Abstract
Background
Prescribing benzodiazepines during buprenorphine treatment is a topic of active discussion. Clinical benefit is unclear. Overdose, accidental injury, and benzodiazepine misuse remain concerns. We examine the relationship between benzodiazepine misuse history, benzodiazepine prescription, and both clinical and safety outcomes during buprenorphine treatment.
Methods
We retrospectively examined outpatient buprenorphine treatment records, classifying patients by past-year benzodiazepine misuse history and approved benzodiazepine prescription at intake. Primary clinical outcomes included 12-month treatment retention and urine toxicology for illicit opioids. Primary safety outcomes included total emergency department (ED) visits and odds of an ED visit related to overdose or accidental injury during treatment.
Results
The 12-month treatment retention rate for the sample (N = 328) was 40%. Neither benzodiazepine misuse history nor benzodiazepine prescription was associated with treatment retention or illicit opioid use. Poisson regressions of ED visits during buprenorphine treatment revealed more ED visits among those with a benzodiazepine prescription versus those without (p < 0.001); benzodiazepine misuse history had no effect. The odds of an accidental injury-related ED visit during treatment were greater among those with a benzodiazepine prescription (OR: 3.7, p < 0.01), with an enhanced effect among females (OR: 4.7, p < 0.01). Overdose was not associated with benzodiazepine misuse history or prescription.
Conclusions
We found no effect of benzodiazepine prescriptions on opioid treatment outcomes; however, benzodiazepine prescription was associated with more frequent ED visits and accidental injuries, especially among females. When prescribing benzodiazepines during buprenorphine treatment, patients need more education about accidental injury risk. Alternative treatments for anxiety should be considered when possible, especially among females.
Keywords: Buprenorphine, Opioid dependence, Benzodiazepine, Accident, Female, Utilization
1. Introduction
Prescribing benzodiazepines during office-based opioid treatment (OBOT) (Gunderson and Fiellin, 2008) with buprenorphine is a practice that evokes intense discussion in clinical settings. Fatal overdoses from mixing benzodiazepines with buprenorphine, especially through concurrent intravenous administration, are a major safety concern (Reynaud et al., 1998b). The Food and Drug Administration required a warning for physicians to use caution when prescribing buprenorphine with benzodiazepines (Reckitt-Benckiser, 2010). Additionally, many clinicians believe that benzodiazepine prescriptions should be avoided because benzodiazepines hinder development of psychological coping strategies (Otto et al., 2005; Soyka, 2010), can be misused (Brunette et al., 2003; Chen et al., 2011) and can contribute to relapse (Brands et al., 2008). In contrast, others argue that use of long-acting benzodiazepine can be helpful in some circumstances, especially to retain people with co-occurring severe anxiety disorders who are receiving treatment for opioid dependence (Bleich et al., 2002; Liebrenz et al., 2010). For instance, 12-month retention in buprenorphine treatment among people with generalized anxiety disorder is low compared to people with major depressive disorder (39% vs. 72%; Gerra et al., 2006) and to the 12-month retention rate reported in several buprenorphine treatment samples (43–49%; Alford et al., 2011; Fiellin et al., 2008; Gerra et al., 2006). In the context of this ongoing debate, some OBOT programs avoid both prescribing benzodiazepines and admitting those who already have a prescription. Alternatively, some programs will accept those who already have a benzodiazepine prescription, while encouraging gradual benzodiazepine reduction within a limited time period (Tyrer, 2010), subject to certain conditions aimed at enhancing safety and limiting aberrant drug use behaviors (Lintzeris and Nielsen, 2010). Furthermore, some clinicians prescribe benzodiazepine maintenance to people with co-occurring anxiety disorders during buprenorphine treatment, believing it helps maintain retention and prevents relapse.
Benzodiazepine use is common among patients prescribed buprenorphine for opioid dependence. For example, in a Norwegian prescription database, 30% of patients on buprenorphine received a benzodiazepine prescription during the previous year (Bramness and Kornor, 2007). Early in treatment, patients receiving buprenorphine treatment frequently request a benzodiazepine prescription to mitigate anxiety and insomnia symptoms (Lintzeris and Nielsen, 2010). Misuse of benzodiazepines is also common; in a French sample, 31% of patients on buprenorphine had problematic use of benzodiazepines in the past month (Lavie et al., 2009).
While benzodiazepines and buprenorphine are each safer than their respective alternatives, barbiturates and methadone, the misuse of benzodiazepines with buprenorphine has resulted in dangerous consequences. Benzodiazepines, unlike barbiturates, do not cause respiratory depression when taken alone (Gasser et al., 1975; Murray et al., 1987), although benzodiazepine use has been associated with sedation, psychomotor impairment, and accidental injuries (Oster et al., 1990). Similarly, buprenorphine is safer than methadone when taken by itself; its partial agonism at central mu opioid receptors results in a “ceiling effect” on respiratory depression (Bell et al., 2009).
A French case series reported details of six overdose deaths related to concomitant use of buprenorphine and benzodiazepines (Reynaud et al., 1998a). When buprenorphine and benzodiazepines were co-administered to rats, the protective bell-shaped dose response effect on respiration was eliminated (Nielsen and Taylor, 2005). Human laboratory studies demonstrated that even when buprenorphine is taken at therapeutic doses, co-administration with supra-therapeutic doses of benzodiazepines can remove buprenorphine's “ceiling effect” on respiratory depression in the same pattern as co-administration with methadone (Lintzeris et al., 2006). In Finland, where buprenorphine is the primary opioid of abuse (Yokell et al., 2011), a retrospective analysis of opioid-associated deaths recorded in the national postmortem toxicology database found 1363 opioid-positive cases, of which 182 had buprenorphine poisoning as the cause of death; either a benzodiazepine or alcohol was found in all but one case, and were present in 82 and 58% of cases, respectively (Hakkinen et al., 2012). The median concentrations of buprenorphine and benzodiazepines in these poisonings were in the therapeutic range (Hakkinen et al., 2012). In contrast, a controlled human laboratory study with eight patients prescribed buprenorphine who were orally co-administered diazepam at therapeutic doses (≤20 mg) did not demonstrate an effect of diazepam on oxygen saturation compared with placebo, even though sedation and performance deficits emerged as the dose was increased (Lintzeris et al., 2006). Additionally, in a cross-sectional survey of 250 people with opioid dependence, overdose events from benzodiazepine use were self-reported ten times less frequently during buprenorphine versus methadone treatment (Nielsen et al., 2007). Taken together, these data suggest, even with therapeutic doses of buprenorphine, co-administration of benzodiazepines may result in lethal overdose when used either at supra-therapeutic dosages or at therapeutic dosages through intravenous injection or in combination with sedatives; however, lethal overdose would be unexpected to occur in the context of controlled oral co-administration of therapeutic dosages of benzodiazepines and buprenorphine. Therefore, clinical investigation of the safety of benzodiazepine treatment prescribed at therapeutic doses during buprenorphine treatment is necessary.
Using a chart review with a naturalistic, quasi-experimental design, we attempted to evaluate the relationship between benzodiazepine prescribing and clinical and safety outcomes during buprenorphine treatment, while also evaluating for effects of historical benzodiazepine misuse. We investigated the relationship between past-year benzodiazepine misuse and benzodiazepine prescription in several areas. Our primary clinical outcomes were 12-month treatment retention and number of months without positive urine toxicology screens for illicit opioids. Our primary safety outcomes were emergency department (ED) visits during treatment and ED visits related to overdose and accidental injury. We hypothesized that the best clinical and safety outcomes would occur among those without any benzodiazepine use history. We further hypothesized that there would be more ED visits due to overdose and accidental injuries, more opioid-positive toxicology screens, and poorer treatment retention among those with both a benzodiazepine prescription and history of benzodiazepine misuse as compared with those without either a benzodiazepine prescription or a history of benzodiazepine misuse.
2. Methods
2.1. Sample selection
This sequential-admission retrospective study was conducted with admissions to a collaborative care OBOT program (Alford et al., 2011; Schuman-Olivier et al., 2010) from November 2007 to June 2010. Two nurse care managers collaborated with multiple buprenorphine prescribers, coordinating urine toxicology screening, monitoring treatment adherence, overseeing medication management and facilitating communication with addiction counselors. Prescribers were affiliated with an academic community healthcare system located in five Boston Metro-North cities, sharing an electronic medical record (EMR). The Cambridge Health Alliance human subjects review board approved this protocol. We conducted the chart review using standardized intake forms, quarterly interviews conducted by nurse care managers, and the EMR.
Nurse care managers conducted a brief screening assessment by telephone or in-person to determine treatment program admission eligibility. All newly eligible patients (n = 386) were included in this sequential admission study. Patients were expected to complete an intake process, including: (1) comprehensive urine toxicology, (2) complete OBOT nurse care manager assessment, and (3) buprenorphine prescription from network physician. We excluded those with an incomplete intake process (n = 35, 9%). To enhance external validity, we excluded special populations not commonly treated in standard buprenorphine treatment or whose response to benzodiazepines could be atypical (i.e., psychotic disorder (n = 2, <1%), pregnancy (n = 3, 1%), and intracranial injury (e.g., traumatic brain injury, stroke; n = 18, 4.6%; Fig. 1). All together, we excluded 15% (n = 58) from the analysis, resulting in 328 patients included in the analysis. Records for the final sample (n = 328) were recorded until the date of OBOT program discharge or until 12 months after intake into OBOT treatment.
Fig. 1.
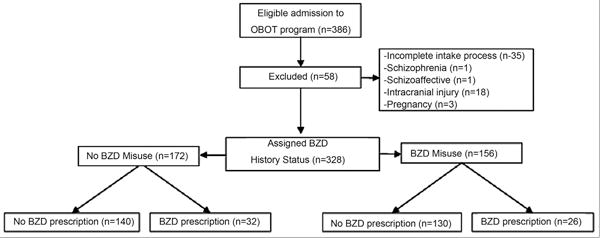
Consort diagram.
2.2. Procedure
2.2.1. Treatment
OBOT consisted of buprenorphine maintenance treatment prescribed by program-affiliated physicians from various medical specialties, including internal medicine, family medicine, and psychiatry. Within this program, clinicians encouraged brief inpatient detoxification before starting buprenorphine maintenance for patients with co-occurring substance dependence or significant medical problems; however, detoxification was not required when opioid dependence was the only substance use disorder present. Standard treatment consisted of buprenorphine initiation during a half-day in-office induction. All buprenorphine prescriptions in this study were buprenorphine/naloxone co-formulation sublingual tablets.
Within this abstinence-focused program, patients typically participated in intensive outpatient programming during the first two weeks of treatment and in response to substance use lapses. The program also required patients to step down into weekly relapse prevention groups unless psychiatric needs precluded participation. The program also provided individual therapy and psychopharmacology based on psychiatric need. When an anxiety disorder diagnosis was made by an in-network physician, recommending a benzodiazepine prescription, the medical director, a board-certified addiction psychiatrist, reviewed the case before granting program approval. Benzodiazepine prescriptions from out-of-network physicians were not approved.
2.2.2. Urine toxicology
Nurse care managers conducted urine toxicology at intake, and then twice weekly during the first month. Weekly urine toxicology was expected for the first six months of treatment. If patients were clinically stable, then the program required at least monthly urine screening for the remainder of the year. If abstinence were violated, urine screening frequency increased. Urine toxicology generally used enzyme-mediated immunoassay techniques (Beckman Coulter, Fullerton, CA), but rapid chromatographic immunoassays were used for buprenorphine and oxycodone (Bio-Rad, Hercules, CA).
2.2.3. Nurse care manager assessments
Nurse care managers conducted structured assessments at intake, every three months thereafter, and at discharge. At intake, they completed a comprehensive interview recording demographic characteristics, treatment utilization and substance use history. At each three-month interview, they recorded retention, ED visits, and benzodiazepine prescription status.
2.3. Measures and materials
2.3.1. Electronic medical record
The EMR recorded descriptions from physician OBOT intake notes and ED visits, urine toxicology results, and medication prescriptions. We obtained these data for each patient until 12 months after OBOT, or until discharge.
2.3.2. Measures
2.3.2.1. Benzodiazepine misuse history
In this study, we assumed the perspective of the OBOT admitting clinician objectively examining available evidence of benzodiazepine misuse. Benzodiazepine misuse was defined as evidence of inappropriate use of a prescribed benzodiazepine (use in greater amounts or for longer periods of time than the prescriber intended) OR use of benzodiazepines recreationally without a prescription (Chen et al., 2011; Griffiths and Johnson, 2005; O'Brien, 2005). We classified patients as having a positive benzodiazepine misuse history if they were positive in any of three categories: (1) intake self-report (endorsed benzodiazepine misuse in the past-year); (2) clinician notes in EMR (clinician intake notes reporting benzodiazepine misuse history in the past-year); or (3) search of EMR prescription and urine toxicology databases in the year before intake (presence of benzodiazepine-positive toxicology with no EMR prescription). We chose the past-year criteria because sustained full remission of substance dependence diagnoses in DSM-IV requires one year when no dependence criteria were met. After we entered all intake data, but before any outcome data were examined, the lead investigators finalized benzodiazepine misuse history assignment labels based on available clinical data at time of intake (Fig. 1). Any disagreements were resolved by review of the record until consensus was reached
2.3.2.2. Benzodiazepine prescription
We included patients with a benzodiazepine prescription approved within one week after OBOT program intake.
2.3.2.3. Urine toxicology
Urine toxicology data collected for all patients included: cocaine, benzodiazepine, and illicit opioid (e.g., heroin, oxycodone, and methadone). Assessments were made during 12 months after intake until date of discharge.
2.3.2.4. Emergency department visits
We recorded all EMR-documented ED visits. We reviewed the discharge summary and diagnoses from each ED visit for evidence of specific events. We classified ED visits into relevant categories: (1) overdose; (2) accidental injury; (3) psychiatric visit; and (4) other medical (e.g., pain, infection). To capture possible out-of-network ED visits, these data were cross-referenced with nurse care manager quarterly reports, which summarized counts of ED visits per quarter. When we found differences between EMR and quarterly records, we reviewed EMR records a second time for details of all clinical notes recorded during the quarter. In order to focus on the prescriber's dilemma regarding safety of benzodiazepine prescribing to people seeking OBOT, we limited our recording of ED visits to the period while people were enrolled in OBOT.
2.3.3. Data management
We merged data from intake and quarterly forms with EMR data. We conducted a second-person, 100% audit of all databases for data entry accuracy, and all discrepancies were resolved with support from original documentation. Databases were merged into SAS 9.3 for analysis.
2.4. Data analysis
We conducted several primary analyses, including clinical (treatment retention, urine toxicology) and safety (number of ED visits, odds of an overdose- or accidental injury-related ED visit) outcomes. We used analysis of variance and chi-square to identify any differences among sample characteristics. Females often receive benzodiazepine prescriptions more frequently than men (Anderson, 1981); therefore, we included both gender and its interaction with benzodiazepine prescription as covariates in all analyses. We used total number of weeks in the treatment program as a covariate in safety and toxicology analyses to adjust for dropout effects.
2.4.1. Clinical outcomes: retention and urine toxicology
We used logistic regression (proc logistic) to identify predictors of 12-month retention, including benzodiazepine prescription, benzodiazepine misuse history, and age. To determine differences in illicit drug use during treatment based on benzodiazepine misuse history and benzodiazepine prescription, we used individual GEE analyses for repeated binary dependent variables, including months without positive urine toxicology for illicit opioids, cocaine, or benzodiazepines; benzodiazepine misuse history and benzodiazepine prescription were predictors for these analyses.
2.4.2. Safety outcomes: ED visits
For number of ED visits during treatment, we used Poisson regression analysis; for this analysis, number of ED visits during treatment was the dependent variable, while benzodiazepine misuse history and benzodiazepine prescription were predictors. We included relevant demographic and treatment utilization variables as covariates. Additionally, we used analysis of variance to establish group ED visit means and effect sizes.
We used individual logistic regression models to determine the odds of an ED visit during treatment related to overdose, accidental injury, psychiatric or medical causes, with benzodiazepine prescription as the predictor. We calculated adjusted odds ratios with benzodiazepine misuse, weeks in treatment, and gender as covariates; as before, we also added, the interaction between gender and prescription as a covariate.
3. Results
3.1. Sample characteristics
Patients with a benzodiazepine misuse history differed from those without a misuse history in several ways (Table 1). Patients with benzodiazepine misuse history more frequently reported misuse of cocaine, amphetamine, and alcohol. Additionally, they more frequently had cocaine positive intake toxicology screens.
Table 1.
Sample characteristics by past-year BZD misuse, BZD Rx status, and their interaction.
| No BZD Rx | BZD Rx | No BZD Misuse Hx | BZD Misuse Hx | BZD Misuse Hx | No BZD Misuse Hx | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| No BZD Rx | BZD Rx | No BZD Rx | BZD Rx | |||||
|
|
||||||||
|
n = 270 Mean (SD) |
n = 58 Mean (SD) |
n = 172 Mean (SD) |
n = 156 Mean (SD) |
n = 130 Mean (SD) |
n = 26 Mean (SD) |
n = 140 Mean (SD) |
n = 32 Mean (SD) |
|
| Demographics | ||||||||
| Gender (% female) | 35.2 | 63.8** | 39.5 | 41.0 | 36.9 | 61.5 | 33.6 | 65.6 |
| Race (% White) | 92.2 | 96.6 | 90.1 | 96.2ˆ | 95.4 | 100.0 | 89.3 | 93.8 |
| Age (in years) | 35.6 (10.5) | 37.7 (11.6) | 35.7 (10.9) | 36.3 (10.6) | 36.2 (10.3) | 36.6 (12.0) | 35.0 (10.7) | 38.6 (11.4) |
| Number of days worked, past month | 4.4 (7.7) | 1.7 (5.6)* | 4.3 (7.6) | 3.6 (7.2) | 4.0 (7.4) | 1.8 (6.1) | 4.9 (8.0) | 1.6 (5.2) |
| Treatment utilization | ||||||||
| Number of ED visits, past year | 0.8 (1.4) | 1.6 (1.9)* | 1.0 (1.6) | 0.9 (1.4) | 2.7 (7.6) | 4.5 (6.4) | 2.5 (5.9) | 4.9 (11.3) |
| Number of detoxification visits, lifetime | 5.1 (9.1) | 3.8 (4.2) | 4.0 (6.5) | 5.8 (10.2) | 6.2 (10.9) | 4.1 (5.0) | 4.1 (7.0) | 3.6 (3.5) |
| Disability income, % | 20.0 | 37.9** | 25.6 | 20.5 | 19.2 | 26.9 | 20.7 | 46.9 |
| History of multiple psychiatric hospitalizations, % | 17.0 | 32.8** | 16.3 | 23.7 | 21.5 | 34.6 | 12.9 | 31.3 |
| Substance use | ||||||||
| Classes of substances misused, lifetime | 6.0 (2.4) | 6.0 (2.3) | 5.3 (2.4) | 6.7 (2.1)ˆˆ | 6.5 (2.2) | 7.3 (1.7) | 4.9 (2.1) | 5.4 (2.4) |
| Heroin use, lifetime self-report, % | 79.6 | 70.7 | 74.4 | 82.1 | 83.1 | 76.9 | 76.4 | 65.6 |
| Cocaine, lifetime self-report, % | 75.6 | 75.9 | 68.0 | 84.0ˆˆ | 83.1 | 88.5 | 68.6 | 65.6 |
| Cocaine, intake tox(+) | 20.7 | 10.3 | 13.4 | 25.0ˆˆ | 26.9 | 15.4 | 15.0 | 6.3 |
| Marijuana, lifetime self-report | 73.3 | 63.8 | 67.4 | 76.3 | 75.4 | 80.8 | 71.4 | 50.0 |
| Marijuana, intake tox(+) | 30.7 | 22.4 | 25.6 | 33.3 | 32.3 | 38.5 | 29.3 | 9.4# |
| Amphetamine, lifetime self-report | 18.9 | 24.1 | 13.4 | 26.9ˆˆ | 23.8 | 42.3 | 14.3 | 9.4 |
| Amphetamine, intake tox(+) | 5.9 | 5.2 | 4.1 | 7.7 | 8.5 | 3.8 | 3.6 | 6.3 |
| Alcohol, lifetime self-report | 80.0 | 75.9 | 71.5 | 87.8ˆˆ | 87.7 | 88.5 | 72.9 | 65.6 |
| Alcohol, intake tox(+) | 5.6 | 5.2 | 6.4 | 4.5 | 5.4 | 0.0 | 5.7 | 9.4 |
| Benzodiazepine, lifetime self-report | 67.0 | 86.2** | 49.4 | 93.6ˆˆ | 88.5 | 100.0 | 34.3 | 28.1 |
| Benzodiazepine, intake tox(+) | 21.1 | 74.1** | 14.5 | 48.1ˆˆ | 43.8 | 69.2 | 0.0 | 78.1 |
Benzodiazepine prescription = BZD Rx.
Note:
p <.05,
p < .01 (BZD Rx vs. No BZD Rx);
p <.05,
p < .01 (BZD misuse vs. No BZD misuse);
p < .05 (BZD misuse × BZD Rx interaction)
Patients with OBOT-approved benzodiazepine prescriptions at intake differed from those without benzodiazepine prescriptions in several ways: they were more frequently female, more likely to receive disability income and work fewer days per month, with more past-year ED visits and more likely to have had multiple psychiatric hospitalizations.
Benzodiazepine prescription use was fairly stable throughout the study; most patients prescribed a benzodiazepine at intake still had an active prescription if they were retained in treatment at 12 months (20/26, 77%). Eighteen patients received a benzodiazepine prescription later during treatment. A sensitivity analysis, removing these patients (n = 310), resulted in no effect on clinical and safety outcomes.
3.2. Treatment outcomes: retention and urine toxicology
We used age as a sole covariate because age was the only covariate with a statistically significant independent effect on retention. Logistic regression demonstrated no statistically significant differences in 12-month treatment retention based on past-year benzodiazepine misuse, benzodiazepine prescription, or their combination. Similar to treatment retention, there were also no significant main effects of benzodiazepine misuse history or benzodiazepine prescription status on screening positive for illicit opioid or cocaine. Unsurprisingly, those without a history of benzodiazepine misuse or benzodiazepine prescription were least likely to have a benzodiazepine-positive urine screen during any given month (Z = 3.73, p < 0.001).
3.3. Safety outcomes: ED visits
We initially found 243 ED visits recorded in the EMR. Care manager records summarized 214 ED visits. We found differences between EMR and care manager records in 60 out of 328 cases. After secondary review of quarters with discrepancies, we identified EMR documentation of 18 additional out-of-network ED visits. The total number of ED visits was 261. The number of ED visits during treatment was greater among patients with a benzodiazepine prescription, (χ2 = 9.1, p < 0.005, Cohen's d = 0.43) with no interaction effect from benzodiazepine misuse history. Benzodiazepine prescription approved at intake predicted the number of ED visits during OBOT treatment even when adjusted for baseline demographic and treatment utilization differences, including number of ED visits in past-year, benzodiazepine misuse history, gender, disability income at intake, history of multiple psychiatric hospitalizations, days worked in the month prior to intake, and number of weeks in treatment program (p < 0.05). Females with a benzodiazepine prescription evidenced a higher frequency of multiple ED visits (≥2 visits during the year of treatment) than all others (including females without a prescription and males with a prescription; χ2 = 4.2, p < 0.05, Cohen's d = 0.51; Fig. 2).
Fig. 2.
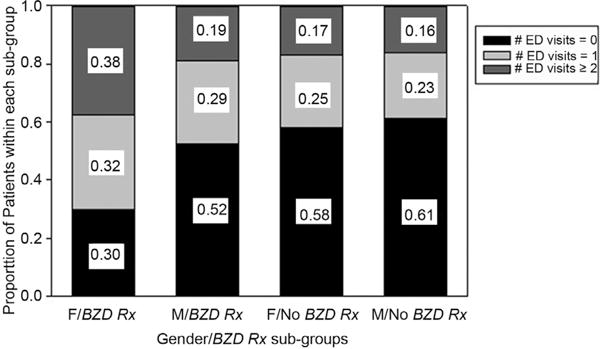
Relationship of BZD prescription (Rx) approval and gender on number of ED visits during treatment.
When examining the type of ED visits patients experienced, we found those with a benzodiazepine prescription had an increased odds of having an ED visit related to accidental injury (OR: 3.75, CI: 1.5–6.8, p < 0.01) and other medical causes (OR: 2.09, CI: 1.15–3.82, p < 0.05), but not overdose. Gender-adjusted logistic modeling demonstrated females with a benzodiazepine prescription had greater odds of having a visit related to accidental injury, medical or psychiatric causes, while men did not (Table 2).
Table 2.
Odds ratios of experiencing a type of ED visit during treatment.
| Type of ED visit | Proportions | ORs | AORsa | AORs (male)b | AORs (female)b | |
|---|---|---|---|---|---|---|
|
| ||||||
| BZD Rx | No BZD Rx | |||||
| Overdose | 0.03 | 0.03 | 1.34 [0.27–6.63] | 1.19 [0.23–6.22] | – [– –]c | 1.80 [0.29–11.34] |
| Accidental injury | 0.26 | 0.09 | 3.75 [1.81–7.75]** | 3.20 [1.50–6.83]** | 1.67 [0.43–6.48] | 4.72 [1.75–12.70]** |
| Psychiatric visit | 0.21 | 0.12 | 1.94 [0.93–4.05] | 1.86 [0.87–3.99] | 0.79 [0.17–3.63] | 2.91 [1.11–7.63]* |
| Medical visit (e.g. infection) | 0.38 | 0.29 | 2.09 [1.15–3.82]* | 1.93 [1.03–3.60]* | 1.21 [0.43–3.36] | 2.65 [1.17–6.01]* |
Benzodiazepine prescription = BZD Rx.
Adjusted odds ratios (AORs): adjusted for total weeks in program, gender, and BZD misuse.
Gender-AORs demonstrate gender/BZD Rx interaction effect.
No overdose-related visits occurred among males with BZD Rx.
p < 0.05.
p < 0.01.
4. Discussion
This investigation of clinical outcomes and safety indicators for patients with varying levels of benzodiazepine prescription and benzodiazepine misuse history had several key findings.
First, when comparing patients with and without a benzodiazepine prescription, we found no significant differences in our primary clinical outcomes, including treatment retention and illicit opioid or cocaine use during 12 months of treatment. People with anxiety disorders who received benzodiazepine prescriptions at OBOT intake maintained similar levels of treatment retention and abstinence from opioids and cocaine as did the general buprenorphine-treated population, irrespective of benzodiazepine misuse history. As such, these findings are in contrast to common worries about benzodiazepine prescriptions interfering with OBOT's main treatment goals.
Second, the total number of ED visits during buprenorphine treatment in the benzodiazepine prescription group was double the number of visits in those without a benzodiazepine prescription; this finding remained statistically significant even after adjusting for relevant demographic and treatment utilization characteristics, including the number of past-year ED visits.
Benzodiazepine use is associated with slowed sensory processing and psychomotor impairment (Barker et al., 2004), potentially resulting in an increase in accidental injuries (Oster et al., 1987), e.g. falls and impaired driving (Bramness et al., 2002). Although we were not surprised to find an increase in accidental injury related to benzodiazepine use, the odds ratio of 3.7 exceeded expectations based on population studies, which have suggested a relative risk of a medical event due to benzodiazepine-related, accidental injury of only 1.15 (Oster et al., 1990). These data suggest clinicians considering benzodiazepine prescriptions during buprenorphine treatment must pay careful attention to the risk of accidental injury to self and others, especially among patients operating motor vehicles.
The frequency of multiple ED visits was greatest among females prescribed benzodiazepines. The high rate of accidental injuries among females with prescriptions might represent greater psychomotor impairment and/or sedation compared to males. Theoretically, this could be caused by higher serum benzodiazepine levels due to (a) lower lean body mass compared to men and/or (b) pharmacokinetic differences resulting from cyclic hormonal variation (Yonkers et al., 1992). Similarly, this could be caused by an interaction with norbuprenorphine levels, which may be higher among females (Moody et al., 2011). Additionally, females are more likely than males to receive prescriptions for psychotropic and controlled substances (Anderson, 1981; Ray et al., 1986), representing a putative prescriber bias reflecting a pattern of more medically inappropriate prescriptions among women. Unmeasured psychological factors might influence ED visit frequency among women with prescriptions (e.g., psychiatric disorders or variation in help-seeking propensity).
In this naturalistic, quasi-experimental study, which was small by epidemiologic standards, benzodiazepine prescriptions, even when in combination with a history of benzodiazepine misuse, were not associated with greater odds of ED visits due to overdoses while patients were active in buprenorphine treatment. Therefore, these data did not support our original hypothesis, nor did they substantiate one of our safety concerns. Moreover, even though 17% of the sample received a benzodiazepine prescription, only 2.7% (n = 9) of 328 patients in buprenorphine treatment had an overdose-related ED visit during their first year of treatment, with no fatalities occurring during OBOT. We do not have data on fatalities after leaving treatment; however, other studies have demonstrated ongoing illicit use of full opioid agonists (e.g., heroin, oxycodone; Bell et al., 2009; SAMHSA, 2011) and dropout from treatment (Clausen et al., 2008; Rosca et al., 2012) both substantially increase risk of fatal overdoses and ED visits as compared to opioid agonist maintenance treatment.
Importantly, clinical guidelines for anxiety disorders do not recommend benzodiazepines as a first line treatment for anxiety disorders, but suggest selective-serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors instead. Because benzodiazepines can cause sedation, cognitive effects, dependence, and loss of anxiolytic efficacy, guidelines for management of anxiety disorders generally support adjunct benzodiazepines only when necessary, with restriction to short-term, scheduled dosing, in those with low risk of misuse (Swinson, 2006).
4.1. Strengths and limitations
This study integrated clinical and laboratory information from a large collaborative care OBOT program coordinating care for buprenorphine prescribers from 5 cities, with EMR-based health records from the regional hospital system, including the primary regional emergency departments. By comparing OBOT clinician records with wider-scale EMR data, we ensured accurate capture of all ED visits during treatment, even those occurring out-of-hospital-network. Sample characteristics were similar to previous studies (Lavie et al., 2009), supporting overall generalizability of study results.
This study had several limitations. First, the use of structured clinical diagnostic interviews (SCIDs) to obtain reliable anxiety disorder diagnoses could have allowed an optimal three-way comparison between those without anxiety disorder, those with anxiety disorder but no benzodiazepine prescription, and those with both anxiety disorder and benzodiazepine prescription. The retrospective quasi-experimental design did not allow for SCIDs, which might have improved reliability of anxiety and mood disorder diagnoses, particularly within the context of substance intoxication and withdrawal symptoms at OBOT intake. Because the clinical psychiatric diagnoses at intake did not utilize SCIDs, the research gold standard, we chose not to include anxiety disorder subtypes in our chart review. The lack of diagnostic specificity is a limitation of this study.
Second, despite the adequate sample size (n = 328), the low incidence of overdose-related ED visits during treatment (n = 9) limited the power of this study to definitively address whether or not benzodiazepine prescriptions, benzodiazepine misuse history, or their combination are associated with increased overdose rates during OBOT treatment. Large prospective studies or epidemiologic analyses of autopsy records are needed given the low incidence of overdose during treatment.
Third, this study did not address program-level implications of prescribing benzodiazepines, including risk of diversion to those without a prescription and potential increased craving among other patients as a result of a peer receiving a benzodiazepine prescription (Greenfield et al., 1992). Also, this study does not provide information about the relationship between benzodiazepine prescribing during treatment and overdoses after discontinuation from buprenorphine treatment.
Fourth, we did not collect information on buprenorphine or ben-zodiazepine dosages, limiting our ability to clearly demonstrate a pharmacokinetic rationale explaining the increased ED visits among females.
Finally, the ongoing use of marijuana or alcohol could influence rates of accidental injury and ED visits, but we were unable to measure these variables objectively in this study, so they were not included in our safety outcome models. Future studies should examine the role played by alcohol and marijuana.
In this study we did not compare different treatment approaches to patients entering OBOT who already have a benzodiazepine prescription and benzodiazepine dependence. Future clinical trials are needed to evaluate if a slow benzodiazepine taper in the context of intensive treatment for co-occurring psychiatric symptoms (Otto et al., 2010) with tight regulations aimed at preventing misuse and diversion (Lintzeris and Nielsen, 2010) would maintain retention and improve safety outcomes.
4.2. Conclusions
Patients receiving buprenorphine and a benzodiazepine prescription had more than three-fold increased odds of accidental injury compared to those without a benzodiazepine prescription, with the greatest odds of injury among females. Patients with anxiety disorders who received a benzodiazepine prescription at intake maintained the same retention rates in buprenorphine treatment at 12 months as the general buprenorphine population, irrespective of their benzodiazepine misuse history.
Acknowledgments
We appreciate the contribution of Kevin Wall to data entry and database auditing. We thank Francyne Puopolo, RN, Lola Roland, RN and David Mysells, MD for their consultation during the data entry process. We appreciate mentoring and editorial support from the MGH Center for Addiction Medicine, especially A. Eden Evins, MD, MPH and John Kelly, PhD.
Role of funding source: Funding for this study was provided through several sources. Dr. Schuman-Olivier conducted this research while receiving salary support through a Harvard Medical School Dupont-Warren Psychiatry Research Fellowship. Dr. Weiss supported the study with funding through NIDA Grant K24DA022288 (RW). In her role as director of statistics at MGH Center for Addiction Medicine, Bettina Hoeppner contributed to statistical analysis while supported by K01DA027097 (BH). Use of REDCAP database services were supported by a NIH grant UL1RR025758 to Harvard Catalyst. Jacob Borodovsky received a summer research scholarship provided by Tufts University Career Services to provide support for his time. No funding sources had any role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors: All authors were involved in the design of the study and contributed the protocol. Zev Schuman-Olivier managed the literature searches and summaries of previous related work. Zev Schuman-Olivier managed the chart review process, sample selection, and database management with collaboration from Jacob Borodovsky and Mark Albanese. Zev Schuman-Olivier and Bettina Hoeppner undertook the statistical analysis, and Zev Schuman-Olivier wrote the first draft of the manuscript. Zev Schuman-Olivier, Jacob Borodovsky, and Bettina Hoeppner contributed to design of tables and figures. All authors contributed to and have approved the final manuscript.
Conflict of interest
Dr. Schuman-Olivier: “I have received salary as medical director at WestBridge Community Services, a non-profit dual diagnosis community treatment program. I have no conflict of interest to report.”
Dr. Hoeppner: “I have no conflicts of interest.”
Dr. Weiss: “I have served as a consultant to Titan Pharmaceuticals and Reckitt Benckiser. None of these funding sources provided support for my involvement in this manuscript. In addition, none of these funding sources were involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.”
Jacob Borodovsky: “No conflict of interest in relation to this research.”
Dr. Shaffer: “I received funding from the following sources for consultative activities, including American Psychological Association, Consulting (editing); bwin.party Interactive Entertainment, grant funding for research; Massachusetts Council on Compulsive Gambling, grant funding for training activities; National Center for Responsible Gaming, grant funding for research; Arcadia Healthcare, Stock (greater than 10K but less than 30K); Daughter derived salary at SFH, St. Francis House (SFH); Consulting income greater than 20K, The DUNES of Easthampton; NIH Research grants (NIAAA; NIMH); Tung Wah Group of Hospitals, Honoraria; and Century Council (research funding). None of these funding sources provided support for my involvement in this manuscript. In addition, none of these funding sources were involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.”
Dr. Albanese: “No funding and no conflicts of interest to report.”
References
- Alford DP, LaBelle CT, Kretsch N, Bergeron A, Winter M, Botticelli M, Samet JH. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Arch Intern Med. 2011;171:425–431. doi: 10.1001/archinternmed.2010.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JE. Prescribing of tranquillizers to women and men. CMAJ. 1981;125:1229–1232. [PMC free article] [PubMed] [Google Scholar]
- Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS Drugs. 2004;18:37–48. doi: 10.2165/00023210-200418010-00004. [DOI] [PubMed] [Google Scholar]
- Bell JR, Butler B, Lawrance A, Batey R, Salmelainen P. Comparing overdose mortality associated with methadone and buprenorphine treatment. Drug Alcohol Depend. 2009;104:73–77. doi: 10.1016/j.drugalcdep.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Bleich A, Gelkopf M, Weizman T, Adelson M. Benzodiazepine abuse in a methadone maintenance treatment clinic in Israel: characteristics and a phar-macotherapeutic approach. Isr J Psychiatry relat Sci. 2002;39:104–112. [PubMed] [Google Scholar]
- Bramness JG, Kornor H. Benzodiazepine prescription for patients in opioid maintenance treatment in Norway. Drug Alcohol Depend. 2007;90:203–209. doi: 10.1016/j.drugalcdep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Bramness JG, Skurtveit S, Morland J. Clinical impairment of benzodiazepines—relation between benzodiazepine concentrations and impairment in apprehended drivers. Drug Alcohol Depend. 2002;68:131–141. doi: 10.1016/s0376-8716(02)00188-6. [DOI] [PubMed] [Google Scholar]
- Brands B, Blake J, Marsh DC, Sproule B, Jeyapalan R, Li S. The impact of benzodiazepine use on methadone maintenance treatment outcomes. J Addict Dis. 2008;27:37–48. doi: 10.1080/10550880802122620. [DOI] [PubMed] [Google Scholar]
- Brunette MF, Noordsy DL, Xie H, Drake RE. Benzodiazepine use and abuse among patients with severe mental illness and co-occurring substance use disorders. Psychiatr Serv. 2003;54:1395–1401. doi: 10.1176/appi.ps.54.10.1395. [DOI] [PubMed] [Google Scholar]
- Chen KW, Berger CC, Forde DP, D'Adamo C, Weintraub E, Gandhi D. Benzodiazepine use and misuse among patients in a methadone program. BMC Psychiatry. 2011;11:90. doi: 10.1186/1471-244X-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Anchersen K, Waal H. Mortality prior to, during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend. 2008;94:151–157. doi: 10.1016/j.drugalcdep.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Moore BA, Sullivan LE, Becker WC, Pantalon MV, Chawarski MC, Barry DT, O'Connor PG, Schottenfeld RS. Long-term treatment with buprenorphine/naloxone in primary care: results at 2–5 years. Am J Addict. 2008;17:116–120. doi: 10.1080/10550490701860971. [DOI] [PubMed] [Google Scholar]
- Gasser JC, Kaufman RD, Bellville JW. Respiratory effects of lorazepam, pentobarbital, and pentazocine. Clin Pharmacol Ther. 1975;18:170–174. doi: 10.1002/cpt1975182170. [DOI] [PubMed] [Google Scholar]
- Gerra G, Leonardi C, D'Amore A, Strepparola G, Fagetti R, Assi C, Zaimovic A, Lucchini A. Buprenorphine treatment outcome in dually diagnosed heroin dependent patients: a retrospective study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:265–272. doi: 10.1016/j.pnpbp.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Weiss RD, Griffin ML. Patients who use drugs during inpatient substance abuse treatment. Am J Psychiatry. 1992;149:235–239. doi: 10.1176/ajp.149.2.235. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66(Suppl. 9):31–41. [PubMed] [Google Scholar]
- Gunderson EW, Fiellin DA. Office-based maintenance treatment of opioid dependence: how does it compare with traditional approaches? CNS Drugs. 2008;22:99–111. doi: 10.2165/00023210-200822020-00002. [DOI] [PubMed] [Google Scholar]
- Hakkinen M, Launiainen T, Vuori E, Ojanpera I. Benzodiazepines and alcohol are associated with cases of fatal buprenorphine poisoning. Eur J Clin Pharmacol. 2012;68:301–309. doi: 10.1007/s00228-011-1122-4. [DOI] [PubMed] [Google Scholar]
- Lavie E, Fatseas M, Denis C, Auriacombe M. Benzodiazepine use among opiate-dependent subjects in buprenorphine maintenance treatment: correlates of use, abuse and dependence. Drug Alcohol Depend. 2009;99:338–344. doi: 10.1016/j.drugalcdep.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Liebrenz M, Boesch L, Stohler R, Caflisch C. Agonist substitution—a treatment alternative for high-dose benzodiazepine-dependent patients? Addiction. 2010;105:1870–1874. doi: 10.1111/j.1360-0443.2010.02933.x. [DOI] [PubMed] [Google Scholar]
- Lintzeris N, Mitchell TB, Bond A, Nestor L, Strang J. Interactions on mixing diazepam with methadone or buprenorphine in maintenance patients. J Clin Psychopharmacol. 2006;26:274–283. doi: 10.1097/01.jcp.0000219050.33008.61. [DOI] [PubMed] [Google Scholar]
- Lintzeris N, Nielsen S. Benzodiazepines, methadone and buprenorphine: interactions and clinical management. Am J Addict. 2010;19:59–72. doi: 10.1111/j.1521-0391.2009.00007.x. [DOI] [PubMed] [Google Scholar]
- Moody DE, Fang WB, Morrison J, McCance-Katz E. Gender differences in pharmacokinetics of maintenance dosed buprenorphine. Drug Alcohol Depend. 2011;118:479–483. doi: 10.1016/j.drugalcdep.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A, Bellville JW, Comer W, Danielson L. Respiratory effects of quazepam and pentobarbital. J Clin Pharmacol. 1987;27:310–313. doi: 10.1002/j.1552-4604.1987.tb03020.x. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Dietze P, Lee N, Dunlop A, Taylor D. Concurrent buprenorphine and benzodiazepines use and self-reported opioid toxicity in opioid substitution treatment. Addiction. 2007;102:616–622. doi: 10.1111/j.1360-0443.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Taylor DA. The effect of buprenorphine and benzodiazepines on respiration in the rat. Drug Alcohol Depend. 2005;79:95–101. doi: 10.1016/j.drugalcdep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- O'Brien CP. Benzodiazepine use, abuse, and dependence. J Clin Psychiatry. 2005;66(Suppl. 2):28–33. [PubMed] [Google Scholar]
- Oster G, Huse DM, Adams SF, Imbimbo J, Russell MW. Benzodiazepine tranquilizers and the risk of accidental injury. Am J Public Health. 1990;80:1467–1470. doi: 10.2105/ajph.80.12.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster G, Russell MW, Huse DM, Adams SF, Imbimbo J. Accident-and injury-related health-care utilization among benzodiazepine users and nonusers. J Clin Psychiatry. 1987;48(Suppl. 17–21) [PubMed] [Google Scholar]
- Otto MW, Bruce SE, Deckersbach T. Benzodiazepine use, cognitive impairment, and cognitive-behavioral therapy for anxiety disorders: issues in the treatment of a patient in need. J Clin Psychiatry. 2005;66(Suppl. 2):34–38. [PubMed] [Google Scholar]
- Otto MW, McHugh RK, Simon NM, Farach FJ, Worthington JJ, Pollack MH. Efficacy of CBT for benzodiazepine discontinuation in patients with panic disorder: further evaluation. Behav Res Ther. 2010;48:720–727. doi: 10.1016/j.brat.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray WA, Schaffner W, Federspiel CF. Differences between female and male children in the receipt of prescribed psychotropic and controlled-analgesic drugs. A five-year epidemiologic study Med Care. 1986;24:801–813. doi: 10.1097/00005650-198609000-00002. [DOI] [PubMed] [Google Scholar]
- Reckitt-Benckiser. Medication Suboxone Guide. Slough, United Kingdom: 2010. [Google Scholar]
- Reynaud M, Petit G, Potard D, Courty P. Six deaths linked to concomitant use of buprenorphine and benzodiazepines. Addiction. 1998a;93:1385–1392. doi: 10.1046/j.1360-0443.1998.93913859.x. [DOI] [PubMed] [Google Scholar]
- Reynaud M, Tracqui A, Petit G, Potard D, Courty P. Six deaths linked to misuse of buprenorphine–benzodiazepine combinations. Am J Psychiatry. 1998b;155:448–449. [PubMed] [Google Scholar]
- Rosca P, Haklai Z, Goldberger N, Zohar P, Margolis A, Ponizovsky AM. Mortality and causes of death among users of methadone maintenance treatment in Israel, 1999–2008. Drug Alcohol Depend. 2012;125:160–163. doi: 10.1016/j.drugalcdep.2012.03.013. [DOI] [PubMed] [Google Scholar]
- SAMHSA. HHS Publication No SMA 11-4618. Rockville, MD: 2011. [Google Scholar]
- Schuman-Olivier Z, Albanese M, Nelson SE, Roland L, Puopolo F, Klinker L, Shaffer HJ. Self-treatment: illicit buprenorphine use by opioid-dependent treatment seekers. J Subst Abuse Treat. 2010;39:41–50. doi: 10.1016/j.jsat.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Soyka M. To substitute or not substitute-optimal tactics for the management of benzodiazepine dependence. Addiction. 2010;105:1876–1877. doi: 10.1111/j.1360-0443.2010.03087.x. [DOI] [PubMed] [Google Scholar]
- Swinson RP. Clinical practice guidelines. Management of anxiety disorders. Can J Psychiatry. 2006;51:9S–91S. [PubMed] [Google Scholar]
- Tyrer P. Benzodiazepine substitution for dependent patients-going with the flow. Addiction. 2010;105:1875–1876. doi: 10.1111/j.1360-0443.2010.03067.x. [DOI] [PubMed] [Google Scholar]
- Yokell MA, Zaller ND, Green TC, Rich JD. Buprenorphine and buprenor-phine/naloxone diversion, misuse, and illicit use: an international review. Curr Drug Abuse Rev. 2011;4:28–41. doi: 10.2174/1874473711104010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Kando JC, Cole JO, Blumenthal S. Gender differences in pharmacokinetics and pharmacodynamics of psychotropic medication. Am J Psychiatry. 1992;149:587–595. doi: 10.1176/ajp.149.5.587. [DOI] [PubMed] [Google Scholar]


