | 74 (44%) | 82 (62%) | 59 (57%) | 0.02 |
| Diabetes, n(%) | 18 (13%) | 4 (2%) | 15 (11%) | 35 (34%) | <0.001 |
| Interleukin-6, pg/mL† | 1.5 (1.1, 2.4) | 1.4 (1.1, 2.1) | 1.8 (1.2, 2.9) | 1.3 (1.1, 2.1) | <0.001 |
| Tumor Necrosis Factor-α, pg/mL† | 1.1 (0.9, 1.3) | 1.0 (0.9, 1.3) | 1.0 (0.8, 1.4) | 1.0 (0.8, 1.3) | 0.16 |
| Adiponectin, ug/mL† | 7.9 (6.1, 11.3) | 13.8 (10.8, 17.7) | 7.9 (5.4, 10.8) | 6.5 (4.1, 10.0) | <0.001 |
| Leptin, ng/mL† | 6.0 (4.1, 8.8) | 14.3 (9.1, 21.5) | 15.8 (21.1, 21.5) | 13.9 (10.6, 18.2) | <0.001 |
For participants with non-missing visceral fat measures
Median (Quartile 1, Quartile 3)
Table 4 shows the multivariable linear regression models of the association between both sitting time and physical activity (in the same model) with regional adiposity measures. Minimally adjusted regression models using tertiles of sitting and activity showed linear patterns for most variables, so continuous measures were used in regression models in order to maximize power. Entering sitting and physical activity separately did not markedly alter results, thus only the simultaneous model is shown to highlight independent associations. After controlling for demographics, greater physical activity was significantly associated with lower pericardial, intra-thoracic, visceral, subcutaneous and intermuscular fat area (for all p < 0.01). The associations with pericardial, intra-thoracic, intermuscular, and subcutaneous fat were reduced after adjusting for CVD risk factors (pericardial) and further adjusting for BMI (intermuscular, intra-thoracic and subcutaneous). However, the association with visceral fat remained highly significant even after adjusting for BMI (β = −1.85 p = 0.004) and inflammatory markers (β = −1.74, p = 0.006).
Table 4. Association of physical activity and leisure sitting time with fat deposition.
| Physical Activity Hours (per week) |
Sitting Hours (per day) |
|||
|---|---|---|---|---|
|
|
||||
| Beta (95% CI) | p-value | Beta (95% CI) | p-value | |
|
|
||||
| Pericardial Fat, cm2 | ||||
| Unadjusteda | −2.53 (−4.66, −0.40) | .020 | 3.19 (0.45, 5.92) | .022 |
| + Demographicsb | −2.68 (−4.67, −0.70) | .008 | 3.94 (1.35, 6.52) | .003 |
| + CVD Risk Factorsc | −1.60 (−3.54, 0.32) | .103 | 3.32 (0.84, 5.81) | .009 |
| + BMI | −0.45 (−2.27, 1.36) | .624 | 2.39 (0.07, 4.72) | .044 |
| + Inflammatory markersd | −0.44 (−2.60, 1.38) | .634 | 2.45 (0.12, 4.77) | .039 |
| Intra-Thoracic Fat, cm2 | ||||
| Unadjusteda | −2.11 (−3.99, −0.23) | .028 | 4.03 (1.71, 6.35) | .001 |
| + Demographicsb | −2.72 (−4.34, −1.09) | .001 | 2.80 (0.67, 4.93) | .010 |
| + CVD Risk Factorsc | −2.01 (−3.64, −0.39) | .015 | 2.20 (0.19, 4.21) | .032 |
| + BMI | −0.85 (−2.20, 0.50) | .217 | 0.72 (−1.07, 2.51) | .429 |
| + Inflammatory markersd | −0.81 (−2.26, 0.64) | .272 | 0.92 (−0.93, 2.77) | .329 |
| Visceral Fat, cm2 | ||||
| Unadjusteda | −3.76 (−5.28, −2.24) | <.001 | 2.19 (0.08, 4.29) | .042 |
| + Demographicsb | −3.79 (−5.14, −2.44) | <.001 | 2.04 (0.03, 4.04) | .046 |
| + CVD Risk Factorsc | −2.88 (−4.23, −1.58) | <.001 | 1.32 (−0.60, 3.23) | .177 |
| + BMI | −1.85 (−3.10, −0.60) | .004 | 0.48 (−1.25, 2.20) | .589 |
| + Inflammatory markersd | −1.74 (−2.99, −0.50) | .006 | 0.44 (−1.29, 2.61) | .620 |
| Intermuscular Fat, cm2 | ||||
| Unadjusteda | −0.49 (−0.77, −0.20) | .001 | 0.26 (−0.09, 0.64) | .186 |
| + Demographicsb | −0.54 (−0.79, −0.28) | <.001 | .030 (−0.27, 0.44) | .883 |
| + CVD Risk Factorsc | −0.44 (−0.69, −0.16) | .002 | −0.05 (−0.35, 0.40) | .800 |
| + BMI | −0.21 (−0.45, −0.03) | .088 | −0.18 (−0.52, 0.15) | .284 |
| + Inflammatory markersd | −0.22 (−0.48, −.032) | .087 | −0.22 (−0.57, 0.14) | .230 |
| Subcutaneous Fat, cm2 | ||||
| Unadjusteda | −6.06 (−8.98, −3.14) | <.001 | 0.31 (−3.83, 4.45) | .883 |
| + Demographicsb | −4.57 (−7.16, −1.98) | .001 | 2.26 (−1.85, 6.37) | .281 |
| + CVD Risk Factorsc | −3.98 (−6.81, −1.15) | .006 | 1.92 (−2.20, 6.04) | .360 |
| + BMI | −0.70 (−2.70, 1.31) | .497 | −0.62 (−3.51, 2.29) | .616 |
| + Inflammatory markersd | −0.70 (−2.70, 1.30) | .494 | −0.78 (−3.66, 2.10) | .594 |
Physical activity and sitting hours entered simultaneously
Adjusted for age, sex, and race/ethnicity
hypertension, diabetes, smoking, HDL, LDL, and triglycerides
adiponectin, IL-6, leptin, and TNF-α
After adjusting for demographics, leisure sitting hours was significantly associated with greater pericardial fat, such that each hour of daily sitting was associated with an increase of 3.94 cm2 of pericardial fat (p = 0.003). This remained significant after controlling for CVD risk factors and BMI, and still significant after further controlling for inflammatory markers (β = 2.45, p = 0.039). Conversely, sitting hours showed no association with intermuscular or subcutaneous fat in any model (for all models p > 0.1). Initial associations with visceral fat and intra-thoracic fat were attenuated after adjusting for CVD risk factors (visceral) and then BMI (intra-thoracic).
Table 5 shows results from multivariate analyses of the association of sitting time and physical activity with total abdominal muscle area and average psoas muscle area. Physical activity was significantly associated with greater total abdominal muscle when adjusting for demographics (β = 0.60, p = 0.015), yet this was attenuated after controlling for CVD risk factors and BMI. Physical activity was not significantly associated with psoas muscle in any model (for all models p > 0.10)
Table 5. Association of physical activity and leisure sitting time with muscle.
| Physical Activity Hours (per week) |
Sitting Hours (per day) |
|||
|---|---|---|---|---|
|
|
||||
| Beta (95% CI) | p-value | Beta (95% CI) | p-value | |
|
|
||||
| Total Abdominal Muscle | ||||
| Unadjusteda | 0.69 (.033, 1.34) | .039 | −0.23 (−1.12, 0.66) | .610 |
| + Demographicsb | 0.60 (0.12, 1.09) | .015 | −0.42 (−1.09, 0.25) | .218 |
| + CVD Risk Factorsc | 0.47 (−0.06, 1.00) | .082 | −0.33 (−1.04, 0.38) | .358 |
| + BMI | 0.31 (−0.18, 0.80) | .219 | −0.15 (−0.84, 0.55) | .676 |
| + Inflammatory markersd | 0.25 (−0.27, 0.77) | .348 | −.017 (−0.73, 0.70) | .963 |
| Average Psoas Muscle | ||||
| Unadjusteda | .051 (−.016, 0.118) | .132 | −.020 (−.111, 0.07) | .657 |
| + Demographicsb | .038 (−.008, 0.085) | .109 | −.063 (−0.128, .001) | .055 |
| + CVD Risk Factorsc | .034 (−.014, .082) | .167 | −.065 (−0.133, .004) | .064 |
| + BMI | .019 (−.029, .066) | .443 | −.050 (−0.118, .017) | .144 |
| + Inflammatory markersd | .024 (−.027, .075) | .359 | −.048 (−0.119, .022) | .179 |
Physical activity and sitting hours entered simultaneously
Adjusted for age, sex, and race/ethnicity
hypertension, diabetes, smoking, HDL, LDL, and triglycerides
adiponectin, IL-6, leptin, and TNF-α
Sitting hours were not associated with total abdominal muscle in any of the models (for all p > 0.20) (Table 5). After adjusting for demographics, sitting hours were moderately and negatively associated with psoas muscle (β = −0.063, p = 0.055), yet this association was attenuated and no longer significant after adjusting for further covariates.
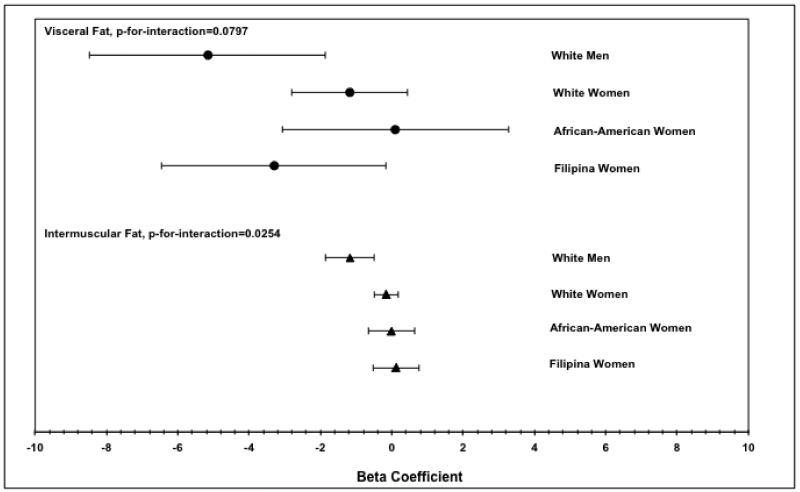
We also examined interactions of sex and racial/ethnic groups with physical activity and with sitting time in models adjusted for age, CVD risk factors, and BMI. There were no significant interactions between sitting time and demographics with any of the physiological outcomes. However, there was a significant interaction of physical activity hours and demographics for intermuscular fat area (p < .05) and a moderate interaction for visceral fat area (p = .08). Examination of the data revealed that for both visceral and intermuscular fat, the inverse association between physical activity and fat was stronger for Caucasian men than for women of any race/ethnicity (Figure 1).
Figure 1. Associations between weekly hours of physical activity and visceral fat (top) and intermuscular fat (bottom) by gender and race/ethnicity.
Lines represent 95% confidence intervals. Betas shown are from models adjusting for age, BMI, smoking status, hypertension, diabetes, LDL, HDL and triglycerides.
Discussion
In this multi-ethnic cross-sectional analysis of older adults, leisure sitting hours and physical activity were differentially associated with distinct measures of regional body composition. Consistent with previous literature, high levels of physical activity were associated with less visceral, even after controlling for demographics and classic CVD risk factors, including BMI(25). Inflammatory markers did not appear to mediate these associations. Conversely, sedentary time showed no significant association with visceral fat. Rather, sitting was significantly associated with a greater area of pericardial fat, and this association continued to be significant after adjusting for all covariates, while the association between physical activity and pericardial fat became increasingly null after controlling for related covariates. Overall, associations between fat and physical activity were stronger than those between fat and sitting time.
While the cross-sectional nature of the study precludes definitive causal conclusions, the greater deposition of pericardial fat in more sedentary individuals could be a partial explanation for the association between sitting time and cardiovascular disease(20). This association persisted after controlling for BMI, suggesting that sitting is related to a selective deposition of fat around the heart, a phenomenon robustly associated with cardiovascular morbidity and mortality(22,23,32,37). These results emphasize that, if the association is causal, the effects of sitting may not be limited to a general increase in overall adiposity. Importantly, this also underscores a limitation of self-reported physical activity as a protective health behavior. Specifically, greater physical activity may decrease overall adiposity and visceral fat specifically, but it does not appear to specifically decrease pericardial fat, and thus may not counteract all the adverse physiological effects of sitting. Individuals who are physically active and have a normal BMI may be unaware of potential cardiovascular risks caused by sedentary behavior.
The strong inverse association between physical activity and visceral fat is consistent with literature reporting selective mobilization of visceral adipose tissue in response to physical activity(33,38). Visceral fat has also emerged as a significant cardiovascular risk factor(8,24,28). Importantly, though, some studies show that visceral and pericardial fat are associated with different risk factors. For example, Rosito et al. showed that pericardial fat was more strongly associated with cardiovascular risk factors, such as coronary artery calcification, while visceral fat was more strongly associated with metabolic risk factors, such as fasting glucose and metabolic syndrome(32). However, sedentary behavior has been linked specifically to metabolic disorders, such as diabetes and metabolic syndrome(17,18). Data from the current study suggest that this is likely due to factors other than changes in regional body fat, and may be due more to overall adiposity and/or other factors, such as glucose metabolism, which recent laboratory studies show is improved by breaks in sitting time(11). More research is needed to determine the distinct pathological effects of each type of fat and how these endpoints might be associated with different behaviors. The strong negative relationship between physical activity and visceral fat, however, emphasizes the importance of physical activity as a health behavior, regardless of levels of sedentary behavior.
The mechanisms responsible for determining fat distribution are not well understood, and the current data cannot speak to why different behaviors were associated with fat deposition in different locations. It has been suggested that visceral fat is especially sensitive to the adrenal-driven adipocyte lipolysis that occurs with vigorous exercise(27), which could explain preferential reductions in visceral fat in more physically active individuals. While past studies have shown significant associations between inflammation and both sedentary behavior(1,15) and physical activity(7,19), our data showed little effect of adjustment for common inflammatory markers, suggesting that inflammation is likely not a mechanism linking these behaviors and fat distribution.
Although the associations were in the anticipated directions (i.e. greater physical activity associated with greater muscle and vice versa), we found no associations between muscle area and physical activity or sedentary time. This is not surprising, as the population was primarily engaged in light cardiovascular exercise (e.g. walking) rather than strengthening exercises.
Interactions were observed between physical activity hours and race/gender group, such that the inverse association between activity and both visceral and intermuscular fat was stronger for Caucasian men than for Caucasian women, African American women, and Filipino women. This association was also more strongly inverse for Filipino women than for other women, though to a much smaller extent than the difference seen in men. This difference could be due to higher fat volumes in men and Filipino women, who have been found to have much higher volumes of visceral fat relative to BMI than women of other races/ethnicities(2). The difference for men could also be partially due to greater muscle volume in men, which could increase metabolism of visceral or intermuscular fat in response to physical activity.
A major limitation to the current data is that physical activity and sedentary time were measured by self-report, and were not further validated. Self-reported sitting time was limited to leisure sitting time, and did not distinguish between leisure activities such as television watching and reading, which may not have equal effects on health(18). Similarly, only leisure time physical activity was measured, thus transportation and occupational activity were not included. Despite this, self-reported physical activity was relatively high for this age group, with an average or 2.56 hours per week, and with >70% of individuals reporting regular walking for exercise. The clear next step in this line of research is to determine if these associations are also observed when using objective measures of activity and sitting, and to examine associations with sitting and activity in other domains such as transport and occupation. That this is a cross-sectional study also limits the interpretations of the findings, though the physical activity data are consistent with randomized trials showing decreases in visceral fat with greater physical activity(21,36). To date, however, there have been no fully powered randomized trials assessing changes in sitting time. While associations have robustly been shown between sitting time and morbidity and mortality in cross-sectional and cohort studies, randomized trials are needed to confirm a causal link between sitting and disease, and to evaluate physiological changes associated with decreased sitting time.
In conclusion, physical activity and sedentary behavior appear to have distinct associations with areas of fat deposition. These data highlight a need for more systematic causal evaluations of the effects of sitting on body composition. The current findings do suggest, however, that sedentary behavior and physical activity are distinct behaviors with unique associations with physiology rather than opposing ends of the same spectrum.
Acknowledgments
The authors would like to thank the participants of the Rancho Bernardo Study, the UCSD Filipino Women’s Health Study, and the HASAAW for their participation in these studies.
Funding Sources
This work was supported by R21HL089622 from the National Heart Lung and Blood Institute to CLW. GAL and MRA were also supported by this award. GAL was supported by an American Heart Association award. BAL was supported by T32HL079891 from the National Heart, Lung, and Blood Institute.
Footnotes
Conflict of Interest
The authors have no conflicts to disclose. The results of the present study do not constitute endorsement by ACSM.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allison MA, Jensky NE, Marshall SJ, Bertoni AG, Cushman M. Sedentary behavior and adiposity-associated inflammation: the Multi-Ethnic Study of Atherosclerosis. Am. J. Prev. Med. 2012;42(1):8–13. doi: 10.1016/j.amepre.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araneta MR, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes. Res. 2005;13(8):1458–1465. doi: 10.1038/oby.2005.176. [DOI] [PubMed] [Google Scholar]
- 3.Bauman AE. Updating the evidence that physical activity is good for health: an epidemiological review 2000-2003. J. Sci. Med. Sport. 2004;7(1 Suppl):6–19. doi: 10.1016/s1440-2440(04)80273-1. [DOI] [PubMed] [Google Scholar]
- 4.Beunza JJ, Martinez-Gonzalez MA, Ebrahim S, et al. Sedentary behaviors and the risk of incident hypertension: the SUN Cohort. Am. J. Hypertens. 2007;20(11):1156–1162. doi: 10.1016/j.amjhyper.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Blanck HM, McCullough ML, Patel AV, et al. Sedentary behavior, recreational physical activity, and 7-year weight gain among postmenopausal U.S. women. Obesity (Silver Spring) 2007;15(6):1578–1588. doi: 10.1038/oby.2007.187. [DOI] [PubMed] [Google Scholar]
- 6.Carroll JF, Chiapa AL, Rodriquez M, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;16(3):600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 7.Colbert LH, Visser M, Simonsick EM, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2004;52(7):1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 8.Despres JP. Cardiovascular disease under the influence of excess visceral fat. Crit Pathw Cardiol. 2007;6(2):51–59. doi: 10.1097/HPC.0b013e318057d4c9. [DOI] [PubMed] [Google Scholar]
- 9.Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am. J. Clin. Nutr. 2009;90(3):499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunstan DW, Barr EL, Healy GN, et al. Television viewing time and mortality: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Circulation. 2010;121(3):384–391. doi: 10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]
- 11.Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35(5):976–983. doi: 10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunstan DW, Thorp AA, Healy GN. Prolonged sitting: is it a distinct coronary heart disease risk factor? Curr. Opin. Cardiol. 2011;26(5):412–419. doi: 10.1097/HCO.0b013e3283496605. [DOI] [PubMed] [Google Scholar]
- 13.Folsom AR, Kaye SA, Sellers TA, et al. Body fat distribution and 5-year risk of death in older women. JAMA. 1993;269(4):483–487. [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 15.Fung TT, Hu FB, Yu J, et al. Leisure-time physical activity, television watching, and plasma biomarkers of obesity and cardiovascular disease risk. Am. J. Epidemiol. 2000;152(12):1171–1178. doi: 10.1093/aje/152.12.1171. [DOI] [PubMed] [Google Scholar]
- 16.Healy GN, Clark BK, Winkler EA, Gardiner PA, Brown WJ, Matthews CE. Measurement of adults’ sedentary time in population-based studies. Am. J. Prev. Med. 2011;41(2):216–227. doi: 10.1016/j.amepre.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healy GN, Wijndaele K, Dunstan DW, et al. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Diabetes Care. 2008;31(2):369–371. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 19.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J. Am. Coll. Cardiol. 2005;45(10):1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 20.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med. Sci. Sports Exerc. 2009;41(5):998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 21.Kay SJ, Fiatarone Singh MA. The influence of physical activity on abdominal fat: a systematic review of the literature. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2006;7(2):183–200. doi: 10.1111/j.1467-789X.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 22.Konishi M, Sugiyama S, Sugamura K, et al. Association of pericardial fat accumulation rather than abdominal obesity with coronary atherosclerotic plaque formation in patients with suspected coronary artery disease. Atherosclerosis. 2010;209(2):573–578. doi: 10.1016/j.atherosclerosis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur. Heart J. 2009;30(7):850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuzawa Y, Nakamura T, Shimomura I, Kotani K. Visceral fat accumulation and cardiovascular disease. Obes. Res. 1995;3(Suppl 5):645S–647S. doi: 10.1002/j.1550-8528.1995.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 25.McGuire KA, Ross R. Incidental physical activity and sedentary behavior are not associated with abdominal adipose tissue in inactive adults. Obesity (Silver Spring) 2012;20(3):576–582. doi: 10.1038/oby.2011.278. [DOI] [PubMed] [Google Scholar]
- 26.Morgan RE, Palinkas LA, Barrett-Connor EL, Wingard DL. Plasma cholesterol and depressive symptoms in older men. Lancet. 1993;341(8837):75–79. doi: 10.1016/0140-6736(93)92556-9. [DOI] [PubMed] [Google Scholar]
- 27.Murphy JC, McDaniel JL, Mora K, Villareal DT, Fontana L, Weiss EP. Preferential reductions in intermuscular and visceral adipose tissue with exercise-induced weight loss compared with calorie restriction. J. Appl. Physiol. 2012;112(1):79–85. doi: 10.1152/japplphysiol.00355.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura T, Tokunaga K, Shimomura I, et al. Contribution of visceral fat accumulation to the development of coronary artery disease in non-obese men. Atherosclerosis. 1994;107(2):239–246. doi: 10.1016/0021-9150(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 29.Owen N, Sparling PB, Healy GN, Dunstan DW, Matthews CE. Sedentary behavior: emerging evidence for a new health risk. Mayo Clin. Proc. 2010;85(12):1138–1141. doi: 10.4065/mcp.2010.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paffenbarger RS, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med. Sci. Sports Exerc. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Reaven PD, Barrett-Connor E, Edelstein S. Relation between leisure-time physical activity and blood pressure in older women. Circulation. 1991;83(2):559–565. doi: 10.1161/01.cir.83.2.559. [DOI] [PubMed] [Google Scholar]
- 32.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117(5):605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 33.Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Med. Sci. Sports Exerc. 2001;33(6 Suppl):S521–527. doi: 10.1097/00005768-200106001-00023. discussion S528-529. [DOI] [PubMed] [Google Scholar]
- 34.Sardinha LB, Andersen LB, Anderssen SA, et al. Objectively measured time spent sedentary is associated with insulin resistance independent of overall and central body fat in 9-to 10-year-old Portuguese children. Diabetes Care. 2008;31(3):569–575. doi: 10.2337/dc07-1286. [DOI] [PubMed] [Google Scholar]
- 35.Sisson SB, Camhi SM, Church TS, et al. Leisure time sedentary behavior, occupational/domestic physical activity, and metabolic syndrome in U.S. men and women. Metab Syndr Relat Disord. 2009;7(6):529–536. doi: 10.1089/met.2009.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slentz CA, Aiken LB, Houmard JA, et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J. Appl. Physiol. 2005;99(4):1613–1618. doi: 10.1152/japplphysiol.00124.2005. [DOI] [PubMed] [Google Scholar]
- 37.Taguchi R, Takasu J, Itani Y, et al. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001;157(1):203–209. doi: 10.1016/s0021-9150(00)00709-7. [DOI] [PubMed] [Google Scholar]
- 38.Thomas EL, Brynes AE, McCarthy J, et al. Preferential loss of visceral fat following aerobic exercise, measured by magnetic resonance imaging. Lipids. 2000;35(7):769–776. doi: 10.1007/s11745-000-0584-0. [DOI] [PubMed] [Google Scholar]
- 39.Thompson P, Buchner D, Pina I, Balady G, Williams M, Marcus BH. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Mebatolism (Subcommittee on Physical Activity) Circulation. 2003;107(24):3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 40.Thorp AA, Healy GN, Owen N, et al. Deleterious associations of sitting time and television viewing time with cardiometabolic risk biomarkers: Australian Diabetes, Obesity and Lifestyle (AusDiab) study 2004-2005. Diabetes Care. 2010;33(2):327–334. doi: 10.2337/dc09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tremblay A, Despres JP, Leblanc C, et al. Effect of intensity of physical activity on body fatness and fat distribution. Am. J. Clin. Nutr. 1990;51(2):153–157. doi: 10.1093/ajcn/51.2.153. [DOI] [PubMed] [Google Scholar]
- 42.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wassel CL, Laughlin GA, Araneta MRG, et al. Associations of pericardial and intra-thoracic fat with coronary calcium presence and progression in a multi-ethnic study. Obesity. doi: 10.1002/oby.20111. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]