Abstract
The clinical application of gene silencing mediated by small interfering RNA (siRNA) has been limited by the lack of efficient and safe carriers. Phospholipid modification of low molecular weight polyethylenimine (PEI 1.8 kDa) dramatically increased its gene down-regulation capacity while keeping cytotoxicity levels low. The silencing efficacy was highly dependent on the nature of the lipid grafted to PEI and the polymer/siRNA ratio employed. Phosphoethanolamine (DOPE and DPPE) and phosphocholine (PC) conjugation did not change the physicochemical properties and siRNA binding capacity of PEI complexes but had a large impact on their transfection and ability to downregulate Green Fluorescent Protein (GFP) expression (60%, 30% and 5% decrease of GFP expression respectively). We found that the micelle-forming structure of DOPE and DPPE-PEI dramatically changed PEI’s interaction with cell membranes and played a key role in promoting PEI 1.8 kDa transfection, completely ineffective in the absence of the lipid modification.
Keywords: siRNA delivery, Polyethylenimine, Lipid conjugation, Phospholipid, Micelle, RNA interference
1. BACKGROUND
RNA interference using a small interfering RNA (siRNA, a double-stranded RNA of 21–23 bp) has emerged as a powerful tool for silencing target genes associated with particular diseases. However, the use of siRNA is linked with some serious clinical limitations including degradation by nucleases in the blood, removal from the circulation by renal clearance, poor cellular penetration and endosomal/lysosomal degradation after siRNA internalization1–3. To overcome these barriers, the siRNA must be associated within a delivery system tailored specifically for high efficiency, and low immunogenicity and toxicity. Apart from virus-based carriers, polycations of a lipidic or polymeric nature have become popular siRNA carriers4–6.
Cationic polyethylenimine (PEI) and its derivatives have a high positive charge density that enables effective condensation of siRNA by electrostatic interactions into a more compact state, referred to as a ‘complex’ or ‘polyplex’. High molecular weight PEI is a popular gene transfection agent, both in vitro and, due to its relatively high efficiency. However, its high toxicity remains a major drawback especially for in vivo applications7–9. Lower molecular weight PEIs have better toxicity profiles but are far less efficient delivery systems achieving less than 5% reduction of gene expression10, 11.
PEI transfection efficiency has been associated with the ability of PEI complexes to avoid lysosomal degradation, which is one of the barriers to successful gene transfer after polyplex endocytosis12. The mechanism of ‘PEI escape’ from the endosomal/lysosomal pathway is still elusive. The ‘proton sponge’ hypothesis postulated by Berh remains the most generally accepted mechanism13. However, this hypothesis has been heavily debated especially in whether the osmotic stress produced by the PEI proton sponge effect can induce the rupture of the endosomes14. Recently, Benjaminsen and co-workers showed that although PEI reaches lysosomes in a high extent, it does not induce any changes in the lysosomal pH. The authors conclude that PEI might work as a proton sponge but the ATPase pump can overcome this effect and stabilize the pH, making the osmotic swelling of endosomes uncertain15. Wu and coworkers investigated the effect of free PEI chains of different length and structures in PEI 25kDa intracellular trafficking and transfection, and similarly concluded that the ‘proton sponge’ effect is not the dominant mechanism in the endosomal release of PEI complexes16. Both groups pointed out as one plausible mechanism that protonated PEI under the acidic pH might interact with endosomal membrane, inducing its destabilization and promoting the formation of pores for the escape of the complexes entrapped inside.
To improve the balance between efficacy and toxicity of PEI, various strategies have been suggested including the conjugation with receptor-specific or PEG moieties to decrease its toxicity, and the lipidation of low molecular weight PEI to increase its interaction with lipophilic cell membranes and facilitate cargo uptake. Early studies by Kim and colleagues demonstrated that lipid substitution of low molecular weight PEI, in particular cholesterol conjugation, gave rise to a “water soluble lipopolymer” (WSLP) that greatly increased plasmid DNA transfection compared to non-modified PEI 1.8 kDa and self-assembly into micelles (CMC=1.43 mg/mL, 7.1×10−4M)17, 18. Since then, a great number of hydrophobic moieties of different length and saturation level have been tested including, alkanes19 fatty acids11, 20, 21 and dialkyl phosphates22, 23. These studies show that lipid substitution of PEI (1:1) is sufficient to maintain the condensation and protection of siRNA and to produce gene down-regulation levels of approximately 60% depending on the lipid moiety used, whereas higher lipid substitutions generally reduced the integrity of such complexes and increased their toxicity19, 24.
Recently, we reported on phospholipid-modified PEI. We showed that micelle-like nanoparticles (MNPs) based on phosphatidyl choline modified-PEI (PC-PEI) delivered plasmid DNA to a distal tumor in vivo25. In addition, MNPs were found to be suitable for siRNA delivery26. On the other hand, MNPs based on phosphatidyl ethanolamine modified PEI (DOPE-PEI) showed higher siRNA silencing transfection than PC-PEIbased ones and mediated a down-regulation of P-glycoprotein expression that overcame doxorubicin resistance in breast cancer cells27. Our focus here is on finding structure/activity relationships for the optimization of these phospholipid-PEI amphiphiles.
For this study, we synthesized a novel conjugate, DPPE-PEI, which differs from DOPEPEI in the degree of saturation of the phospholipid fatty chains. We compared the PCPEI, DOPE-PEI and DPPE-PEI potential as siRNA carriers, and discuss the effect of the phospholipid components and the amount of conjugate on their in vitro performance. In particular, we evaluated phospholipid-PEI conjugates for self-assembly, condensation and protection of siRNA, delivery of siRNA to the cellular cytoplasm and downregulation of Green Fluorescent Protein (GFP) expression.
2. MATERIALS AND METHODS
2.1. Materials
All materials used were purchased from Sigma-Aldrich unless otherwise stated. Branched polyethylenimines (PEI) with molecular weights of 1.8 kDa and 25 kDa were purchased from Polysciences, Inc (Warrington, PA). Glutaryl head-modified phospholipids 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(glutaryl) (NG-DOPE) and dipamitoyl-sn-glycero-3-phosphoethanolamine-N-(glutaryl) (NG-DPPE), the oxidized phospholipid 1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine (Az PC Ester) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethyleneglycol)-2000] (PEG-PE) were purchased from Avanti Polar Lipids (Alabaster, AL). A siRNA duplex targeting Green Fluorescent Protein (GFP-siRNA): 5’-AUGAACUUCAGGGUCAGCUdTdT-3’ (sense), a non-targeting control duplex, (Negative-siRNA): 5’-AGUACUGCUUACGAUACGGdTdT-3’ (sense) and DY-547 (Red) or 6-FAM (Green) fluorescence-labeled siRNA were purchased from Dharmacon (Lafayette, CO). RNase III was purchased from Ambion/Life Technologies (Carlsbad, CA). The CellTiter-Blue® Cell Viability Assay was purchased from Promega (Madison, WI). Nuclease-free water was purchased from Qiagen (Germantown, MD).
The cell lines c166 (mouse yolk sac embryo) and c166-GFP (c166 cells stably transfected with a plasmid reporter vector, pEGFP-N1, encoding for the enhanced green fluorescent protein, GFP) were obtained from the American Type Culture Collection (Manassas, VA). Cells were grown at 37°C under 5% CO2 in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 units/mL) and streptomycin (100 µg/mL). For c166-GFP cells, DMEM media was supplemented with 10% FBS and 0.2 mg/mL of Geneticin (G-418, Life Technologies, Carlsbad, CA). Cell culture media and penicillin/streptomycin stock solutions were purchased from Cellgro (Herndon, VA). Heat-inactivated fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA).
2.2. Methods
2.2.1 Synthesis of phospholipid-polyethylenimine conjugates
The DOPE-PEI and DPPE-PEI conjugates were synthesized from PEI 1.8 kDa and the glutaryl head-modified phospholipids: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(glutaryl) (DOPE-NG) and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(glutaryl) (DPPE-NG) as described in28. Briefly, NG-DOPE or NG-DPPE (5.5 µM) in chloroform (1mL) were activated with N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide HCl (EDCI) and N-hydroxysuccinimide (NHS) followed by addition of PEI 1.8kDa (5.5 µM). The mixture was incubated with 4 µL of triethylamine (TEA) at room temperature for 24 h with stirring. The chloroform was removed under nitrogen gas and the residue was suspended with 1 mL of dH2O. The products were purified by dialysis (MWCO 2000 Da) against dH2O and lyophilized.
The PC-PEI conjugate was synthesized from PEI 1.8 kDa and the oxidized phospholipid 1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine (Az PC Ester) as described in25. Briefly, 12 mg of the branched PEI (7µmol) were dissolved in 0.5 mL of chloroform and mixed with 5 mg of the oxidized PC (AzPC Ester, 7µmol) dissolved in 1 mL of chloroform. Carbonyldiimidazole (CDI, 0.5mg, 3µmol) was added to the above solution for the activation of acid by formation of an imidazole derivative. The mixture was incubated with 10 µL of TEA at room temperature for 24 h with stirring. The chloroform was removed under nitrogen gas and the residue was suspended with 2 mL of dH2O. The products were purified by dialysis (MWCO 2000 Da) against dH2O and lyophilized.
The synthesized conjugates were characterized by 1H nuclear magnetic resonance (NMR) spectroscopy and MALDI-TOF mass spectrometry. NMR spectroscopy was performed using a Varian 400 MHz spectroscope.
2.2.2. Determination of critical micelle concentration (CMC) of the conjugates
CMC was estimated by the pyrene method29. Briefly, phospholipid-PEI conjugates were serially diluted with dH2O to obtain 10 −7to 10 −4M solutions. Then, 1mL of each dilution was added to pyrene-coated glass tubes containing 0.5 mg of dry pyrene crystals. The mixtures were kept overnight in a shaking incubator at 150 rpm at 25 °C. Free pyrene was removed by filtration through polycarbonate membranes with a 0.2 µm pore size. The filtered samples were transferred to a 96-well plate and the fluorescence was measured using a plate reader (Multiscan MCC/340, Fisher Scientific) at excitation and emission wavelengths of λex 360 and λem 460 nm, respectively. CMC values were determined from the pyrene fluorescence in solution as a function of conjugate concentration, and corresponded to concentrations at which a sharp increase in the solution fluorescence occurred. The amphiphile PEG-PE was used as a reference.
2.2.3. Cytotoxicity of phospholipid-PEI conjugates
For cytotoxicity studies, c166 GFP cells were seeded in 96-well plates (104 cells/well). After 24 h, the media was replaced with 100 µL/well serial dilutions of PL-PEI conjugates and non-modified 1.8 kDa PEI. Additionally, 25kDa PEI was used as a control. After 4 hours of incubation, the cells were washed twice with fresh media and returned to complete media (100 µL). After 24 h incubation, 20 µL of CellTiter-Blue®(Promega) was added to each well and the plates re-incubated for 2 h. The fluorescence was measured at excitation and emission wavelengths of 560 nm and 590 nm, respectively. When possible, sigmoidal curves of cell viability vs log concentration were transformed into pseudo-Hill plots, and the polymer concentrations at 50% cell death (IC50) were calculated.
2.2.4. Preparation and characterization of phospholipid-PEI/siRNA complexes
Complexes were prepared by mixing a fixed amount of siRNA and varying amounts of phospholipid-PEI conjugates that were diluted separately in equal volumes (50 µL) of buffered HEPES glucose (BHG, pH 7.4, nuclease-free buffer). The siRNA solution was transferred to the polymer solution, mixed by smooth pipetting and incubated for 15–20 min. The polymer/siRNA ratio was expressed as the nitrogen/phosphate (N/P) ratio and calculated assuming that 43 g/mol corresponded to each repeating unit of PEI containing one amine and 316 g/mol corresponded to each repeating unit of siRNA containing one phosphate. The molecular weight of the grafted lipids was also factored in to calculate the amount of polymer in the complex.
Complex formation was studied by gel retardation. Complexes containing 750 ng of siRNA with varying amounts of phospholipid-PEI were electrophoresed through a 0.8% agarose gel, using the E-Gel electrophoresis system (Life Technologies) and evaluated under UV-light.
Nuclease resistance of the siRNA in phospholipid-PEI complexes was evaluated by incubation of phospholipid-PEI/siRNA complexes at an N/P ratio of 16 with 1U of RNase III/ µg siRNA for 2h at 37°C. The progression of the enzymatic degradation was stopped by the incubation of each sample with 30mM EDTA for 5 min. Complexes were then disassembled by adding heparin (10U/ µg siRNA) and analyzed by agarose gel electrophoresis. The integrity of siRNA in complexes in the presence or the absence of enzyme was compared with that of naked siRNA under the same experimental conditions.
The particle size and zeta potential of the formulations were measured using a Zeta Plus Particle Analyzer (Brookhaven Instruments Corp, Santa Barbara, CA). Scattered light intensity (DLS) was detected at 25°C at an angle of 90°. Samples (100 µL) of complexes were diluted in 1.7 mL of nuclease-free water and measured immediately after preparation. The final siRNA concentration in the samples was 5–10 µg/mL.
Phospholipid-modified PEI, siRNA and complexes were characterized by Atomic Force Microscopy (AFM) (see Supplementary Material).
2.2.5. Green Fluorescent Protein (GFP) silencing
In vitro GFP silencing experiments were performed in stably transfected c166 GFP cells using GFP-siRNA. A non-targeting control duplex (Negative-siRNA, scramble siRNA) was used as a non-specific control siRNA. In a typical experiment, cells were seeded 24 h prior to transfection in 12-well plates at a density of 5 × 104 per well and complete medium was replaced with fresh serum-free medium. Phospholipid-PEI complexes at varying N/P ratios were added to cells to yield a final siRNA concentration of 100 nM. After 4h of incubation, the complexes were removed and fresh complete media was added. The cells were further incubated for 48 h. Thereafter, the cells were washed, detached by trypsinization, and GFP down-regulation analyzed by flow cytometry. In selected experiments, c166 GFP cells were pre-incubated for 1h with chloroquine (50 µM) or bafilomycin A1 (175 nM) before siRNA transfection.
2.2.6. Intracellular delivery of phospholipid-PEI/siRNA complexes
The ability of phospholipid-PEI complexes to deliver siRNA into the cells was studied by microscopy and flow cytometry using a fluorescence-labeled siRNA. The condensation of fluorescent siRNA with the different conjugates did not affect the intrinsic fluorescence of siRNA. Briefly, cells were seeded into 12-well plates (5 × 104 cells/well) and incubated 24 h prior to transfection. Cells were treated with complexes prepared with a siRNA concentration of 100 nM at various N/P ratios. After incubation for 1 h or 4 h with complexes, the cells were washed with PBS and trypsinized. The fluorescence from siRNA inside the cells was detected with a Becton-Dickinson FACSort™ flow cytometer (Franklin Lakes, NJ). For the distinction between internalized and surface-bound siRNA, trypan blue (1.2 mg/ml) was used to quench surface-bound fluorescence. Data was analyzed with CellQuest™ software (Becton-Dickinson).
For better visualization of the siRNA in microscopy experiments, non-GFP expressing c166 cells were employed. Briefly, cells were seeded into 6-well plates (3 × 103 cells /well) and incubated 24 h prior transfection. Cells were treated with complexes for 4 h, washed with PBS and incubated with Hoechst 33342 for 15 min. The cells were washed several times with PBS. The cover slips were mounted with Fluoromount-G medium and examined with a Confocal Zeiss LSM 700 microscope. Zeiss ZEN2009 software was used for set up, and Image J software was used for image processing.
2.2.7. Statistical analysis
Results are presented as mean ± SD. Unless otherwise stated, comparisons between the groups were made using Student’s t test. P values less than 0.05 were considered to indicate a significant difference.
3. RESULTS
3.1. Biophysical characterization and enzymatic stability of phospholipid-PEI/siRNA complexes
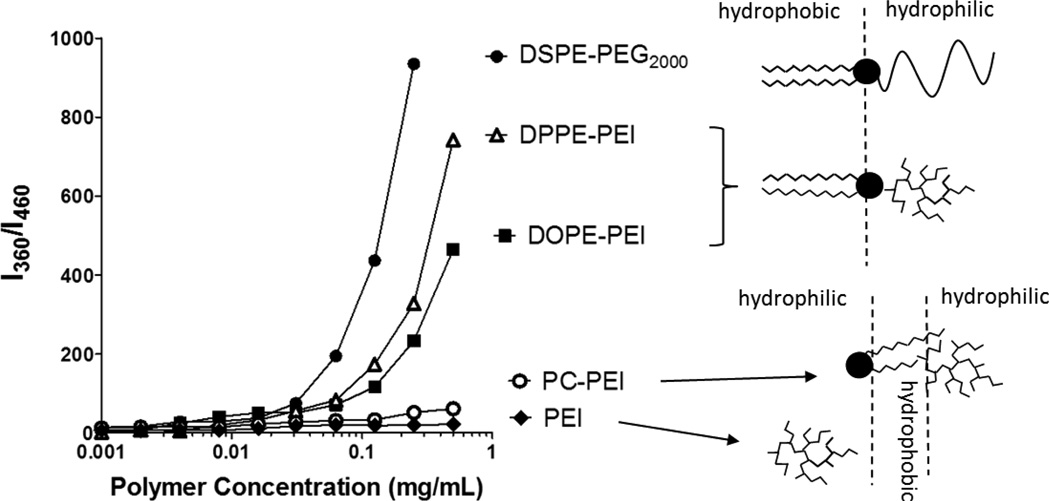
Polyethylenimine (PEI 1.8kDa) was modified with phosphoethanolamine (DPPE-PEI, DOPE-PEI) or phosphocholine phospholipids (PC-PEI) to obtain different structures (Supplementary Figure 1). The molar ratio between PEI and phospholipids was kept at 1:1. After synthesis followed by purification, the polymers were characterized by 1H-NMR spectroscopy. The characteristic peaks of PEI and the lipids in the NR spectra of the conjugates indicate successful conjugation of lipids with PEI (Supplementary Figure 1). Conjugates between hydrophilic PEI and hydrophobic phospholipids self-assembled into micellar structures due to their amphiphilic nature. The self-association of the different conjugates is shown in Figure 1. Both DOPE-PEI and DPPE-PEI conjugates with a hydrophilic-hydrophobic di-block structure assembled with critical micelle concentrations (CMC) of 97 µg/mL and 72 µg/mL, respectively, into 20 nm-micellar structures (mean diameter by DLS). The CMC measured for PEG2000-DSPE used as a reference amphiphile was 43µg/mL (1.5 × 105M) in agreement with reported values30. The hydrophobic-hydrophilic-hydrophobic architecture of PC-PEI did not facilitate micellation. The absence of a sharp increase in the fluorescence indicated the minimal solubilization capacity of the PC-PEI conjugate which was similar to that of nonmodified PEI. The detailed structure of the amphiphile assemblies was investigated by AFM (Supplementary Figure 2).
Figure 1.
Self-association of phospholipid-PEI conjugates. The critical micelle concentration (CMC) of conjugates was determined from fluorescence of the pyrene incorporated into the hydrophobic core of the micelles. The absence of a sharp increase in the fluorescence indicated the minimal solubilization capacity of the PC-PEI conjugate and non-modified PEI.
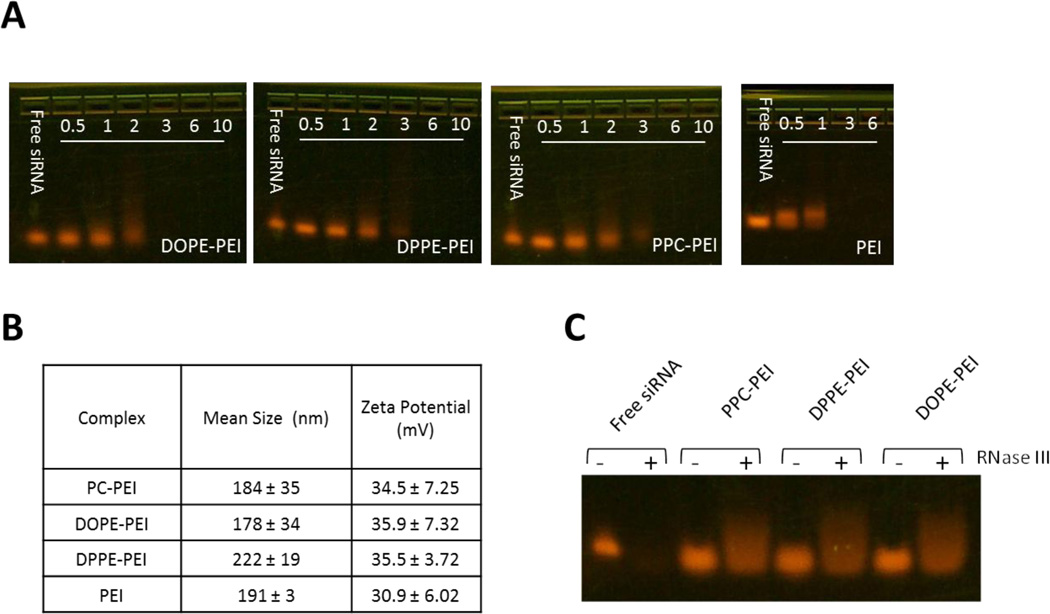
Phospholipid-grafting of PEI did not affect the siRNA condensation ability of PEI or the size and the zeta potential of the complexes. All the conjugates (PC-PEI, DPPE-PEI and DOPE-PEI) neutralized the anionic charge of siRNA and spontaneously collapsed into small and positively charged particles with a mean diameter of 150–200 nm and a positive zeta potential of 30–35 mV (N/P 16) (Figure 2B). Complexes prepared at N/P ratios of 3 or higher completely retarded the migration of siRNA in the agarose gel (Figure 2A). The formation of complexes between siRNA and lipid-modified PEI was also confirmed by AFM. The images showed complex structures that were different from individual polymers or free uncondensed siRNA (Supplementary Figure 3).
Figure 2.
Analysis of complex formation. (A) Gel retardation of complexes at varying N/P ratios. No migration of siRNA into the gel indicates the complex formation at N/P ≥ 3. (B) Particle size and zeta potential of complexes as at N/P ratio of 16. (C) Protection of siRNA within complexes against RNAse III degradation.
The stability of the condensed siRNA against enzymatic degradation within the different formulations was tested by using RNase III. The integrity of siRNA was checked by agarose electrophoresis and quantified by Image J software (Figure 2C). Control naked siRNA was completely digested after the enzymatic treatment. By contrast, condensed siRNA was protected from nuclease degradation. Due to interference of the enzyme, the migration of siRNA was slightly retarded but the overall intensity of the siRNA bands before and after the enzymatic treatment was the same for all the formulations.
3.2. In vitro gene silencing
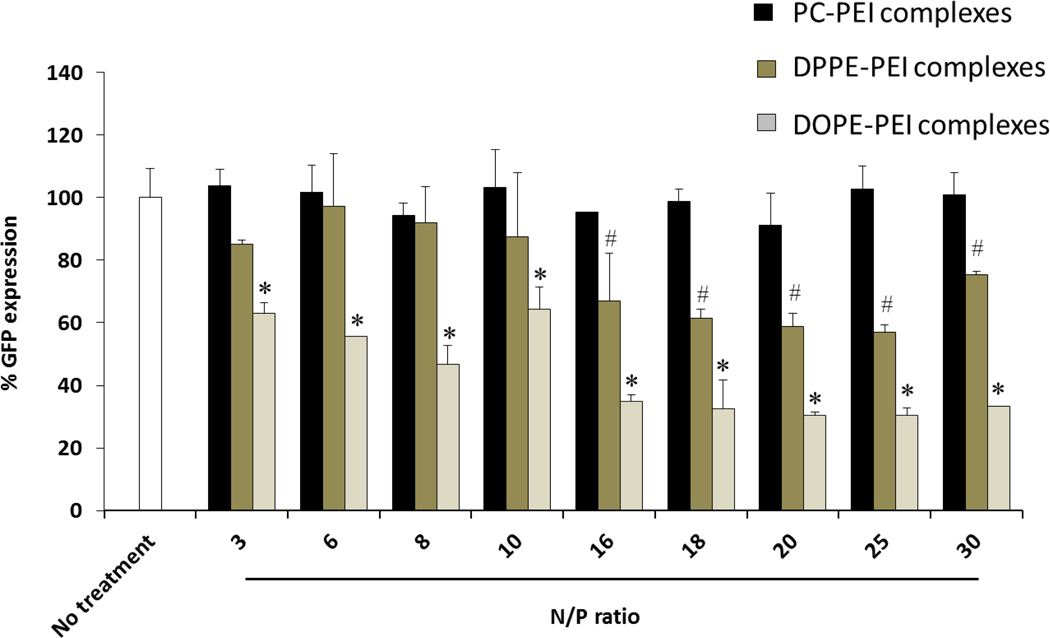
The silencing efficacy of PC-PEI, DPPE-PEI and DOPE-PEI was evaluated in c166 stably expressing GFP (c166-GFP) using GFP-targeted siRNA or scrambled siRNA. The silencing of GFP was measured by the decrease in the mean fluorescence of the cells after siRNA treatment. The complexes were prepared at a range of N/P ratios from 3 to 30. No detectable GFP suppression was observed with non-modified PEI (1.8 kDa) based-complexes regardless of the N/P employed (data not-shown). At N/P ratios ≥16, DOPE-PEI complexes produced the highest protein suppression (60% reduction of GFP expression) followed by DPPE-PEI complexes (30% reduction). PC-PEI complexes were the least effective with less than a 5% of protein content reduction (Figure 3).
Figure 3.
GFP silencing efficacy of phospholipid-PEI/siRNA complexes (PC-PEI, DPPE-PEI and DOPE-PEI) prepared at various N/P ratios. Data are expressed as the mean ± SD (n=3). (ANOVA, *# P< 0.05 vs scramble siRNA formulations).
3.3. Cellular association and intracellular delivery
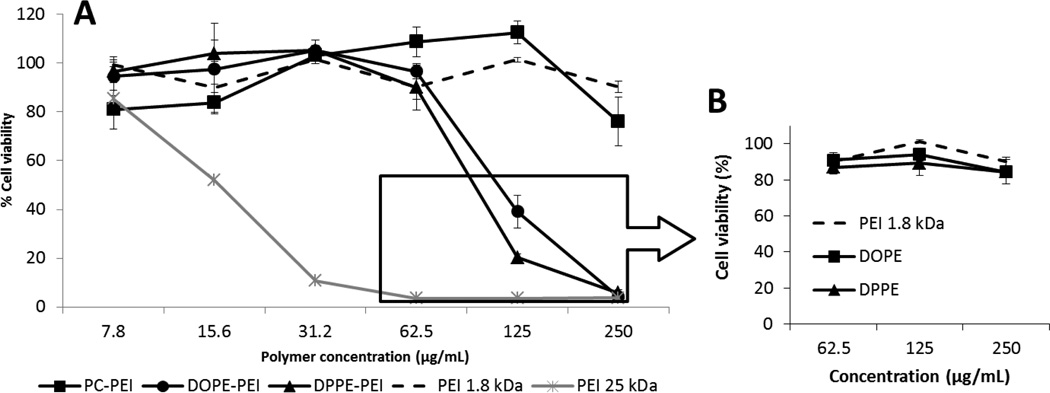
We asked whether the differences observed in the gene silencing of the conjugates could be explained by their different interaction with cell membranes. First, we studied the toxicity of the conjugates as a function of their concentration (Figure 4A). An increment of phospholipid-modified PEI interaction with cells should eventually translate into progressive membrane disruption and cell death. However, PC-PEI conjugates and non-modified PEI 1.8 kDa remained non-toxic for the cells regardless of the polymer concentration employed. DOPE-PEI and DPPE-PE showed no toxic effect unless high concentrations (≥ 125 µg/mL) were reached. The IC50 values were 123 µg/mL and 104 µg/mL respectively for DOPE-PEI, DPPE-PEI conjugates. In contrast, PEI 25kDa was highly toxic for cells. (IC50 =15.4 µg/mL). Interestingly, the same high concentrations of the conjugate components themselves, free lipids (DOPE and DPPE) and free non-modified PEI 1.8 kDa were non-toxic (Figure 4B) supporting the conclusion that the increase in the interaction of the conjugates with cells can be attributed specifically to the anchoring of DOPE and DPPE phospholipids to PEI. It is also worth mentioning that the highest concentration of phospholipid-PEI conjugates used for the formulation of siRNA in our in vitro experiments was 8 µg/mL (final concentration per well) and therefore non-toxic for cells.
Figure 4.
Cytotoxicity of free phospholipid-PEI conjugates (A) and free phospholipids (B) towards GFP-c166 cells. Data are expressed as the mean ±SD (n=3).
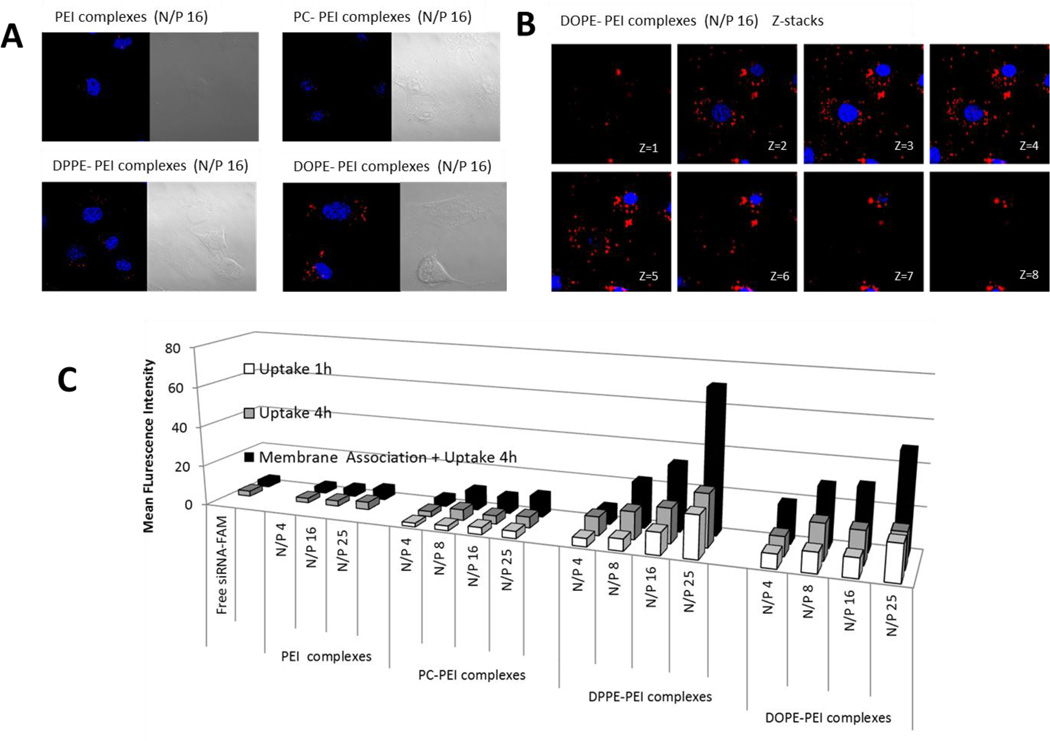
Next, we studied and compared the cellular uptake of the different complexes prepared with fluorescently-labeled siRNA. Cells treated with DPPE-PEI and DOPE-PEI complexes showed increased intracellular fluorescence compared to non-modified PEI and PC-PEI complexes (red dots in Figure 5A). Images from selected planes at various depths within the cells (Z-stack images) confirmed the presence of siRNA associated both with the cell membrane and homogenously distributed within the cytoplasm (red dots in Figure 5B). The siRNA inside the cells was quantified by flow cytometry (Figure 5C). The membrane association and uptake of complexes was strongly influenced by the lipid grafted to PEI (PC, DPPE, DOPE) and by the amount of conjugate (N/P ratio). The mean fluorescence of cells treated with DPPE-PEI, DOPEPEI and PC-PEI complexes was 23-, 16- and 3-fold higher, respectively, than that of non-modified PEI. DPPE-PEI and DOPE-PEI complexes were better taken up by cells. They showed at least a 3-fold increased cellular association and uptake compared to PCPEI complexes. In addition, the comparison between the 1 h and 4 h fluorescence uptake values showed that 50–60% of the total siRNA is internalized during the first hour of incubation. This percentage rose to 80% and 100% respectively for DPPE-PEI and DOPE-PEI complexes prepared at the highest N/P ratio of 25, supporting the conclusion that an excess of conjugate in the complex not only promotes cellular association but accelerates the internalization of siRNA.
Figure 5.
Intracellular delivery mediated by phospholipid –PEIs conjugates in c166 cells. (A) Cellular uptake of phospholipid-PEIs prepared at N/P 16 ratio after 4 h of incubation. The nuclei (blue) were stained with Hoechst dye. The internalized DY-547-siRNA appears in red. (B) Intracellular trafficking of DOPE-PEI/siRNA complexes after 4h of incubation. Selected images from sequentially numbered z-stacks are shown. C) Cellular uptake of the complexes prepared with fluorescence-labeled siRNA after 1h and 4 h of treatment with the complexes. The mean fluorescence intensity of cells after treatment with the complexes is shown. Data are expressed as the mean ± SD (n=3).
3.4. Effect of chloroquine and bafilomycin on the gene silencing mediated by DOPEPEI and DPPE-PEI complexes
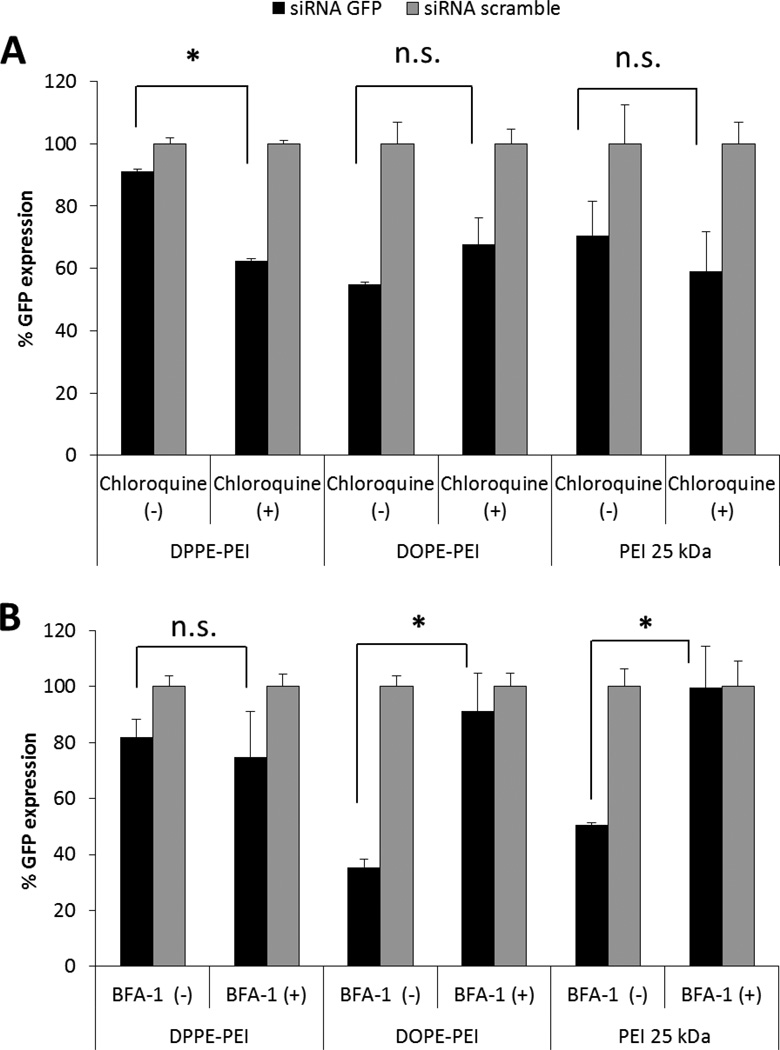
Although DPPE-PEI and DOPE-PEI carriers showed similar cell membrane interaction and siRNA uptake, DOPE-PEI displayed a more effective gene silencing. A possible reason for DOPE-PEI superiority may be the improved intracellular trafficking of siRNA complexes due to a greater escape from endosomes which is known to be a major barrier in gene delivery. In order to elucidate the mechanistic differences in the productive cytosolic entry of the conjugates, the silencing efficacy of DOPE-PEI and DPPE-PEI complexes was measured after cell pre-incubation with chloroquine, (the low molecular weight drug, which buffers lysosomes and is commonly used to improve the non-viral transfection) and bafilomycin A1 (the vacuolar ATPase proton pump inhibitor). Akinc and co-workers showed that bafilomycin dramatically decreased the transfection activity of PEI 25 kDa by preventing the acidification of endosomes and thus, the further protonation of PEI, whereas chloroquine did not affect PEI activity but increased that of quaternized counterparts31. On the other hand, PEI 1.8 kDa was completely ineffective in silencing GFP protein independently of the addition of chloroquine or bafilomycin A1, even at a N/P ratio of 30 (data not-shown). Although the buffering properties of the low molecular weight PEI have been described10, the poor cellular uptake of PEI 1.8 kDa complexes may make any later effects of these agents inside the endosomes undetectable. Therefore, PEI 25 kDa was selected as the control polymer for the mechanistic studies, and the corresponding complexes were prepared with the N/P of 6, reported to be the maximum amount of polymer that can be used to avoid toxicity7–9. As with the PEI 25kDa complexes, the silencing efficacy of DOPE-PEI complexes was not affected by the pre-treatment of cells with chloroquine. However, the silencing mediated by DPPE-PEI increased significantly (by 20%) after chloroquine treatment (Figure 6A). Similarly to PEI 25kDa, the inhibition of the ATPase by bafilomycin A1 significantly decreased the GFP-downregulation mediated by DOPE-PEI complexes while DPPE-PEI efficacy was not affected (Figure 6B).
Figure 6.
Analysis of endosomal escape of DOPE-PEI and DPPE-PEI complexes (N/P 16). The silencing efficacy of complexes was measured after pre-incubation of cells with the endosomal acidification inhibitors chloroquine and bafilomycin A1 and compared with PEI 25 kDa (N/P 4). (one-way ANOVA, Turkey's test, * P< 0.05, n.s. no significant differences)
4. DISCUSSION
The development of novel materials and the optimization of existing ones for safe and efficient siRNA delivery are key to successful clinical application of gene silencing in cancer and other diseases. PEI-based delivery systems are attractive carriers because they can protect and efficiently deliver nucleotide-based molecules to target cells. Despite broad experience in the use of PEI, the balance between the efficacy and toxicity of this carrier is still sub-optimal. Recently, we found that phospholipid modification of low molecular weight PEI dramatically increased its gene downregulation capacity while keeping cytotoxicity levels low27. In the present study, we aimed for a deeper understanding of the detailed structure and impact of DOPE-, DPPEand PC-PEI conjugations on the complexes’ properties, intracellular delivery and gene down-regulation mediated by these PEI derivatives.
Lipidation of PEI has usually been carried out in the context of plasmid DNA gene expression by cholesterol modification17. Regarding siRNA delivery, the modification of PEI with different fatty acids or alkane chains was reported to improve gene downregulation efficiency that appeared to be dependent mostly on the level of substituted lipid and the ratio of polymer to siRNA. These features affect the siRNA binding affinity and other complex properties such as surface charge, which in turns affects uptake and intracellular trafficking11, 24. For example, Schroeder and collaborators have performed gel retardation studies demonstrating that the binding affinity of alkylated PEI compounds to siRNA decreased as the conjugation levels increased, and by reducing the binding affinity within the complex, the siRNA was readily released into the cytoplasm after cellular internalization19.
Our results indicate that although the physicochemical properties and siRNA binding capacity of the conjugates were the same, their cellular interaction and silencing potency varied dramatically. DOPE, DPPE and PC conjugation did not change the size or the zeta potential of PEI complexes (see Figure 3B, N/P 16) but had a large impact on their transfection and ability to down regulate GFP expression (60%, 30% and 5% decrease of GFP expression respectively, at a ratio of N/P 16). We attributed the specificity displayed by the phospholipid-PEI conjugates to a structure-specific interaction of the conjugates with the cell surface. In particular, the modification of PEI with DOPE and DPPE produced di-block amphiphiles able to self-assembly into micellar aggregates that completely changed PEI’s interaction with cell membranes. The interaction of phospholipid-PEI conjugates either in free form (Figure 4) or as complexes (Figure 5) with the cells was dose-dependent. The amount of siRNA formulated with DOPE-PEI or DPPE-PEI associated with cells increased with increasing N/P ratio. This was in sharp contrast with non-micellizable PC-PEI, which was unable to interact with cells in free form or as a complex with siRNA. Interestingly, we observed that an excess of conjugate not only increases the cellular association, as previously reported for other lipid-PEI derivatives32, but rather promotes and accelerates siRNA internalization. As shown in Figure 5C, more than 80% of the siRNA was already internalized during the first hour of incubation, when complexes were prepared at the N/P ratio of 25. Regarding the interaction with cells of the conjugates in their free form, no toxic effect was observed, unless high concentrations (≥ 125 µg/mL) were reached, in contrast to the noticed toxicity of other derivatives even at concentrations of only 10–20µg/mL11, 20. The phospholipid-PEIs were 10 times less toxic than PEI 25 kDa (IC50 = 123 and 100µg/mL vs IC50 = 15µg/mL). Interestingly, their cytotoxicity became noticeable due to the excessive cell interaction once the critical micellar concentration was exceeded (CMC= 97 µg/mL and 72 µg/mL, for DOPE-PEI and DPPE-PEI, respectively) supporting the idea of the structure-specific interaction of these conjugates with cells.
Although DPPE-PEI and DOPE-PEI carriers showed similar cell membrane interaction and siRNA uptake, DOPE-PEI displayed a more effective gene silencing. Mechanistic studies, the results of which are presented in Figure 6, show a positive lysomotropic chloroquine dependency of DPPE-PEI but not DOPE-PEI, and conversely a negative effect of bafilomycin on DOPE-PEI, but not on DPPE-PEI, pointing out the mechanistic differences in the cytosolic trafficking of these conjugates. Chloroquine improved the silencing efficacy of DPPE-PEI, indicating the incomplete release of the internalized complexes from the endosomal compartment in the absence of this buffering agent (Figure 6A). On the other hand, bafilomycin inhibition of the active influx of water and ions essential for endosome acidification abolished the GFP down-regulation mediated by DOPE-PEI complexes and PEI 25 kDa, while DPPE-PEI efficacy was not affected (Figure 6B). The pH dependent-membrane activity of DOPE unit but not of DPPE is in agreement with previous studies with lipoplexes containing DOPE as a helper lipid for fusogenic functionality33–35. Zurhorn and co-workers showed that with the substitution of DOPE for DPPE, the transfection activity of the lipoplex decreased dramatically as a result of the inhibition of the intracellular delivery, confirming the fusogenic role of DOPE36. In addition, palmitic acid-substituted PEI (0.6–2kDa) did not assist the endosomal release and was affected by chloroquine treatment20, pointing out the advantages of DOPE conjugation over other lipidic moieties in the optimization of lipid-PEI gene delivery systems.
The ability to condense siRNA, hydrodynamic size, zeta potential and enzymatic stability are often considered as important criteria to estimate the efficacy of lipid-PEI derivatives for delivery siRNA. This study highlights the importance of the amphiphile structure and the lipid unit in promoting the interaction with cell membranes, triggering the siRNA uptake and intracellular delivery. In addition to higher transfection efficacy and safer toxicity profile of PEI 1.8 kDa compared to PEI 25 kDa, phospholipid-PEI conjugate can self-assemble into the micellar structures and incorporate poorly soluble compounds in their core. These improved features open new possibilities for the application of such nanocarriers as a potential co-delivery platform for siRNA/poorly soluble drugs combinations in a single carrier.
Supplementary Material
Acknowledgments
This work was funded by a fellowship from the Department of Education of the Navarra Regional Government (Spain) to GN and by the NIH grant RO1CA121838 and 1U54CA151881 to V.P. Torchilin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No competing interests are present.
REFERENCES
- 1.de Wolf HK, Snel CJ, Verbaan FJ, Schiffelers RM, Hennink WE, Storm G. Effect of cationic carriers on the pharmacokinetics and tumor localization of nucleic acids after intravenous administration. International journal of pharmaceutics. 2007;331:167–175. doi: 10.1016/j.ijpharm.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 2.van de Water FM, Boerman OC, Wouterse AC, Peters JG, Russel FG, Masereeuw R. Intravenously administered short interfering RNA accumulates in the kidney and selectively suppresses gene function in renal proximal tubules. Drug metabolism and disposition: the biological fate of chemicals. 2006;34:1393–1397. doi: 10.1124/dmd.106.009555. [DOI] [PubMed] [Google Scholar]
- 3.Zuckerman JE, Choi CH, Han H, Davis ME. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3137–3142. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Martimprey H, Vauthier C, Malvy C, Couvreur P. Polymer nanocarriers for the delivery of small fragments of nucleic acids: oligonucleotides and siRNA. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2009;71:490–504. doi: 10.1016/j.ejpb.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 5.David S, Pitard B, Benoit JP, Passirani C. Non-viral nanosystems for systemic siRNA delivery. Pharmacological research : the official journal of the Italian Pharmacological Society. 2010;62:100–114. doi: 10.1016/j.phrs.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Bruno K. Using drug-excipient interactions for siRNA delivery. Advanced drug delivery reviews. 2011;63:1210–1226. doi: 10.1016/j.addr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oskuee RK, Philipp A, Dehshahri A, Wagner E, Ramezani M. The impact of carboxyalkylation of branched polyethylenimine on effectiveness in small interfering RNA delivery. The journal of gene medicine. 2010;12:729–738. doi: 10.1002/jgm.1490. [DOI] [PubMed] [Google Scholar]
- 8.Merkel OM, Librizzi D, Pfestroff A, Schurrat T, Buyens K, Sanders NN, et al. Stability of siRNA polyplexes from poly(ethylenimine) and poly(ethylenimine)-g-poly(ethylene glycol) under in vivo conditions: effects on pharmacokinetics and biodistribution measured by Fluorescence Fluctuation Spectroscopy and Single Photon Emission Computed Tomography (SPECT) imaging. Journal of controlled release : official journal of the Controlled Release Society. 2009;138:148–159. doi: 10.1016/j.jconrel.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Navarro G, Maiwald G, Haase R, Rogach AL, Wagner E, de Ilarduya CT, et al. Low generation PAMAM dendrimer and CpG free plasmids allow targeted and extended transgene expression in tumors after systemic delivery. Journal of controlled release : official journal of the Controlled Release Society. 2010;146:99–105. doi: 10.1016/j.jconrel.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Grayson AC, Doody AM, Putnam D. Biophysical and structural characterization of polyethylenimine-mediated siRNA delivery in vitro. Pharmaceutical research. 2006;23:1868–1876. doi: 10.1007/s11095-006-9009-2. [DOI] [PubMed] [Google Scholar]
- 11.Aliabadi HM, Landry B, Bahadur RK, Neamnark A, Suwantong O, Uludag H. Impact of lipid substitution on assembly and delivery of siRNA by cationic polymers. Macromolecular bioscience. 2011;11:662–672. doi: 10.1002/mabi.201000402. [DOI] [PubMed] [Google Scholar]
- 12.Wang T, Upponi JR, Torchilin VP. Design of multifunctional non-viral gene vectors to overcome physiological barriers: dilemmas and strategies. International journal of pharmaceutics. 2012;427:3–20. doi: 10.1016/j.ijpharm.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Behr JP. The proton sponge: A trick to enter cells the viruses did not exploit. Chimia. 1997;51:34–36. [Google Scholar]
- 14.Won YY, Sharma R, Konieczny SF. Missing pieces in understanding the intracellular trafficking of polycation/DNA complexes. Journal of controlled release : official journal of the Controlled Release Society. 2009;139:88–93. doi: 10.1016/j.jconrel.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL. The possible "proton sponge" effect of polyethylenimine (PEI) does not include change in lysosomal pH. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:149–157. doi: 10.1038/mt.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yue Y, Jin F, Deng R, Cai J, Dai Z, Lin MC, et al. Revisit complexation between DNA and polyethylenimine--effect of length of free polycationic chains on gene transfection. Journal of controlled release : official journal of the Controlled Release Society. 2011;152:143–151. doi: 10.1016/j.jconrel.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Han S, Mahato RI, Kim SW. Water-soluble lipopolymer for gene delivery. Bioconjugate chemistry. 2001;12:337–345. doi: 10.1021/bc000120w. [DOI] [PubMed] [Google Scholar]
- 18.Wang DA, Narang AS, Kotb M, Gaber AO, Miller DD, Kim SW, et al. Novel branched poly(ethylenimine)-cholesterol water-soluble lipopolymers for gene delivery. Biomacromolecules. 2002;3:1197–1207. doi: 10.1021/bm025563c. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder A, Dahlman JE, Sahay G, Love KT, Jiang S, Eltoukhy AA, et al. Alkane-modified short polyethyleneimine for siRNA delivery. Journal of controlled release : official journal of the Controlled Release Society. 2012;160:172–176. doi: 10.1016/j.jconrel.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahadur KC, Landry B, Aliabadi HM, Lavasanifar A, Uludag H. Lipid substitution on low molecular weight (0.6–2.0 kDa) polyethylenimine leads to a higher zeta potential of plasmid DNA and enhances transgene expression. Acta biomaterialia. 2011;7:2209–2217. doi: 10.1016/j.actbio.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 21.Hsu CY, Hendzel M, Uludag H. Improved transfection efficiency of an aliphatic lipid substituted 2 kDa polyethylenimine is attributed to enhanced nuclear association and uptake in rat bone marrow stromal cell. The journal of gene medicine. 2011;13:46–59. doi: 10.1002/jgm.1526. [DOI] [PubMed] [Google Scholar]
- 22.Dewa T, Ieda Y, Morita K, Wang L, MacDonald RC, Iida K, et al. Novel polyamine-dialkyl phosphate conjugates for gene carriers. Facile synthetic route via an unprecedented dialkyl phosphate. Bioconjugate chemistry. 2004;15:824–830. doi: 10.1021/bc049925k. [DOI] [PubMed] [Google Scholar]
- 23.Dewa T, Asai T, Tsunoda Y, Kato K, Baba D, Uchida M, et al. Liposomal polyamine-dialkyl phosphate conjugates as effective gene carriers: chemical structure, morphology, and gene transfer activity. Bioconjugate chemistry. 2010;21:844–852. doi: 10.1021/bc900376y. [DOI] [PubMed] [Google Scholar]
- 24.Alshamsan A, Haddadi A, Incani V, Samuel J, Lavasanifar A, Uludag H. Formulation and delivery of siRNA by oleic acid and stearic acid modified polyethylenimine. Molecular pharmaceutics. 2009;6:121–133. doi: 10.1021/mp8000815. [DOI] [PubMed] [Google Scholar]
- 25.Ko YT, Kale A, Hartner WC, Papahadjopoulos-Sternberg B, Torchilin VP. Self-assembling micelle-like nanoparticles based on phospholipid-polyethyleneimine conjugates for systemic gene delivery. Journal of controlled release : official journal of the Controlled Release Society. 2009;133:132–138. doi: 10.1016/j.jconrel.2008.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navarro G, Sawant RR, Essex S, Tros de Ilarduya C, Torchilin VP. Phospholipid–polyethylenimine conjugate-based micelle-like nanoparticles for siRNA delivery. Drug Delivery and Translational Research. 2011;1:25–33. doi: 10.1007/s13346-010-0004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarro G, Sawant RR, Biswas S, Essex S, Tros de Ilarduya C, Torchilin VP. P-glycoprotein silencing with siRNA delivered by DOPE-modified PEI overcomes doxorubicin resistance in breast cancer cells. Nanomedicine (Lond) 2012;7:65–78. doi: 10.2217/nnm.11.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawant RR, Sriraman SK, Navarro G, Biswas S, Dalvi RA, Torchilin VP. Polyethyleneimine-lipid conjugate-based pH-sensitive micellar carrier for gene delivery. Biomaterials. 2012;33:3942–3951. doi: 10.1016/j.biomaterials.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukyanov AN, Gao Z, Mazzola L, Torchilin VP. Polyethylene glycoldiacyllipid micelles demonstrate increased acculumation in subcutaneous tumors in mice. Pharmaceutical research. 2002;19:1424–1429. doi: 10.1023/a:1020488012264. [DOI] [PubMed] [Google Scholar]
- 30.Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Advanced drug delivery reviews. 2004;56:1273–1289. doi: 10.1016/j.addr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. The journal of gene medicine. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 32.Cullis PR, de Kruijff B, Verkleij AJ, Hope MJ. Lipid polymorphism and membrane fusion. Biochemical Society transactions. 1986;14:242–245. doi: 10.1042/bst0140242. [DOI] [PubMed] [Google Scholar]
- 33.Cullis PR, Hope MJ, Tilcock CP. Lipid polymorphism and the roles of lipids in membranes. Chemistry and physics of lipids. 1986;40:127–144. doi: 10.1016/0009-3084(86)90067-8. [DOI] [PubMed] [Google Scholar]
- 34.Wrobel I, Collins D. Fusion of cationic liposomes with mammalian cells occurs after endocytosis. Biochimica et biophysica acta. 1995;1235:296–304. doi: 10.1016/0005-2736(95)80017-a. [DOI] [PubMed] [Google Scholar]
- 35.Almofti MR, Harashima H, Shinohara Y, Almofti A, Baba Y, Kiwada H. Cationic liposome-mediated gene delivery: biophysical study and mechanism of internalization. Archives of biochemistry and biophysics. 2003;410:246–253. doi: 10.1016/s0003-9861(02)00725-7. [DOI] [PubMed] [Google Scholar]
- 36.Zuhorn IS, Bakowsky U, Polushkin E, Visser WH, Stuart MC, Engberts JB, et al. Nonbilayer phase of lipoplex-membrane mixture determines endosomal escape of genetic cargo and transfection efficiency. Molecular therapy : the journal of the American Society of Gene Therapy. 2005;11:801–810. doi: 10.1016/j.ymthe.2004.12.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.