Abstract
Background
Tobacco use has significant adverse effects on oral health. Oral health professionals in the dental office or community setting have a unique opportunity to increase tobacco abstinence rates among tobacco users.
Objectives
This review assesses the effectiveness of interventions for tobacco cessation delivered by oral health professionals and offered to cigarette smokers and smokeless tobacco users in the dental office or community setting.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialized Register (CENTRAL), MEDLINE (1966‐November 2011), EMBASE (1988‐November 2011), CINAHL (1982‐November 2011), Healthstar (1975‐November 2011), ERIC (1967‐November 2011), PsycINFO (1984‐November 2011), National Technical Information Service database (NTIS, 1964‐November 2011), Dissertation Abstracts Online (1861‐November 2011), Database of Abstract of Reviews of Effectiveness (DARE, 1995‐November 2011), and Web of Science (1993‐November 2011).
Selection criteria
We included randomized and pseudo‐randomized clinical trials assessing tobacco cessation interventions conducted by oral health professionals in the dental office or community setting with at least six months of follow‐up.
Data collection and analysis
Two authors independently reviewed abstracts for potential inclusion and abstracted data from included trials. Disagreements were resolved by consensus. The primary outcome was abstinence from smoking or all tobacco use (for users of smokeless tobacco) at the longest follow‐up, using the strictest definition of abstinence reported. The effect was summarised as an odds ratio, with correction for clustering where appropriate. Heterogeneity was assessed using the I² statistic and where appropriate a pooled effect was estimated using an inverse variance fixed‐effect model.
Main results
Fourteen clinical trials met the criteria for inclusion in this review. Included studies assessed the efficacy of interventions in the dental office or in a community school or college setting. Six studies evaluated the effectiveness of interventions among smokeless tobacco (ST) users, and eight studies evaluated interventions among cigarette smokers, six of which involved adult smokers in dental practice settings. All studies employed behavioral interventions and only one required pharmacotherapy as an interventional component. All studies included an oral examination component. Pooling all 14 studies suggested that interventions conducted by oral health professionals can increase tobacco abstinence rates (odds ratio [OR] 1.71, 95% confidence interval [CI] 1.44 to 2.03) at six months or longer, but there was evidence of heterogeneity (I² = 61%). Within the subgroup of interventions for smokers, heterogeneity was smaller (I² = 51%), but was largely attributable to a large study showing no evidence of benefit. Within this subgroup there were five studies which involved adult smokers in dental practice settings. Pooling these showed clear evidence of benefit and minimal heterogeneity (OR 2.38, 95% CI 1.70 to 3.35, 5 studies, I² = 3%) but this was a posthoc subgroup analysis. Amongst the studies in smokeless tobacco users the heterogeneity was also attributable to a large study showing no sign of benefit, possibly due to intervention spillover to control colleges; the other five studies indicated that interventions for ST users were effective (OR 1.70; 95% CI 1.36 to 2.11).
Authors' conclusions
Available evidence suggests that behavioral interventions for tobacco cessation conducted by oral health professionals incorporating an oral examination component in the dental office or community setting may increase tobacco abstinence rates among both cigarette smokers and smokeless tobacco users. Differences between the studies limit the ability to make conclusive recommendations regarding the intervention components that should be incorporated into clinical practice, however, behavioral counselling (typically brief) in conjunction with an oral examination was a consistent intervention component that was also provided in some control groups.
Keywords: Humans; Dental Offices; Counseling; Oral Health; Randomized Controlled Trials as Topic; Schools; Smoking Cessation; Smoking Cessation/methods; Smoking Cessation/psychology; Tobacco Use Cessation; Tobacco Use Cessation/methods; Tobacco Use Cessation/psychology; Tobacco, Smokeless; Tobacco, Smokeless/adverse effects; Universities
Can interventions delivered by dental professionals help tobacco users to quit?
In addition to the well‐known harmful effects of smoking on respiratory and cardiovascular systems, tobacco use is associated with an increased risk for oral disease, including cancer and gum disease. Dental professionals are in a unique position to help tobacco users who present for dental care by providing assistance to help them stop smoking or using other tobacco products. Combined findings from 14 studies including over 10,500 participants showed that tobacco interventions by dental professionals helped tobacco users to quit. These findings are similar for smokeless tobacco users and smokers, and the body of evidence reveals a significant increase in demonstrated benefit compared to earlier findings of this review.
Background
In addition to the well‐known harmful effects of smoking on respiratory and cardiovascular systems, tobacco use has significant adverse effects on oral health (Warnakulasuriya 2010). Cigarette smoking is associated with an increased risk for oral disease (Gelskey 1999; Mecklenburg 1998; Salvi 2000). Tobacco exposes the oral cavity to toxic carcinogens that may have a role in initiation and promotion of cancer (Mirbod 2000). Tobacco is the major inducer of oral squamous cell carcinoma (SCC) and is considered to be responsible for 50% to 90% of oral cancer cases worldwide (Epstein 1992; Holleb 1996). The incidence of oral SCC is four to seven times greater in smokers than non‐smokers (Piyathilake 1995). Oral cancer and pre‐cancer occurs more frequently in smokers, and quitting smoking decreases the risk for oral cancer within 5 to 10 years (EU Working Group 1998). Tobacco exposure is also harmful to periodontal health, and smoking status is an important factor in the prognosis for periodontal therapy, oral wound healing, implant therapy, and cosmetic dentistry (Mecklenburg 1998). Smoking results in discoloration of both teeth and dental restorations, and is associated with halitosis, diminished taste, and an increased prevalence and severity of periodontal disease (EU Working Group 1998). Cigarette smoking is causally associated with an increased prevalence and severity of periodontitis (Gelskey 1999), even when adequate oral hygiene is practiced (Kerdvongbundit 2002). Cessation of smoking may halt disease progression and improve outcomes of periodontal therapy (EU Working Group 1998).
Smokeless tobacco use has been reported to cause tooth decay (Tomar 1999) and discoloration of dental restorations (Walsh 2000). Chewing tobacco, in particular, is associated with an increased risk for dental caries due to high sugar content and increased gingival recession. Abrasive particles in chewing tobacco may contribute to significant dental attrition which may require dental restorations in advanced cases (Bowles 1995; Milosevic 1996). Cross‐sectional studies have suggested that smokeless tobacco users with co‐existing gingivitis have high rates of gingival recession, mucosal pathology, and dental caries (Offenbacher 1985). Smokeless tobacco use has also been associated with irreversible gingival attachment loss resulting in root exposure (Ernster 1990). Effects of smokeless tobacco use are typically observed at anatomical locations where the tobacco contacts the mucosa, such as the labial vestibule and adjacent periodontium. Both the prevalence and severity of tobacco‐related oral lesions demonstrate a dose‐response relationship with the amount, frequency and duration of smokeless tobacco exposure (Little 1992a). Chronic exposure can lead to leukoplakia (Hirsch 1982), a premalignant condition (Silverman 1984; Silverman 1976). Smokeless tobacco use in the United States has been associated with an increased risk for oral cancer in a dose‐response fashion (Stockwell 1986; Williams 1977; Winn 1981). Risk may vary depending upon the type of smokeless tobacco used, as the highest rates of oral cancer are observed in countries where smokeless tobacco is consumed with additives (e.g., areca nut) (Critchley 2003).
The dental practice setting provides a unique opportunity to assist tobacco users in achieving tobacco abstinence (Christen 1990; Needleman 2010; Ramseier 2010). Widespread acceptance of tobacco use interventions in the dental setting have been lacking and limitations in primary care resources have curtailed further efforts (Warnakulasuriya 2002). Compared to other health care providers, dentists more accurately estimate patient tobacco use (Block 1999). However, dental practitioners are less consistent with and supportive of intervention, less likely to report having strong knowledge or skill levels regarding tobacco cessation, and more likely to perceive barriers to tobacco intervention (Block 1999). More than 40% of dentists do not routinely ask about tobacco use and 60% do not routinely advise tobacco users to quit (Tomar 2001).
While 61.5% of dentists believe their patients do not expect tobacco cessation services, 58.5% of their patients felt such services should be provided (Campbell 1999). Barriers to providing tobacco cessation service include concern for patient resistance (Campbell 1994), lack of knowledge, lack of time (Dolan 1997), lack of financial reimbursement (Fried 1992), and a concern for poor co‐ordination of care between dentistry and tobacco cessation services (Campbell 1994).
Objectives
We assess the effectiveness of interventions for tobacco cessation offered by oral health professionals to cigarette smokers and smokeless tobacco users in the dental office or community setting. We were interested in testing the following hypotheses in regards to increasing tobacco abstinence rates among tobacco users:
In dental settings, brief counselling cessation interventions are more effective than usual care.
Brief counselling cessation interventions conducted by dental professionals combined with nicotine replacement therapy (NRT) are more effective than NRT alone.
Tobacco use interventions incorporating personalized feedback from an oral examination are more effective than interventions without personalized feedback from an oral examination.
Tobacco use interventions conducted by dental health professionals are more effective than interventions conducted by other healthcare professionals.
Methods
Criteria for considering studies for this review
Types of studies
All randomized and pseudo‐randomized (i.e., by patient number, date of birth, day of attendance) controlled trials with at least six months follow‐up were included. The unit of randomization was the dentist or practice for the studies in the dental office setting, and college or high school for the studies in the community setting.
Types of participants
Patients or subjects of any age reporting tobacco use and receiving oral health interventions by dental professionals were included. Subject recruitment and participation included both those actively seeking treatment and those who did not express an interest in quitting. All tobacco users (cigarette, cigar, and pipe smokers, and smokeless tobacco users) were included.
Types of interventions
We included any intervention to promote tobacco use cessation (intervention versus usual care or placebo, and/or intervention versus other intervention) which included a component delivered by a dentist, dental hygienist, dental assistant or office staff in the dental practice setting and any combination of these, as well as the same individuals providing intervention as part of a community effort. Interventions could include brief advice to quit, provision of self‐help materials, counselling, pharmacotherapy or any combination of these, or referral to other sources of support. Interventions that were directed at both smokers and smokeless tobacco users were included, as were interventions aimed at the training of dental health professionals.
Types of outcome measures
The outcome measure was smoking and tobacco use cessation, assessed at least six months from the delivery of the intervention. Trials which did not report tobacco use outcomes or did not have sufficiently long follow‐up were excluded. Biochemical validation of self‐reported cessation was not required but was recorded and used where available.
Search methods for identification of studies
The Specialized Registers of the Cochrane Tobacco Addiction Group (most recent search November 2011) and the Cochrane Oral Health Group (Cochrane Central Register of Controlled Trials, Cochrane Library 2011) were searched for references to tobacco use interventions by dental health professionals, in the dental practice setting or otherwise. We also searched the following electronic retrieval systems and databases:
The Cochrane Central Register of Controlled Trials (CENTRAL)
MEDLINE (1966‐ November 2011)
EMBASE (1988‐ November 2011)
CINAHL (1982‐ November 2011)
Healthstar (1975‐ November 2011)
ERIC (1967‐ November 2011)
PsycINFO (1984‐ November 2011)
National Technical Information Service database (NTIS, 1964‐ November 2011)
Dissertation Abstracts Online (1861‐ November 2011)
Database of Abstract of Reviews of Effectiveness (DARE, 1995‐ November 2011)
Web of Science (1993‐ November 2011)
The following terms were used to describe the participants: smokers; smoking; cigarettes; smokeless tobacco; chewing tobacco; oral tobacco; spit tobacco; snuff; quid; chew; plug; tobacco use(rs). The following terms described the interventions: randomized; dentists; dental; hygienists; dental‐patient relations; behavior modification; conditioning therapy; therapy; behavior; conditioning; group therapy; cognitive therapy; counselling; behavioral intervention; pharmacotherapy; drug; patient education; health promotion. The following terms were used to describe the outcomes: tobacco use cessation; smoking abstinence; tobacco abstinence. The following terms described the intervention environment: dentists; dental; hygienists; dental‐patient relations; oral health.
The Medical Subject Headings (MeSH) used in MEDLINE and CINAHL were also used to focus on the dental environment: limit retrieval to the dentistry journals subset; or subject headings Oral Health/ or exp Dentistry/ or exp Dental Staff/ or exp DENTISTS/ or DENTIST'S PRACTICE PATTERNS/ or exp dental auxiliaries/ or dental hygienists. Keywords of the various oral specialties orthodont$, periodont$ and endodont$ were also searched. There were no language restrictions. In general, records were searched by conducting searches the following way: (participants OR outcomes) AND interventions. We contacted experts in the area to locate unpublished studies in an effort to minimise publication bias.
Data collection and analysis
Two authors screened the records retrieved by the searches for potential relevance against stated inclusion criteria: randomized/pseudo‐randomized clinical trial, dental setting, tobacco cessation interventions, and cessation measures of six‐month minimum follow‐up. Two authors checked studies of possible relevance for inclusion or exclusion, and independently extracted and compared data. We resolved disagreements by discussion and consensus (referring to a colleague or Cochrane Review Group staff when necessary).
We extracted the following information about each study:
Site, including country and type of dental practice
Method of randomization and allocation concealment, and whether individual or cluster randomized
Method of participant selection
Characteristics of the intervention (behavioral/pharmacologic, delivered by whom)
Characteristics of participants (type of tobacco use, interest in quitting)
Outcome assessment (length of follow‐up, definition of quitting, method for validation of self‐report)
For each study we selected the outcome with the most rigorous definition available with regards to maintenance of abstinence (i.e., continuous versus point prevalence) and type of tobacco abstinence (i.e., all tobacco versus smokeless tobacco only) (Hughes 2003). Rates were based on an intention‐to‐treat analysis with drop‐outs and losses to follow‐up assumed to be continuing tobacco users. We noted any difference in numbers lost to follow‐up between intervention and control groups.
The risk of randomization and allocation concealment were judged to be low if the method was described in sufficient detail to ensure that allocation was blinded until after trial enrolment, unclear if there was insufficient detail with which to judge, and high if allocation was not concealed (as in use of patient record numbers, day of attendance, etc.). We also assessed the risk of attrition bias in included studies. Where there appears to have been a large loss to follow‐up we assessed whether the findings were sensitive to the use of different denominators. In addition to the judgements above, we assessed bias impact on strength of the evidence by identifying trials with multiple sources of bias, and we comment on the potential impact of the bias on the overall treatment effect.
The outcome from each trial was expressed as an odds ratio (OR). Where cessation is the outcome this was defined as (number of quitters in treatment group/number of smokers in treatment group)/(number of quitters in control group/number of smokers in control group). The OR was greater than 1 if people were more likely to quit in the treatment group. For cluster randomized trials we adjusted for clustering using the reported intraclass correlation coefficient (ICC) and the average cluster size. We calculated the logarithm of the adjusted or natural OR and its standard error and pooled studies using the generic inverse variance method (Higgins 2011, Section 16.3.4). We have displayed the raw data in Analysis 1.4.
Analysis 1.4.
Comparison 1 Behavioral Interventions versus control, Outcome 4 Tobacco abstinence at longest follow‐up. Raw data for all studies.
Tobacco abstinence at longest follow‐up. Raw data for all studies
| Study | Intervention | Control | Abstinence def | Notes |
|---|---|---|---|---|
| Cigarette Smokers | ||||
| Binnie 2007 | 3/59 | 2/57 | 12m, prolonged (repeated PP), all tobacco | Validated |
| Ebbert 2007 | 15/60 | 6/22 | 6m, PP, all tobacco | Cluster RCT, ICC 0.001 |
| Gordon 2010a | 24/793 | 8/550 | 12m, sustained, all tobacco | Cluster RCT, ICC 0.012 27/817 quit in 5As |
| Gordon 2010b | 74/1394 | 22/1155 | 7.5m, prolonged, all tobacco | Cluster RCT, ICC 0.009 |
| Hanioka 2010 | 12/33 | 3/23 | 12m, continuous, all tobacco | |
| Lando 2007 | 4/61 | 7/63 | 12m, PP (30 day), smoking | |
| Nohlert 2009 | 27/150 | 13/150 | 12m, sustained, smoking | |
| Severson 1998 | 35/1374 | 32/1350 | 12m, sustained, all tobacco | Cluster RCT, ICC 0.0004 |
| Smokeless Tobacco Users | ||||
| Andrews 1999 | 40/394 | 8/239 | 12m, sustained, all tobacco | Cluster RCT, ICC 0.0009 |
| Gansky 2005 | 103/285 | 130/352 | 12m, PP, ST | Cluster RCT, ICC 0.0074 |
| Severson 2009 | 53/393 | 22/393 | 6m, prolonged, ST | |
| Stevens 1995 | 25/245 | 19/273 | 12m, sustained, all tobacco | |
| Walsh 1999 | 60/171 | 30/189 | 12m, PP, ST | Cluster RCT, ICC 0.02 |
| Walsh 2003 | 32/141 | 21/166 | 24m, sustained, ST | Cluster RCT, ICC 0.04 12m outcome was 38/141 vs 23/166 |
We hypothesized that the following would explain heterogeneity which was explored through subgroup analyses: 1) Patients ‐ smokers (cigarette, cigar, pipe) versus smokeless tobacco users, patients enrolled based on their interest in tobacco cessation versus patients enrolled regardless of interest in quitting (e.g., subjects enrolled in a study requiring informed consent to participate versus subjects enrolled in a study implemented in a dental practice enrolling all patients who are treated clinically), highly dependent versus less dependent tobacco users using the Fagerström Tolerance Questionnaire or modifications of the this dependence measure (to the extent that dependence is similarly categorized across trials), specialty practice versus general practice dental settings; 2) Interventions ‐ interventions delivered by dentists versus dental hygienists or other dental staff, behavioral interventions versus pharmacologic interventions; 3) Outcomes ‐ all tobacco abstinence versus tobacco‐specific (cigarette smoking, smokeless tobacco) outcomes; 4) Method of randomization ‐ cluster versus individual. We assessed heterogeneity using the I² statistic (Higgins 2003). Sensitivity analyses included assessment of changes in the estimate of the treatment effect using the random effects model compared with the fixed effect model.
We include in this updated review the Cochrane Tobacco Addiction Group's Glossary of smoking‐related terms (Appendix 1).
Results
Description of studies
The review included 14 studies involving over 10,500 participants (Andrews 1999; Binnie 2007; Ebbert 2007; Gordon 2010a; Gordon 2010b; Gansky 2005; Hanioka 2010; Lando 2007; Nohlert 2009; Severson 1998; Severson 2009; Stevens 1995; Walsh 1999; Walsh 2003). Although Andrews 1999 and Severson 1998 reported the same trial they are treated here as separate studies since Andrews 1999 focused on outcomes in smokeless tobacco users and Severson 1998 on smokers. One eligible study had to be excluded due to unavailable subgroup denominator values (Cohen 1989). Other papers identified as potentially relevant but not meeting all inclusion criteria are listed in Characteristics of excluded studies along with the reason for exclusion.
The dental offices involved in the included studies were a heterogeneous group: six studies were conducted in private practice office settings (Andrews 1999; Ebbert 2007; Gordon 2010a; Hanioka 2010; Nohlert 2009; Severson 1998;); one study involved community public health dental clinics (Gordon 2010b); one was set in a hospital‐based periodontal clinic (Binnie 2007); two took place in managed care clinics (Lando 2007; Stevens 1995); and one took place in military clinics (Severson 2009). Three involved oral health professionals (dentists and dental hygienists) providing interventions to athletes within high school or college community settings (Gansky 2005; Walsh 1999; Walsh 2003). The school community studies included a dental professional intervention component as a major part of the intervention.
Eight studies targeted smokers (Binnie 2007; Ebbert 2007; Gordon 2010a; Gordon 2010b; Hanioka 2010; Lando 2007; Nohlert 2009; Severson 1998). Five studies involved dental practice settings where adult smokers were provided at least brief behavioral counselling as part of the intervention (Binnie 2007; Ebbert 2007; Hanioka 2010; Gordon 2010b; Gordon 2010a). One study targeted both smokers and smokeless tobacco users; the data for the two types of participant were reported separately and are treated as two studies, Severson 1998 covering smokers and Andrews 1999 covering smokeless tobacco users. Six studies targeted smokeless tobacco users (Andrews 1999; Walsh 2003; Gansky 2005; Severson 2009; Stevens 1995; Walsh 1999). In the dental office studies, studies included tobacco users not actively seeking treatment (Andrews 1999; Gordon 2010a; Severson 1998; Stevens 1995) and those willing to participate (Binnie 2007; Ebbert 2007; Gordon 2010b; Hanioka 2010; Lando 2007; Nohlert 2009; Severson 2009).
Interventions in the dental office setting occurred during hygiene visits in general dental practices (Andrews 1999; Binnie 2007; Ebbert 2007; Gordon 2010a; Gordon 2010b; Hanioka 2010; Lando 2007; Nohlert 2009; Severson 1998; Stevens 1995), and involved either: 1) brief advice plus quitline referral (Ebbert 2007), brief advice plus motivational interviewing (Lando 2007), brief advice plus video‐based cessation program with phone follow‐up (Andrews 1999; Severson 1998; Severson 2009), or 2) counselling using the 5 A's plus nicotine replacement therapy (NRT) (Binnie 2007), 5 A's plus NRT and population‐specific printed material (Gordon 2010b), 3 A's plus pharmacotherapy and referral as needed (Gordon 2010a). One study used target population‐specific videos as an adjunct to counselling (Severson 2009). Two studies used a high intensity intervention where intensity was gauged as frequency of personal contact, and occurred five times or more (Hanioka 2010; Nohlert 2009).
In the school community studies, tobacco users had to agree to participate and informed consent was obtained (Gansky 2005; Walsh 1999; Walsh 2003). Some dental office studies restricted enrolment to 15 years of age or older (Andrews 1999; Severson 1998; Stevens 1995), one of which placed gender restrictions on inclusion, while the remaining targeted adults. All of the school community studies based their intervention on the Cognitive Social Learning Theory (Bandura 1986), two of which reported that the Diffusion of Innovation Theory (Rogers 1983) was instrumental for incorporating the use of peer leaders. No such theoretical foundation was mentioned for the interventions applied to the dental office studies.
Nicotine replacement therapy in the form of gum (2 mg) was used in one of the school community studies (Walsh 1999). The gum was reinforced with counselling by a dental professional. In the majority of the studies, dental professionals (dentists and dental hygienists) provided counselling interventions which most often included combinations of an oral examination, feedback from the examination as to oral effects of tobacco use, a message to quit, motivational counselling using printed material or media presentations, and self‐help aids. In five dental office studies, the usual care group included was either not described (Hanioka 2010) or involved no structured intervention (Andrews 1999; Gordon 2010a; Gordon 2010b; Stevens 1995), and in all the school community studies the control schools received no formal training.
In five of the dental office studies, the dental office was the unit of randomization (Andrews 1999; Ebbert 2007, Gordon 2010a, Gordon 2010b, Severson 1998), and in six studies, the patient was the unit of randomization (Binnie 2007, Hanioka 2010, Lando 2007, Nohlert 2009, Severson 2009, Stevens 1995). In the school community studies, the school was the unit of randomization following stratification based on baseline prevalence of tobacco use.
In the included trials, participants were followed for a maximum of six months (Ebbert 2007; Severson 2009), seven and a half months (Gordon 2010b), 12 months (Andrews 1999; Binnie 2007; Gansky 2005; Gordon 2010a; Hanioka 2007; Lando 2007; Nohlert 2009; Severson 1998; Stevens 1995; Walsh 1999), and 24 months (Walsh 2003, two year outcomes reported in Gansky 2002).
Of the studies targeting smokeless tobacco users, three reported all tobacco abstinence outcomes (Andrews 1999; Severson 1998; Stevens 1995). Point prevalence was reported as the primary outcome in three studies (Gansky 2005; Stevens 1995; Walsh 1999). Two studies reported abstinence as one week (seven day) point prevalence (Ebbert 2007; Stevens 1995), while four reported 30‐day point prevalence abstinence (Gansky 2005; Lando 2007, Walsh 1999; Walsh 2003). Seven studies used continuous (sustained or prolonged) abstinence requiring either no tobacco use at both 3 and 12 months (Andrews 1999; Severson 1998), 12 months continuous abstinence (Hanioka 2007), six months continuous abstinence (Nohlert 2009), prolonged abstinence (Gordon 2010a, Gordon 2010b), or no current tobacco use at both 12 and 24 months after quitting before the one‐month follow‐up (Walsh 2003, reported in Gansky 2002).
Details of included studies can be found in Characteristics of included studies.
Risk of bias in included studies
The risk of selection bias, judged on the basis of random sequence generation, was considered low in 12 studies (Binnie 2007; Ebbert 2007; Gansky 2005; Gordon 2010a; Gordon 2010b; Hanioka 2010; Lando 2007; Nohlert 2009; Severson 1998; Severson 2009; Walsh 1999; Walsh 2003), unclear in Andrews 1999, and high in Stevens 1995. Methods of allocation concealment were judged to be at low risk of bias in 11 studies (Andrews 1999; Binnie 2007; Gansky 2005; Gordon 2010a; Gordon 2010b; Hanioka 2010; Nohlert 2009; Severson 1998; Severson 2009; Walsh 1999; Walsh 2003), at unclear risk of bias in Ebbert 2007 and Lando 2007, and at high risk of bias in Stevens 1995. The risk of attrition bias was judged to be low in nine studies (Andrews 1999; Gansky 2005; Gordon 2010a; Gordon 2010b; Hanioka 2010; Severson 1998; Severson 2009; Walsh 1999; Walsh 2003), unclear in three studies (Binnie 2007; Ebbert 2007; Nohlert 2009), and high in two studies (Lando 2007; Stevens 1995).
Biochemical confirmation was used to validate self report in two studies (Binnie 2007; Hanioka 2010). In three other studies, biochemical confirmation was initially utilized and abandoned due to poor compliance (Stevens 1995), or used as a strategy to enhance self report (Walsh 1999; Walsh 2003) (i.e., the 'bogus pipeline' method). The remaining studies did not use biochemical confirmation.
The control of detection bias through the blinding participants or oral health care personnel was limited due to the nature of the behavioral interventions evaluated.
In one school‐based study (Gansky 2005), the authors describe a 'spill‐over' effect between the intervention and control group that was felt to bias the results of the trial. In one study which took place in a managed care setting, the authors describe a host of process constraints limiting the effectiveness of achieving study goals, therefore impacting the outcomes (Lando 2007).
Rationale for risk of bias judgements can be found in Characteristics of included studies.
Effects of interventions
When the 14 clinical trials of dental interventions compared to usual care, no contact, or less treatment intensive controls are pooled (including all tobacco users), a statistically significant increase in the odds of tobacco abstinence at 6 to 24 months is observed (odds ratio [OR] 1.71, 95% confidence interval [CI] 1.44 to 2.03, Figure 1, Analysis 1.1,) but with evidence of heterogeneity between the studies (I² = 61%).
Figure 1.

Forest plot of comparison: 1 Behavioral interventions versus control, outcome: 1.1 Abstinence at longest follow‐up.
Analysis 1.1.
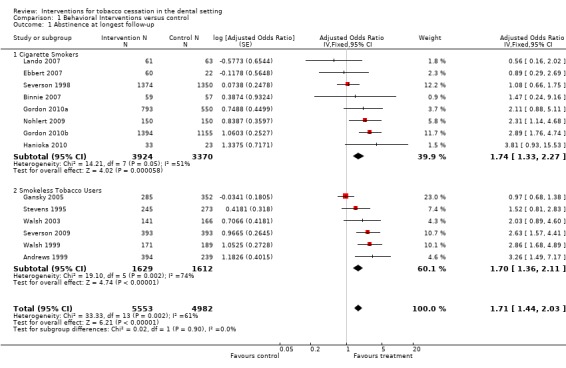
Comparison 1 Behavioral Interventions versus control, Outcome 1 Abstinence at longest follow‐up.
Heterogeneity was explored by assessing the prespecified potential explanations.
Patients
Heterogeneity is not fully explained by separating studies targeting cigarette smokers (I² = 51%) and smokeless tobacco (ST) users (I² = 74%). The estimated effect size was similar in both subgroups. In both groups the study contributing the greatest weight had a point estimate close to no effect, with confidence intervals ruling out large benefits or harms. In the subgroup of interventions for smokers, the largest trial, Severson 1998, failed to detect any effect of an extended intervention compared to a brief intervention by dental hygienists. The same intervention showed a benefit in ST users recruited as part of the same study (Andrews 1999). The next three largest studies amongst smokers did detect significant or near significant benefits and the pooled estimate indicated a clinically and statistically significant effect (OR 1.74, 95% CI 1.33 to 2.27, 8 studies, I² = 51%). In a sensitivity analysis using a random effects model the pooled estimate was similar and the confidence intervals still excluded one.
Heterogeneity may be due in part to type of practice. The included studies took place in dental settings characterized as either a specialty dental practice, general dental practices, federally funded dental practices, or managed care dental practices. Some targeted adolescents and adults while others focused only on adults. Recognizing these different features, we conducted a post hoc subgroup analysis of studies conducted in the settings most representative of typical dental environments (general dental practitioners seeing adult smokers). Dental practices providing, at a minimum, brief counselling to adult smokers showed a significant benefit of intervention when compared to usual care or less treatment intensive controls (OR 2.38, 95% CI 1.70 to 3.35, Analysis 1.2 ) with no evidence of heterogeneity (I² = 3%).
Analysis 1.2.

Comparison 1 Behavioral Interventions versus control, Outcome 2 Abstinence at longest follow‐up. Subgroup of trials in adult smokers seen by general dental practitioners.
In the ST use subgroup, Gansky 2005 had narrow CIs ruling out large benefits or harms, but all other studies suggested benefit and three showed significant effects. As noted above, Gansky 2005 is likely have been affected by spill‐over of intervention to control groups. The estimate including Gansky 2005 indicated a clinically and statistically significant effect (OR 1.70, 95% CI 1.36 to 2.11, 6 studies, I² = 74%). In a sensitivity analysis using a random effects model the pooled estimate was larger and the confidence intervals still excluded 1. A sensitivity analysis omitting Gansky 2005 removed all evidence of heterogeneity and increased the effect estimate (OR 2.40, 95% CI 1.82 to 3.18).
Odds ratios were similar in a subgroup analysis comparing three studies in which participants were actively seeking treatment with three studies in which participants were not actively seeking treatment (actively seeking treatment: OR 1.41, 95% CI 1.07 to 1.86; not actively seeking treatment: OR 1.48, 95% CI 1.05 to 2.09, analysis not shown).
Interventions
Interventions in all studies were a team effort involving brief dental encounters plus additional behavioral interventions and/or pharmacotherapy. Interventions differed in intensity as measured by number of planned contacts but there was no clear indication of a dose‐response relationship. Gordon 2010a had three arms: a '3 As' intervention using a 'fax‐to‐quit' referral form; a '5As' arm with Quitline referral at the provider's discretion; and a usual care control arm. Our analysis uses the '3As' intervention, but opting to use the '5As' intervention arm would not alter the findings.
Outcomes
There was no evidence that the length of follow‐up or definition of abstinence explained heterogeneity between studies.
Method of randomization
The group of six trials that were individually randomized had lower heterogeneity (I² = 27%), although this may be due to chance, because the two studies contributing most to heterogeneity were both cluster randomized (Severson 1998 and Gansky 2005). The effect estimate in the individually randomized study subgroup, although indicating significant benefit, was not clinically large (OR 1.46, 95% CI 1.06 to 2.01, Analysis 1.3).
Analysis 1.3.

Comparison 1 Behavioral Interventions versus control, Outcome 3 Abstinence at longest follow‐up. Subgroups by method of randomization.
Discussion
The findings of our review are consistent with the hypothesis that dental interventions conducted in the dental office and school community setting are more effective than usual care for promoting tobacco use cessation. When dental interventions were compared to usual care, no contact, or less treatment intensive controls, the pooled odds ratio for abstinence at a follow‐up of between 6 and 24 months was 1.71 (95% CI 1.44 to 2.03), although this estimate must be viewed cautiously because of unexplained heterogeneity (I² = 61%).
Current results are based on a much greater body of research on cigarette smokers when compared to the previous version of this review (Carr 2006), with eight studies of interventions for cigarette smoking now included compared to just one (Severson 1998). Findings now suggest a significant benefit of intervention for increasing tobacco abstinence rates. Findings may be of particular interest to dental care providers whose practices focus on adult patients, as adult smokers may be particularly responsive to the effect of an intervention in this setting (OR 2.38, 95% CI 1.70 to 3.35, Analysis 1.2 ).
Results should also be viewed cautiously due to the inability to blind, unclear methods of treatment allocation, a lack of biochemical validation of self reported tobacco cessation in most studies and inconsistent content and delivery of dental‐specific interventions within the pooled studies. Although all of the included studies contained a dental intervention component, they involved varying dental settings, personnel, and interventions, and statistical heterogeneity was evident. The source of heterogeneity was unclear, and seemed largely attributable to null effects in two large trials. In one of these, the control communities may have been exposed to intervention (Gansky 2005). For the other (Severson 1998), there was no clear reason for the lack of effect of the intervention.
All of the studies included in this review included brief advice to quit by an oral health professional. Brief advice from physicians has been shown to be an effective means to promote cessation (Lancaster 2008), and this review suggests the same can be expected from dental professionals interacting with smokeless tobacco users. Clinical practice guidelines advise brief interventions in the clinical setting where patients are asked about their tobacco use and then advised to quit. If the user is ready to quit, the clinician can offer specific assistance and provide follow‐up care. An insufficient number of studies are available to determine what specific assistance measures delivered by a dental professional provide additional effectiveness beyond brief advice.
The public health benefits of tobacco cessation interventions within the dental setting are potentially significant. The findings for both the smokeless tobacco users and smokers in this review suggest that there is an advantage of cessation interventions using dental professionals. However, the limited number of studies reviewed does not allow identification of intervention components most critical for cessation.
Authors' conclusions
Interventions for tobacco users delivered by oral health professionals, either in the dental office or in the school community, increase the odds of quitting tobacco. Insufficient evidence exists to make conclusions about the effectiveness of specific intervention components, but behavioral counselling (typically brief) is a consistent component.
Additional study of interventions for tobacco cessation within the dental office setting is important to identify critical intervention components which are effective for this group of providers in this clinical setting.
Acknowledgements
We would like to acknowledge Sylvia Bickley of the Cochrane Oral Health Group for her assistance in obtaining references for this review, and Rafael Perera of the Tobacco Addiction Group for his statistical assistance with adjustment for clustering effects. We would also like to thank Patricia Erwin, MLS, for her assistance with search strategy development and execution. We would like to thank Dr. Kristen Vickers‐Douglas for her review of the study protocol, and Drs Ian Needleman and Helen Worthington for comments and suggestions on the full review draft.
We would like to acknowledge the assistance of the Tobacco Addiction Group staff in this update. We especially want to acknowledge Lindsay Stead for her assistance with obtaining references, review input, statistical analysis, and general facilitation with this update.
Appendices
Appendix 1. Glossary of terms
| Term | Definition |
| Abstinence | A period of being quit, ie stopping the use of cigarettes or other tobacco products, May be defined in various ways; see also: point prevalence abstinence; prolonged abstinence; continuous/sustained abstinence |
| Biochemical verification | Also called 'biochemical validation' or 'biochemical confirmation': A procedure for checking a tobacco user's report that he or she has not smoked or used tobacco. It can be measured by testing levels of nicotine or cotinine or other chemicals in blood, urine, or saliva, or by measuring levels of carbon monoxide in exhaled breath or in blood. |
| Bupropion | A pharmaceutical drug originally developed as an antidepressant, but now also licensed for smoking cessation; trade names Zyban, Wellbutrin (when prescribed as an antidepressant) |
| Carbon monoxide (CO) | A colourless, odourless highly poisonous gas found in tobacco smoke and in the lungs of people who have recently smoked, or (in smaller amounts) in people who have been exposed to tobacco smoke. May be used for biochemical verification of abstinence. |
| Cessation | Also called 'quitting' The goal of treatment to help people achieve abstinence from smoking or other tobacco use, also used to describe the process of changing the behaviour |
| Continuous abstinence | Also called 'sustained abstinence' A measure of cessation often used in clinical trials involving avoidance of all tobacco use since the quit day until the time the assessment is made. The definition occasionally allows for lapses. This is the most rigorous measure of abstinence |
| 'Cold Turkey' | Quitting abruptly, and/or quitting without behavioural or pharmaceutical support. |
| Craving | A very intense urge or desire [to smoke]. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614 |
| Dopamine | A neurotransmitter in the brain which regulates mood, attention, pleasure, reward, motivation and movement |
| Efficacy | Also called 'treatment effect' or 'effect size': The difference in outcome between the experimental and control groups |
| Harm reduction | Strategies to reduce harm caused by continued tobacco/nicotine use, such as reducing the number of cigarettes smoked, or switching to different brands or products, e.g. potentially reduced exposure products (PREPs), smokeless tobacco. |
| Lapse/slip | Terms sometimes used for a return to tobacco use after a period of abstinence. A lapse or slip might be defined as a puff or two on a cigarette. This may proceed to relapse, or abstinence may be regained. Some definitions of continuous, sustained or prolonged abstinence require complete abstinence, but some allow for a limited number or duration of slips. People who lapse are very likely to relapse, but some treatments may have their effect by helping people recover from a lapse. |
| nAChR | [neural nicotinic acetylcholine receptors]: Areas in the brain which are thought to respond to nicotine, forming the basis of nicotine addiction by stimulating the overflow of dopamine |
| Nicotine | An alkaloid derived from tobacco, responsible for the psychoactive and addictive effects of smoking. |
| Nicotine Replacement Therapy (NRT) | A smoking cessation treatment in which nicotine from tobacco is replaced for a limited period by pharmaceutical nicotine. This reduces the craving and withdrawal experienced during the initial period of abstinence while users are learning to be tobacco‐free The nicotine dose can be taken through the skin, using patches, by inhaling a spray, or by mouth using gum or lozenges. |
| Outcome | Often used to describe the result being measured in trials that is of relevance to the review. For example smoking cessation is the outcome used in reviews of ways to help smokers quit. The exact outcome in terms of the definition of abstinence and the length of time that has elapsed since the quit attempt was made may vary from trial to trial. |
| Pharmacotherapy | A treatment using pharmaceutical drugs, e.g. NRT, bupropion |
| Point prevalence abstinence (PPA) | A measure of cessation based on behaviour at a particular point in time, or during a relatively brief specified period, e.g. 24 hours, 7 days. It may include a mixture of recent and long‐term quitters. cf. prolonged abstinence, continuous abstinence |
| Prolonged abstinence | A measure of cessation which typically allows a 'grace period' following the quit date (usually of about two weeks), to allow for slips/lapses during the first few days when the effect of treatment may still be emerging. See: Hughes et al 'Measures of abstinence in clinical trials: issues and recommendations'; Nicotine & Tobacco Research, 2003: 5 (1); 13‐25 |
| Relapse | A return to regular smoking after a period of abstinence |
| Secondhand smoke | Also called passive smoking or environmental tobacco smoke [ETS] A mixture of smoke exhaled by smokers and smoke released from smouldering cigarettes, cigars, pipes, bidis, etc. The smoke mixture contains gases and particulates, including nicotine, carcinogens and toxins. |
| Self‐efficacy | The belief that one will be able to change one's behaviour, e.g. to quit smoking |
| SPC [Summary of Product Characteristics] | Advice from the manufacturers of a drug, agreed with the relevant licensing authority, to enable health professionals to prescribe and use the treatment safely and effectively. |
| Tapering | A gradual decrease in dose at the end of treatment, as an alternative to abruptly stopping treatment |
| Tar | The toxic chemicals found in cigarettes. In solid form, it is the brown, tacky residue visible in a cigarette filter and deposited in the lungs of smokers. |
| Titration | A technique of dosing at low levels at the beginning of treatment, and gradually increasing to full dose over a few days, to allow the body to get used to the drug. It is designed to limit side effects. |
| Withdrawal | A variety of behavioural, affective, cognitive and physiological symptoms, usually transient, which occur after use of an addictive drug is reduced or stopped. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614 |
Data and analyses
Comparison 1.
Behavioral Interventions versus control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence at longest follow‐up | 14 | 10535 | Adjusted Odds Ratio (Fixed, 95% CI) | 1.71 [1.44, 2.03] |
| 1.1 Cigarette Smokers | 8 | 7294 | Adjusted Odds Ratio (Fixed, 95% CI) | 1.74 [1.33, 2.27] |
| 1.2 Smokeless Tobacco Users | 6 | 3241 | Adjusted Odds Ratio (Fixed, 95% CI) | 1.70 [1.36, 2.11] |
| 2 Abstinence at longest follow‐up. Subgroup of trials in adult smokers seen by general dental practitioners | 5 | Adjusted odds ratio (Fixed, 95% CI) | 2.38 [1.70, 3.35] | |
| 3 Abstinence at longest follow‐up. Subgroups by method of randomization | 14 | Adjusted odds ratio (Fixed, 95% CI) | 1.56 [1.32, 1.85] | |
| 3.1 Cluster Randomization | 8 | Adjusted odds ratio (Fixed, 95% CI) | 1.61 [1.32, 1.96] | |
| 3.2 Individual Randomization | 6 | Adjusted odds ratio (Fixed, 95% CI) | 1.46 [1.06, 2.01] | |
| 4 Tobacco abstinence at longest follow‐up. Raw data for all studies | Other data | No numeric data | ||
| 4.1 Cigarette Smokers | Other data | No numeric data | ||
| 4.2 Smokeless Tobacco Users | Other data | No numeric data |
What's new
| Date | Event | Description |
|---|---|---|
| 10 April 2012 | New citation required and conclusions have changed | Conclusions updated to include interventions among cigarette smokers as well as among smokeless tobacco users. New included studies increase strength of effect. |
| 10 April 2012 | New search has been performed | 8 new included studies added, evaluating interventions among cigarette smokers. |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 22 February 2012 | New search has been performed | Updated search to November 2011 |
| 29 July 2008 | Amended | Converted to new review format. |
| 5 September 2006 | New search has been performed | Updated for issue 1 2007. No new studies identified. Two studies reviewed and added to excluded studies list. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Country: USA Recruitment: Hygiene patients in private dental practices Cluster randomized trial |
|
| Participants | 633 ST users >= 15 years of age | |
| Interventions | 1. Intervention: Determine tobacco use, identify oral disease, strong advice to quit, set quit date within 2w, motivation video, written material, call patient within 2w. 2. Usual care | |
| Outcomes | 12m 'sustained' abstinence from ST and all tobacco: subjects must have reported 7‐day PP ST and all tobacco abstinence at both 3m and 12m. Abstinence verification: None | |
| Notes | Data for smokers in the same trial reported in Severson 1998 ICC calculated < 0.0009. Intervention group more likely to have previously been advised by a dental care provider to quit use of ST and were less likely to be single. Study reports only those using smokeless tobacco. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomized: Practices were blocked (by average number of hygiene visits per week and years dentists had been in practice). Method not described |
| Allocation concealment (selection bias) | Low risk | At hygiene visit all patients completed health survey; those responding 'Yes' to current use of tobacco were enrolled. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was 26% (102/394) in intervention and 26% (62/239) in the control group. |
| Methods | Country: UK Recruitment: occurred in a single hospital‐based periodontal clinic Randomized controlled trial |
|
| Participants | 118 smoking adults attending consultant clinics in an outpatient dental hospital periodontal clinic | |
| Interventions | 1. Intervention group ‐ 5 A's, NRT prn (patches, gum) 2. Usual care ‐ Received information regarding the role of tobacco in periodontal disease, 'very brief' advice to quit smoking |
|
| Outcomes | 3, 6, 12m PP tobacco abstinence. Repeated PP (3, 6, 12m) used in analyses 12m cotinine measured for self reported quitters. |
|
| Notes | CO <8 ppm, COT <20 ng/ml cutoffs for quit status | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization process was set up by the project statistician and was implemented independently from the recruitment process. After recruitment, the patient's name was transcribed into a log book containing sequential patient log numbers and the allocated hygienist. |
| Allocation concealment (selection bias) | Low risk | After allocating the patient to a hygienist, the patient was allocated to either intervention or control group using the minimization method. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Significant loss to follow‐up for both groups; 34/57 for control, 26/59 for intervention. |
| Methods | Country: USA Recruitment: General practice attending smokers Cluster‐randomized controlled trial |
|
| Participants | Adult smokers attending 8 dental practices in south east Minnesota (60 intervention/22 control) | |
| Interventions | 1. Quitline referral ‐ Brief counselling plus quitline referral (Fax‐to‐quit referral form) 2. Usual care ‐ Brief counselling plus patient education brochure |
|
| Outcomes | 7‐day PP at 6m | |
| Notes | Quitline dose‐response trend noted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random table used for random assignment of clinics to intervention or usual care |
| Allocation concealment (selection bias) | Unclear risk | Clinic allocation not concealed at time of participant recruitment, and lower participation rate amongst control clinic patients |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition from 3 to 6m in responders. Only 17 of 60 quitline subjects received any follow‐up counselling and 32 of the 60 in the quitline group received no quitline contact. |
| Methods | Country: USA Recruitment: Contacted athletic trainers at California colleges Cluster‐randomized controlled trial |
|
| Participants | College baseball athletes who use ST | |
| Interventions | Based upon the innovation theory and social learning theory. 1. Intervention consisted of the following components:
2. Nonintervention ‐ (not described) |
|
| Outcomes | 30‐day point‐prevalence ST abstinence at 12m Abstinence verification: None | |
| Notes | Spillover of cessation intervention seen in control groups via ATC activity. ICC: 0.0197. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster randomized: Schools stratified by tertiles of baseline ST use then within strata colleges were randomized to intervention or control group. |
| Allocation concealment (selection bias) | Low risk | Intervention assignment determined by the allocation of the school (cluster) they attended; no differential recruitment suspected. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 76% of ST users, 81% nonusers completed 1 yr follow‐up [non significant after adjustment]; no differential drop‐out seen between groups. |
| Methods | Country: USA Recruitment: Tobacco users in private practice clinics Cluster‐randomized controlled trial of 1 year duration |
|
| Participants | 2160 tobacco users attending 68 dental clinics in Mississippi | |
| Interventions | 1. '3 As' intervention; ask, advise, arrange quitline referral using 'fax‐to‐quit referral form. 2. '5 As' intervention; ask, advise, assess, assist, arrange counselling with Quitline referral as an option at provider's discretion 3. Usual care ‐ Practitioners provided usual tobacco‐use cessation services |
|
| Outcomes | 12m prolonged abstinence (9m without tobacco use with 3m grace period) | |
| Notes | 1 vs. 3 used in meta‐analyses. 5As results were very similar (27/817, 3.3% quit). ICC .012 for 9m prolonged abstinence. Significant impact on study by Hurricane Katrina. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified approximately 17 clinics in each cohort (4) by location and assigned each of them randomly to one of the three study conditions. Probably done through the investigating institution. |
| Allocation concealment (selection bias) | Low risk | Probably done through the investigating institution. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data fairly well balanced (usual care 26.4%, 5 A's 26.4%, 3 A's 31.3%); reasons not likely study related. |
| Methods | Country: USA Recruitment: Smokers attending federally funded public health dental clinics in Mississippi, New York & Oregon Cluster randomized controlled trial |
|
| Participants | 2549 adult smokers attending public dental clinics for non‐emergency visits | |
| Interventions | 1. Intervention ‐ Brief 'tailored' tobacco advice, assistance, & NRT 2. Usual Care ‐ Tobacco cessation methods as standard practice |
|
| Outcomes | Prolonged abstinence at 7.5m | |
| Notes | Did not use small participant group of ST only (2.4%) and ST/smoked tobacco (1%) users in analysis. ICC for prolonged abstinence at 7.5m: 0.009. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clinics were stratified by size and patient ethnicity, and randomized to one of two study conditions. Probably done through investigating institution. |
| Allocation concealment (selection bias) | Low risk | Centrally through Oregon Research Institute by clinic assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Usual care response 73.9%, Intervention 69.3%; relatively equivalent attrition. Women more likely to respond than men; respondents were older, smoked longer, and more educated; impact of responder profile for significant bias likely low. |
| Methods | Country: Japan Recruitment: Adults willing to stop smoking within 1m Randomized controlled multi‐clinic trial |
|
| Participants | Adult smokers attending dental clinics in Japan | |
| Interventions | 1. Intervention ‐ behavioral and pharmacological (nicotine patch and gum) relapse strategies; counselled at initial 2 visits and at 2, 4, 8, and 12w 2. Nonintervention ‐ (not described) |
|
| Outcomes | 3, 6, 12m continuous abstinence (intention‐to‐treat analyses) Validation by saliva cotinine < 20 ng/ml |
|
| Notes | Results for willing to quit cohort. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment cards in envelopes provided a priori to clinics; allocated as subjects agreed to participate/consented |
| Allocation concealment (selection bias) | Low risk | Low risk with stated allocation process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comparable numbers lost to follow‐up |
| Methods | Country: USA Recruitment: Adolescents attending hygiene visits within multi‐clinic managed care organization Randomized controlled trial |
|
| Participants | Adolescents (14 to 17 years old) attending dental offices for hygiene care | |
| Interventions | 1. Intervention ‐ provider advice plus motivational interviewing/follow‐up phone calls 2. Usual care ‐ provider advice |
|
| Outcomes | Smoking abstinence within past 30 days at 1 year | |
| Notes | Significant process errors impacting recruitment and limiting amount of useful study data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A table of random numbers in sequential order was used with odd numbers assigned to intervention and even number assigned to control. |
| Allocation concealment (selection bias) | Unclear risk | A prominent sticker was placed in the charts of subjects in both groups to prompt staff hygienists and dentists to provide tobacco‐related advice. Unclear if this identification could have impacted subsequent intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors report that administrative database problems had significant impact on study data. |
| Methods | Country: Sweden Recruitment: smokers identified via dental and health care personnel screening over 18m Randomized controlled trial |
|
| Participants | 300 smokers in a mixed urban/rural general dental setting; counselling conducted by dental hygienists at local dental clinics | |
| Interventions | 1. High intensity ‐ 8 x 40 minute counselling sessions over 4m; mixed behavioral, coaching, and pharmacological advice 2. Low intensity ‐ 1 x 30 minute counselling session explaining a self‐help program [8 week program] Information on NRT given to both groups but no recommendation about whether to use or not. |
|
| Outcomes | PP & 'Continuous' (previous 6m) smoking abstinence at 1 year; ITT analyses | |
| Notes | Did not exclude ST users from obtaining cessation support; smokers meeting inclusion criteria were randomized. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by an independent person using an envelope technique in blocks of four |
| Allocation concealment (selection bias) | Low risk | Assignment done through central location after consent/baseline questionnaire received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 29% of high intensity treatment; 18% of low intensity treatment |
| Methods | Country: USA Recruitment: Hygiene patients in 75 private practices Cluster randomized clinical trial |
|
| Participants | 4029 cigarette smokers >= 15 years of age | |
| Interventions | 1. Minimal intervention:
2. Extended intervention: as per minimal intervention, plus asked the patient to set a quit date within 2w of visit, gave the patient a motivational video, and called the patient within 2w after the hygiene visit to ask if he/she read the materials, watched the video, and either quit or is now willing to set a quit date. 3. Usual care |
|
| Outcomes | 12m 'sustained' abstinence from ST and all tobacco: subjects must have reported 7‐day PP ST and all tobacco abstinence at both 3m and 12m. Abstinence verification: None | |
| Notes | 2 vs 3 (extended intervention compared to usual care) in analyses. Minimal intervention quit rate similar (34/1305, 2.6%) Data for ST users reported in Andrews 1999. ICC for cigarette smoking was 0.00004. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster randomized: Practices were blocked by average number of hygiene visits per week and number of years dentists had been in practice, then randomized to usual care, minimal intervention, or extended intervention |
| Allocation concealment (selection bias) | Low risk | Patient treatment assignment determined by the allocation of the practice (cluster) they attended; no differential recruitment suspected. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24.3% loss to follow‐up not broken down by study arm or type of tobacco used. Non responders counted as smokers. |
| Methods | Country: USA Recruitment: Active duty military ST users attending annual examination at military dental clinics, asked to participate irrespective of motivation to quit |
|
| Participants | 785 active‐duty military personnel using ST | |
| Interventions | 1. Minimal contact behavioral treatment consisting of ST cessation manual, videotape cessation guide tailored for military personnel, 3 x15 min telephone counselling sessions using motivational interviewing methods 2. Usual care: recommendations to quit using ST and referral to extant local tobacco cessation programs |
|
| Outcomes | PP, repeated PP (3 & 6m, all tobacco), and prolonged abstinence at 3 and 6 mo (ST only). Prolonged ST abstinence at 6m used in analyses. | |
| Notes | Though minimal in face‐to‐face contact, which apparently occurred only at the annual evaluation session and then for recruitment, the intervention was not minimal in time expenditure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Enrollment material mailed to Oregon Research Institute where participants were randomized. |
| Allocation concealment (selection bias) | Low risk | Names/phone numbers of behavioral intervention participants sent to military phone counselling staff. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data not different in terms of condition, race/ethnicity, rank, readiness to quit, age, first tobacco use, or time to 1st chew. Completed 6m assessment ‐ Intervention 69.9% & usual care 75.6% |
| Methods | Country: USA Recruitment: Hygiene patients in HMO dental offices | |
| Participants | 518 male ST users | |
| Interventions | Intervention: soft‐tissue exam, cleaning, patient education, feedback on oral health and advice on self care, report of keratotic lesions asking where tobacco was placed, hygienist‐directed advice to quit, dentists' strong advice to quit, 9 min video, setting a quit date, self‐help booklet, 24‐hour advice phone line, kit providing oral substitutes and tip sheets with advice on how to quit, 1w follow‐up call by hygienist, plus monthly mailing of tip sheets and newsletter Control: usual care | |
| Outcomes | 12m 7‐day PP all tobacco abstinence 12m 7‐day PP ST abstinence 12m all tobacco sustained abstinence: subjects must have reported no tobacco use in the last 7 days at the 3m and 12m assessments (used in analyses) 12m ST tobacco abstinence: subjects must have reported no ST use in the last 7 days at the 3m and 12m assessments Abstinence verification: None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐randomized by clinic identification number |
| Allocation concealment (selection bias) | High risk | Allocation not concealed at time of enrolment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High numbers lost to follow‐up: 51.9% (intervention) and 53.7% (control) |
| Methods | Country: USA Recruitment: Publicly‐supported colleges were contacted for permission to recruit athletes | |
| Participants | ST users among college‐baseball and football athletes | |
| Interventions | Intervention: 3‐5 min dental exam, advice to quit, discussed ST‐related tissue changes, photographs of facial disfigurement due to oral cancer, self‐help guide, offered a 10‐15 minute counselling session by the hygienist which included nicotine gum, review of addiction nature of ST and nicotine withdrawal, setting a quit date, developing a plan to quit, and identifying triggers for tobacco use. Phone calls were conducted by the hygienist on the quit date and 1m later. Control: No intervention. | |
| Outcomes | 30‐day PP ST abstinence at 12m Abstinence verification: None | |
| Notes | ICC: 0.02 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster randomized: Colleges were pair‐matched based on baseline prevalence of ST use and 1 randomized to intervention, the other to control |
| Allocation concealment (selection bias) | Low risk | Patient treatment assignment determined by the allocation of the school (cluster) they attended; no differential recruitment suspected. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low loss to follow‐up: 10% (intervention) and 5% (control) |
| Methods | Country: USA Recruitment: Principals from randomly selected high schools were contacted | |
| Participants | High school baseball team members who use ST | |
| Interventions | Intervention:
Control: Usual care. |
|
| Outcomes | Repeated PP smokeless tobacco abstinence at 1m, 12m & 24m Abstinence verification: at 12m only | |
| Notes | Walsh 2003 is the main trial report but only reported 12m findings. Abstinence at 24m reported in Gansky 2002 is used in the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster randomized: High schools stratified by baseline number and size of teams and baseline prevalence of ST use, then within strata schools were randomized to intervention or control groups. |
| Allocation concealment (selection bias) | Low risk | Patient treatment assignment determined by the allocation of the school (cluster) they attended; no differential recruitment suspected. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported < 10% loss to follow‐up |
ATC: athletic training coach; CO: carbon monoxide; COT: cotinine; HMO: Health Maintenance Organization; ICC: intraclass correlation; m: month(s); PP: point prevalence abstinence; prn: when necessary; ST: smokeless tobacco; w: week(s)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albert 2004 | No tobacco use outcomes reported. Study assessed the effectiveness of academic detailing. |
| Barker 1995 | Not an RCT. School‐wide tobacco cessation effort. |
| Barker 2001 | Not an RCT. Survey of cessation practice behavior of hygienists and dentists. |
| Barnfather 2005 | Short follow‐up (8 weeks). Intervention included exam and counselling for both arms, with point‐of‐care test for salivary nicotine as the exposure variable. |
| Binnie 2003 | 3‐month outcomes only. RCT assessing the effectiveness of smoking cessation counselling and nicotine replacement delivered by dental hygienists. |
| Boundouki 2004 | Not an RCT. Use of a patient‐information leaflet to improve knowledge of mouth cancer. |
| Campbell 1997 | No tobacco use outcomes reported. This report describes the recruitment strategy and response rate for a 3 year RCT to test the effectiveness of a dissemination strategy aimed at improving the tobacco cessation services offered by rural dental practices. |
| Christen 1984 | 15‐week outcomes only. Assessed the efficacy of nicotine gum vs. advice to quit and videotape. |
| Christen 1985 | Not an RCT. Assessed nicotine effects on oral health. |
| Cohen 1987 | No tobacco use outcomes reported. Results of exit survey conducted during a study of the impact of nicotine gum and chart reminders on tobacco cessation. |
| Cohen 1989 | Data reported in composite, without subgroup denominator values. There was no non‐behavioral control group. Unable to contact corresponding author, and co‐authors did not have access to the data. |
| Cooper 1989 | Not an RCT. Hospital‐based smoking cessation program using behavioral modification and pharmacotherapy. |
| Gelskey 2002 | Not an RCT. No tobacco cessation outcomes. Study of tobacco use cessation counselling by oral health professionals. |
| Glasgow 1993 | Methods of individual clinical trials are not included. Description of efforts to biochemically validate self‐reports of smoking cessation from participants in four large‐scale randomized trials. Study of RCT in dental clinics is reviewed elsewhere in this systematic review. See Little 1992 /Stevens 1995). |
| Gordon 2002 | Not an RCT. Assessed the effectiveness of tobacco use counselling through public health dental clinics. |
| Gordon 2005a | Not an RCT. Assessed the effectiveness of a behavioral intervention delivered through public health dental clinics. |
| Gordon 2005b | No tobacco use outcomes. Compared different methods of training on hygienists tobacco use cessation activities. |
| Gorin 2004 | Meta‐analysis included 5 dental intervention studies of 3m duration and Stevens 1995, which is included in the review. |
| Gould 1998 | Not an RCT. Survey of participants in an NCI training program for delivering tobacco use interventions. |
| Greene 1994 | 3‐month outcomes only. Assessed the effectiveness of two interventions for smokeless tobacco cessation. |
| Gritz 1993 | Not in a dental setting. Hospital‐based study assessing the impact of tobacco use counselling on head and neck cancer patients. Only 7/110 health care professionals were dental providers. |
| Hanioka 2007 | The effectiveness of intervention was evaluated with respect to attempts to quit and progression through the stages of behavioral changes involved in quitting using the standardized questionnaire ‐ not abstinence. |
| Houston 2008 | No tobacco use outcomes reported. Assessed an internet‐delivered intervention to increase implementation of brief provider advice. |
| Hovell 1995 | No tobacco use outcomes reported. Assessed the distribution of anti‐tobacco materials in orthodontic offices. |
| Hovell 2001 | Not an RCT. Assessed the effectiveness of a behavioral intervention delivered by orthodontists in preventing pre‐teens from initiating tobacco use. |
| Johnston 1996 | Not an RCT. The questionnaire was being developed as part of a 2‐year RCT of the effect of a multifaceted oral health education program on tobacco use among elementary school children in Ontario, CA. This is a report of pretest evaluation for the questionnaire. |
| Jones 1993 | Not an RCT. Baseline survey of tobacco use cessation activity and attitudes in community practices. |
| Kentala 1999 | Prevention study. Assessed the effectiveness of behavioral counselling on preventing or treating adolescent smoking. |
| Kirkwood 2001 | 4‐week outcomes only. Assessed the efficacy of a smoking deterrent mouthwash. No tobacco use outcomes reported. |
| Kirkwood 2002 | 4‐week outcomes only. Assessed the efficacy of a smoking deterrent breath spray. Outcome is smoking reduction not cessation. |
| Koerber 2003 | No tobacco use outcomes reported. Assessed the effects of teaching dental students brief motivational interviewing. |
| Little 2009 | No tobacco use outcomes reported. Evaluated assisted referral. |
| Maassen 2008 | Not an RCT. Study sought to determine guideline implementation parameters in a trial of 12 dental practices ‐ measured patient receptiveness to cessation advice. |
| Macgregor 1996 | Not an RCT. Evaluated the effectiveness of dental health advice for a reduction in cigarette smoking. |
| Masouredis 1997 | 3‐month outcomes only. Assessed the effectiveness of a smokeless tobacco intervention in colleges. |
| Morgan 2000 | Not an RCT. Recommendations for oral health professionals for addressing patient tobacco use. |
| Nasry 2006 | Not an RCT. Single cohort of smokers in a periodontal clinic provided counselling and pharmacotherapy as needed. |
| NCI 1994 | Collection of monographs addressing smoking cessation in medical and dental environments. Data from primary literature are covered elsewhere in this review. See Cohen 1987, Cohen 1989, Gritz 1993. |
| NCI 1995 | Intervention not confined to the dental setting. Community‐based interventions with communities as the unit of randomization. Tobacco control activities were promoted through medical and dental office settings. |
| O'Keefe 1995 | Not an RCT. Study of dental practitioner compliance with tobacco use intervention training. |
| Olson 1985 | 15‐week outcomes only of salivary parameters before and after among smokers using nicotine‐containing chewing gum. No tobacco cessation outcomes. |
| Secker‐Walker 1988 | Not an RCT. Pilot study of smoking cessation advice among patients in a periodontal practice. |
| Smith 1998 | Not an RCT. Case series of smoking cessation programs conducted in dental practices in the UK. |
| Walsh 2010 | No tobacco use outcomes. |
| Williams 2002 | Abstract unavailable. No additional information supplied by author. |
| Wood 1997 | Not an RCT. 3‐month data only. Office‐based training in tobacco cessation for dentists. |
RCT: randomized controlled trial
Contributions of authors
JOE and ABC both conceived and planned the review, sought trials and extracted data; both were equally involved in writing the review.
Sources of support
Internal sources
No sources of support supplied
External sources
1R21DE016024, National Institute for Dental and Craniofacial Research, USA.
Declarations of interest
None.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Andrews JA, Severson H, Lichtenstein E, Gordon JS, Barckley MF. Evaluation of a dental office tobacco cessation program: effects on smokeless tobacco use. Annals of Behavioral Medicine 1999;21:48‐53. [DOI] [PubMed] [Google Scholar]; Gordon JS, Severson HH. Tobacco cessation through dental office settings. Journal of Dental Education 2001;65(4):354‐63. [PubMed] [Google Scholar]; Severson HH, Andrews JA, Lichtenstein E, Gordon JS, Barckley MF. Using the hygiene visit to deliver a tobacco cessation program: results of a randomized clinical trial. Journal of the American Dental Association 1998;129(7):993‐9. [DOI] [PubMed] [Google Scholar]
- Binnie VI, McHugh S, Jenkins W, Borland W, MacPherson LM. A randomised controlled trial of a smoking cessation intervention delivered by dental hygienists: A feasibility study. BMC Oral Health 2007;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert JO, Carr AB, Patten CA, Morris RA, Schroeder DR. Tobacco use quitline enrollment through dental practices: a pilot study. Journal of the American Dental Association 2007;138(5):595‐601. [DOI] [PubMed] [Google Scholar]
- Ellison J, Gansky SA, Kavanagh C, Rudy D, et al. An Athletic Trainer‐Mediated Spit Tobacco Prevention and Cessation Program (IADR San Diego 2002 abstracts). Journal of Dental Research 2002;81(March, Special Issue A):A‐494 (Abs 4047). [Google Scholar]; Gansky SA, Ellison JA, Rudy D, Bergert N, Letendre MA, Nelson L, et al. Cluster randomized controlled trial of an athletic trainer‐directed spit (smokeless) tobacco intervention for college baseball athletes: results after one year. Journal of Athletic Training 2005;40(2):76‐87. [PMC free article] [PubMed] [Google Scholar]
- Gordon JS, Andrews JA, Crews KM, Payne TJ, Severson HH, Lichtenstein E. Do faxed quitline referrals add value to dental office‐based tobacco‐use cessation interventions?. JADA Aug 2010;141(8):2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JS, Albert DA, Andrews JA, Crews KM, Payne TJ, Severson HH. Tobacco cessation in public dental clinics: short‐term outcomes (PA14‐5). Society for Research on Nicotine and Tobacco 14th Annual Meeting February 26‐March 1, Portland, Oregon. 2008. [Google Scholar]; Gordon JS, Andrews JA, Albert DA, Crews KM, Payne TJ, Severson HH. Tobacco cessation via public dental clinics: results of a randomized trial. American Journal of Public Health 2010;100(7):1307‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanioka T, Ojima M, Tanaka H, Naito M, Hamajima N, Matsuse R. Intensive smoking‐cessation intervention in the dental setting. Journal of Dental Research 2010;89(1):66‐70. [DOI] [PubMed] [Google Scholar]
- Lando HA, Hennrikus D, Boyle R, Lazovich D, Stafne E, Rindal B. Promoting tobacco abstinence among older adolescents in dental clinics. Journal of Smoking Cessation 2007;2(1):23‐30. [Google Scholar]
- Nohlert E, Tegelberg A, Tillgren P, Johansson P, Rosenblad A, Helgason AR. Comparison of a high and a low intensity smoking cessation intervention in a dentistry setting in Sweden: a randomized trial. BMC Public Health 2009;9:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JS, Severson HH. Tobacco cessation through dental office settings. Journal of Dental Education 2001;65(4):354‐63. [PubMed] [Google Scholar]; Severson HH, Andrews JA, Lichtenstein E, Gordon J. A dental office intervention for smokeless tobacco cessation. 10th World Conference on Tobacco or Health 24‐28 August Beijing Abstract book. 1997. [Google Scholar]; Severson HH, Andrews JA, Lichtenstein E, Gordon JS. Smokeless tobacco cessation through dental offices: An intervention that works (AADR Abstract No. 1206). Journal of Dental Research 1988;77(AADR Abstracts):256. [Google Scholar]; Severson HH, Andrews JA, Lichtenstein E, Gordon JS, Barckley MF. Using the hygiene visit to deliver a tobacco cessation program: results of a randomized clinical trial. Journal of the American Dental Association 1998;129(7):993‐9. [DOI] [PubMed] [Google Scholar]
- Severson HH, Gordon J, Andrews J, Peterson AL, Cigrang J. Evaluating motivational interview phone support for smokeless tobacco cessation with military personnel (PA10‐6). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006. [Google Scholar]; Severson HH, Peterson AL, Andrews JA, Gordon JS, Cigrang JA, Danaher BG, et al. Smokeless tobacco cessation in military personnel: a randomized controlled trial. Nicotine & Tobacco Research 2009;11:730‐38. [DOI] [PubMed] [Google Scholar]
- Gordon JS, Severson HH. Tobacco cessation through dental office settings. Journal of Dental Education 2001;65(4):354‐63. [PubMed] [Google Scholar]; Little SJ, Stevens VJ, Severson HH, Lichtenstein E. Effective smokeless tobacco intervention for dental hygiene patients. Journal of Dental Hygiene 1992;66(4):185‐90. [PubMed] [Google Scholar]; Stevens VJ, Severson H, Lichtenstein E, Little SJ, Leben J. Making the most of a teachable moment: a smokeless‐tobacco cessation intervention in the dental office. American Journal of Public Health 1995;85(2):231‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MM, Hilton JF, Masouredis C, Gee L, Chesney MA, Ernster VL. A randomized controlled intervention trial to promote spit tobacco cessation among college athletes. Journal of Dental Research 1995;Special Issue ‐ Abstracts of Papers (73rd General Session and Exhibition of the International Association for Dental Research June 28‐July 1, 1995, Singapore):458 (Abs No 463). [Google Scholar]; Walsh MM, Hilton JF, Masouredis CM, Gee L, Chesney MA, Ernster VL. Smokeless tobacco cessation intervention for college athletes: results after 1 year. American Journal of Public Health 1999;89(2):228‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansky SA, Ellison JA, Kavanagh C, Hilton JF, Walsh MM. Oral screening and brief spit tobacco cessation counseling: a review and findings. Journal of Dental Education 2002;66(9):1088‐98. [PubMed] [Google Scholar]; Walsh MM, Ellison J, Hilton JF, Kavanagh C, Chesney M, Ernster V. Spit tobacco cessation: high school baseball athletes, 2 year findings. Journal of Dental Research 2001;80(January 2001 Special Issue AADR Abstracts):116 (Abstract 645). [Google Scholar]; Walsh MM, Hilton JF, Ellison JA, Gee L, Chesney MA, Tomar SL, et al. Spit (smokeless) tobacco intervention for high school athletes: results after 1 year. Addictive Behaviors 2003;28(6):1095‐113. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Albert DA, Anluwalia KP, Ward A, Sadowsky D. The use of 'academic detailing' to promote tobacco‐use cessation counseling in dental offices. Journal of the American Dental Association 2004;135(12):1700‐6. [DOI] [PubMed] [Google Scholar]
- Barker GJ, Taylor TS, Barker BF. Implementation of a tobacco cessation program in the student clinics. Journal of Dental Education 1995;59(8):850‐5. [PubMed] [Google Scholar]
- Barker GJ, Williams KB, Taylor TS, Barker BF. Practice behaviors of alumni trained as students in tobacco use cessation interventions. Journal of Dental Hygiene 2001;75(2):165‐9. [PubMed] [Google Scholar]
- Barnfather KD, Cope GF, Chapple IL. Effect of incorporating a 10 minute point of care test for salivary nicotine metabolites into a general practice based smoking cessation programme: randomised controlled trial. BMJ 2005;331:979‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnie V, McHugh S, MacPherson LM, Jenkins W, et al. Dental hygienists' delivery of smoking cessation: 3‐month outcomes (JDR Abstract). Journal of Dental Research 2003;82(Special Issue B, June):B‐373. [Google Scholar]
- Boundouki G, Humphris G, Field A. Knowledge of oral cancer, distress and screening intentions: longer term effects of a patient information leaflet. Patient Education & Counseling 2004;53(1):71‐7. [DOI] [PubMed] [Google Scholar]
- Campbell HS, Petty TL, Meadows LM, Simpson L, Jennett PA. Improving tobacco cessation services in rural dental offices ‐ recruitment to a randomized controlled trial. Canadian Journal of Community Dentistry 1997;12(2):15‐22. [Google Scholar]
- Christen AG, McDonald JL Jr, Olson BL, Drook CA, Stookey GK. Efficacy of nicotine chewing gum in facilitating smoking cessation. Journal of the American Dental Association 1984;108(4):594‐7. [DOI] [PubMed] [Google Scholar]
- Christen AG, Beiswanger BB, Mallatt ME, Tomich CE, Drook CA, McDonald JL Jr, et al. Effects of nicotine‐containing chewing gum on oral soft and hard tissues: A clinical study. Oral Surgery, Oral Medicine and Oral Pathology 1985;59(1):37‐42. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Christen AG, Katz BP, Drook CA, Davis BJ, Smith DM, et al. Counseling medical and dental patients about cigarette smoking: the impact of nicotine gum and chart reminders. American Journal of Public Health 1987;77(3):313‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Stookey GK, Katz BP, Drook CA, Christen AG. Helping smokers quit: a randomized controlled trial with private practice dentists. Journal of the American Dental Association 1989;118(1):41‐5. [DOI] [PubMed] [Google Scholar]
- Cooper TM, Clayton RR. Stop‐smoking program using nicotine reduction therapy and behavior modification for heavy smokers. Journal of the American Dental Association 1989;118(1):47‐51. [DOI] [PubMed] [Google Scholar]
- Gelskey SC. Impact of a dental/dental hygiene tobacco‐use cessation curriculum on practice. Journal of Dental Education 2002;66(9):1074‐8. [PubMed] [Google Scholar]
- Glasgow RE, Mullooly JP, Vogt TM, Stevens VJ, Lichtenstein E, Hollis JF, et al. Biochemical validation of smoking status: Pros, cons, and data from four low‐intensity intervention trials. Addictive Behaviors 1993;18(5):511‐27. [DOI] [PubMed] [Google Scholar]
- Gordon JS, Andrews JA, Severson HH, Stewart DCL. Tobacco cessation via public health dental clinics: preliminary results. Society for Research on Nicotine and Tobacco 8th Annual Meeting Rapid Communications Posters, Savannah, Georgia. 2002:RP‐64. [Google Scholar]
- Gordon JS, Andrews JA, Lichtenstein E, Severson HH. The impact of a brief tobacco cessation intervention in public health dental clinics. Journal of the American Dental Association 2005;136(2):179‐86. [DOI] [PubMed] [Google Scholar]
- Akers L, Gordon JS, Andrews JA, Barckley M, Lichtenstein E, Severson HH. Cost effectiveness of changing health professionals' behavior: Training dental hygienists in brief interventions for smokeless tobacco cessation. Preventive Medicine 2006;43(6):482‐7. [DOI] [PubMed] [Google Scholar]; Gordon JS, Andrews JA, Lichtenstein E, Severson HH, Akers L. Disseminating a smokeless tobacco cessation intervention model to dental hygienists: A randomized comparison of personalized instruction and self‐study methods. Health Psychology 2005;24(5):447‐55. [DOI] [PubMed] [Google Scholar]
- Gorin SS, Heck JE. Meta‐analysis of the efficacy of tobacco counseling by health care providers. Cancer Epidemiology, Biomarkers & Prevention 2004;13:2012‐22. [PubMed] [Google Scholar]
- Gould KA, Eickhoff‐Shemek JM, Stacy RD, Mecklenburg RE. The impact of National Cancer Institute training on clinical tobacco use cessation: services by oral health teams. Journal of the American Dental Association 1998;129(10):1442‐9. [DOI] [PubMed] [Google Scholar]
- Greene JC, Walsh MM, Masouredis C. A program to help major league baseball players quit using spit tobacco. Journal of the American Dental Association 1994;125(5):559‐68. [DOI] [PubMed] [Google Scholar]
- Gritz ER, Carr CR, Rapkin D, Abemayor E, Chang LJ, Wong WK, et al. Predictors of long‐term smoking cessation in head and neck cancer patients. Cancer Epidemiology, Biomarkers & Prevention 1993;2(3):261‐70. [PubMed] [Google Scholar]; Gritz ER, Carr CR, Rapkin DA, Chang C, Beumer J, Ward PH. A smoking cessation intervention for head and neck cancer patients: trial design, patient accrual, and characteristics. Cancer Epidemiology, Biomarkers & Prevention 1991;1(1):67‐73. [PubMed] [Google Scholar]
- Hanioka T, Ojima M, Hamajima N, Naito M. Patient feedback as a motivating force to quit smoking. Community Dentistry & Oral Epidemiology 2007;35(4):310‐7. [DOI] [PubMed] [Google Scholar]
- Houston TK, Richman JS, Coley HL, Ray MN, Allison JJ, Gilbert GH, et al. Does delayed measurement affect patient reports of provider performance? Implications for performance measurement of medical assistance with tobacco cessation: a Dental PBRN study. BMC Health Services Research 2008;8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]; Houston TK, Richman JS, Ray MN, Allison JJ, Gilbert GH, Shewchuk RM, et al. Internet delivered support for tobacco control in dental practice: randomized controlled trial. Journal of Medical Internet Research 2008;10(5):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovell MF, Russos S, Beckhelm MK, Jones JA, Burkham‐Kreitner SM, Slymen DJ, et al. Compliance with primary prevention in private practice: creating a tobacco‐free environment. American Journal of Preventive Medicine 1995;11(5):288‐93. [PubMed] [Google Scholar]
- Hovell MF, Jones JA, Adams MA. The feasibility and efficacy of tobacco use prevention in orthodontics. Journal of Dental Education 2001;65(4):348‐53. [PubMed] [Google Scholar]
- Johnston DW, O'Shea RM. Testing an evaluation instrument in a tobacco use intervention study. Journal of Dental Research 1996;75(5 (Divisional Abstracts)):1241 (Abstract 16). [Google Scholar]
- Jones RB, Pomrehn PR, Mecklenburg RE, Lindsay EA, Manley M, Ockene JK. The COMMIT dental model: tobacco control practices and attitudes. Journal of the American Dental Association 1993;124(9):92‐104; discussion 106‐8. [DOI] [PubMed] [Google Scholar]
- Kentala J, Utriainen P, Pahkala K, Mattila K. Can brief intervention through community dental care have an effect on adolescent smoking?. Preventive Medicine 1999;29(2):107‐11. [DOI] [PubMed] [Google Scholar]
- Kirkwood KL, Ciancio SG, Mather ML. Clinical evaluation of a smoking deterrent mouthwash. Journal of Dental Research 2001;80(January 2001 Special Issue AADR Abstracts):223 (Abstract 1504). [Google Scholar]
- Kirkwood KL, Ciancio SG, Mather ML. Clinical evaluation of a smoking deterrent breathspray compared to a placebo spray. Journal of Dental Research 2002;81(March 2002 Special Issue A):A‐295 (Abstract 2328). [Google Scholar]
- Koerber A, Crawford J, O'Connell K. The effects of teaching dental students brief motivational interviewing for smoking‐cessation counseling: a pilot study. Journal of Dental Education 2003;67(4):439‐47. [PubMed] [Google Scholar]
- Fellows JL, Hollis JF, Fleming C, Little SJ, Snyder J. Randomized trial of a tobacco "Assisted Referral" programme in a large dental office (RPOS 3‐69). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006. [Google Scholar]; Little SJ, Hollis JF, Fellows JL, Snyder JJ, Dickerson JF. Implementing a Tobacco Assisted Referral Program in Dental Practices. Journal of Public Health Dentistry 2009. [DOI] [PubMed] [Google Scholar]
- Maassen IT, Jacobs JE, Plasschaert AJ, Allard RH, Schattenberg G, Hilberink SR. [Stop smoking advice for patients who smoke: feasible in the dental practice?]. [Dutch]. Nederlands Tijdschrift Voor Tandheelkunde 2008;115(9):460‐5. [PubMed] [Google Scholar]
- Macgregor ID. Efficacy of dental health advice as an aid to reducing cigarette smoking. British Dental Journal 1996;180(8):292‐6. [DOI] [PubMed] [Google Scholar]
- Masouredis CM, Hilton JF, Grady D, Gee L, Chesney M, Hengl L, et al. A spit tobacco cessation intervention for college athletes: three‐month results. Advances in Dental Research 1997;11(3):354‐9. [DOI] [PubMed] [Google Scholar]
- Morgan ML. Are you doing your part to help stamp out tobacco use?. Journal of the Oklahoma Dental Association 2000;90(4):30‐3. [PubMed] [Google Scholar]
- Nasry HA, Preshaw PM, Stacey F, Heasman L, Swan M, Heasman PA. Smoking cessation advice for patients with chronic periodontitis. British Dental Journal 2006;200(5):272‐5. [DOI] [PubMed] [Google Scholar]
- NCI (National Cancer Institute). Tobacco and the Clinician: Interventions for Medical and Dental Practice. Smoking and Tobacco Control Monograph 5. Washington DC: NTIS, 1994.
- NCI (National Cancer Institute). Community‐Based Interventions for Smokers: The COMMIT Field Experience.. Smoking and Tobacco Control Monograph 6. Bethesda MD: National Cancer Institute, 1995:280 p.. [Google Scholar]
- O'Keefe J, Lessio A, Kassirer B. A pilot smoking cessation program involving dental offices in the borough of East York, Ontario: an initial evaluation. Journal of the Canadian Dental Association 1995;61(1):65‐7. [PubMed] [Google Scholar]
- Olson BL, McDonald JL Jr, Gleason MJ, Stookey GK, Schemehorn BR, Drook CA, et al. Comparisons of various salivary parameters in smokers before and after the use of a nicotine‐containing chewing gum. Journal of Dental Research 1985;64(5):826‐30. [DOI] [PubMed] [Google Scholar]
- Secker‐Walker RH, Solomon LJ, Haugh LD, Welsh D, Tatro M, Witham L, et al. Smoking cessation advice delivered by the dental hygienist. A pilot study. Dental Hygiene (Chicago) 1988;62(4):186‐92. [PubMed] [Google Scholar]
- Smith SE, Warnakulasuriya KAAS, Johnson NW, Cooper DJ, Feyerabend C, Belcher M. A smoking cessation programme conducted through dental practices in the UK. British Dental Journal 1998;185(6):299‐303. [DOI] [PubMed] [Google Scholar]
- Walsh MM, Belek MG, Prakash P, Grimes B, Heckman B, Kaufman N, et al. Changing dentists' tobacco control attitudes and behaviors. (PA9‐2). Society for Research on Nicotine and Tobacco 16th Annual Meeting February 24‐27, Baltimore, MD. 2010. [Google Scholar]
- Williams NJ. Comparison of effectiveness of a dental school based nicotine dependence program to dental hygiene student lead tobacco intervention counseling. Society for Research on Nicotine and Tobacco 8th Annual Meeting, Savannah, Georgia. 2002:Sym 6a. [Google Scholar]
- Wood GJ, Cecchini JJ, Nathason N, Hiroshige K. Office‐based training in tobacco cessation for dental professionals. Journal of the American Dental Association 1997;128(2):216‐24. [DOI] [PubMed] [Google Scholar]
Additional references
- Bandura A. Social foundations of thought and action: a social cognitive theory. New Jersey: Prentice‐Hall, Inc., 1986. [Google Scholar]
- Block DE, Block LE, Hutton SJ, Johnson KM. Tobacco counseling practices of dentists compared to other health care providers in a midwestern region. Journal of Dental Education 1999;63(11):821‐7. [PubMed] [Google Scholar]
- Bowles WH, Wilkinson MR, Wagner MJ, Woody RD. Abrasive particles in tobacco products: a possible factor in dental attrition. Journal of the American Dental Association 1995;126(3):327‐31. [DOI] [PubMed] [Google Scholar]
- Campbell HS, Macdonald JM. Tobacco counselling among Alberta dentists. Journal of the Canadian Dental Association1994; Vol. 60, issue 3:218‐20, 223‐6. [PubMed]
- Campbell HS, Sletten M, Petty T. Patient perceptions of tobacco cessation services in dental offices. Journal of the American Dental Association 1999;130(2):219‐26. [DOI] [PubMed] [Google Scholar]
- Christen AG, Klein JA, Christen JA, McDonald JL Jr, Guba CJ. How‐to‐do‐it quit‐smoking strategies for the dental office team: an eight‐step program. Journal of the American Dental Association 1990;Suppl:20S‐27S. [DOI] [PubMed] [Google Scholar]
- Critchley JA, Unal B. Health effects associated with smokeless tobacco: a systematic review. Thorax 2003;58(5):435‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan TA, McGorray SP, Grinstead‐Skigen CL, Mecklenburg R. Tobacco control activities in U.S. dental practices. Journal of the American Dental Association 1997;128(12):1669‐79. [DOI] [PubMed] [Google Scholar]
- Epstein JB, Silverman S Jr. Head and neck malignancies associated with HIV infection. Oral Surgery, Oral Medicine and Oral Pathology 1992;73(2):193‐200. [DOI] [PubMed] [Google Scholar]
- Ernster VL, Grady DG, Greene JC, Walsh M, Robertson P, Daniels TE, et al. Smokeless tobacco use and health effects among baseball players. JAMA 1990;264(2):218‐24. [PubMed] [Google Scholar]
- EU Working Group on Tobacco and Oral Health. EU Working Group on Tobacco and Oral Health Consensus Meeting. Abstracts. Oral Disease 1998;4(1):48‐67. [PubMed] [Google Scholar]
- Fried JL, Cohen LA. Maryland dentists' attitudes regarding tobacco issues. Clinical Preventive Dentistry 1992;14(2):10‐16. [PubMed] [Google Scholar]
- Gansky SA, Ellison JA, Kavanagh C, Hilton JF, Walsh MM. Oral screening and brief spit tobacco cessation counseling: a review and findings. Journal of Dental Education 2002;66(9):1088‐98. [PubMed] [Google Scholar]
- Gelskey SC. Cigarette smoking and periodontitis: methodology to assess the strength of evidence in support of a causal association. Community Dentistry and Oral Epidemiology 1999;27(1):16‐24. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
- Hirsch JM, Heyden G, Thilander H. A clinical, histomorphological and histochemical study on snuff‐induced lesions of varying severity. Journal of Oral Pathology 1982;11(5):387‐98. [DOI] [PubMed] [Google Scholar]
- Holleb AI. Comments from the former Chief Medical Officer of the American Cancer Society (1968‐1988). Cancer 1996;78(12):2590‐1. [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip‐Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine and Tobacco Research 2003;5(1):13‐25. [PubMed] [Google Scholar]
- Kerdvongbundit V, Wikesjo UM. Prevalence and severity of periodontal disease at mandibular molar teeth in smokers with regular oral hygiene habits. Journal of Periodontology 2002;73(7):735‐40. [DOI] [PubMed] [Google Scholar]
- Stead L, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 42. [DOI: 10.1002/14651858.CD000165.pub3] [DOI] [PubMed] [Google Scholar]
- Little SJ, Stevens VJ, LaChance PA, Severson HH, Bartley MH, Lichtenstein E, et al. Smokeless tobacco habits and oral mucosal lesions in dental patients. Journal of Public Health Dentistry 1992;52(5):269‐76. [DOI] [PubMed] [Google Scholar]
- Mecklenburg RE. Tobacco: addiction, oral health, and cessation. Quintessence International 1998;29(4):250‐2. [PubMed] [Google Scholar]
- Milosevic A, Lo MS. Tooth wear in three ethnic groups in Sabah (northern Borneo). International Dental Journal 1996;46(6):572‐8. [PubMed] [Google Scholar]
- Mirbod SM, Ahing SI. Tobacco‐associated lesions of the oral cavity: Part II. Malignant lesions. Journal of the Canadian Dental Association 2000;66(6):308‐11. [PubMed] [Google Scholar]
- Needleman IG, Binnie VI, Ainamo A, Carr AB, Fundak A, Koerber A, et al. Improving the effectiveness of tobacco use cessation (TUC). International Dental Journal 2010;60(1):50‐9. [PubMed] [Google Scholar]
- Offenbacher S, Weathers DR. Effects of smokeless tobacco on the periodontal, mucosal and caries status of adolescent males. Journal of Oral Pathology 1985;14(2):169‐81. [DOI] [PubMed] [Google Scholar]
- Piyathilake CJ, Macaluso M, Hine RJ, Vinter DW, Richards EW, Krumdieck CL. Cigarette smoking, intracellular vitamin deficiency, and occurrence of micronuclei in epithelial cells of the buccal mucosa. Cancer Epidemiology, Biomarkers and Prevention 1995;4(7):751‐8. [PubMed] [Google Scholar]
- Ramseier CA, Warnakulasuriya S, Needleman IG, Gallagher JE, Lahtinen A, Ainamo A, et al. Consensus Report: 2nd European Workshop on Tobacco Use Prevention and Cessation for Oral Health Professionals. International Dental Journal 2010;60(1):3‐6. [PubMed] [Google Scholar]
- Rogers E. Diffusion of Innovations. New York: Free Press, 1983. [Google Scholar]
- Salvi GE, Lawrence HP, Offenbacher S, Beck JD. Influence of risk factors on the pathogenesis of periodontitis. Periodontology 2000;14:173‐201. [DOI] [PubMed] [Google Scholar]
- Silverman S, Bhargava K, Smith LW, Malaowalla AM. Malignant transformation and natural history of oral leukoplakia in 57,518 industrial workers of Gujarat, India. Cancer 1976;38(4):1790‐5. [DOI] [PubMed] [Google Scholar]
- Silverman S Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow‐up study of 257 patients. Cancer 1984;53(3):563‐8. [DOI] [PubMed] [Google Scholar]
- Stockwell HG, Lyman GH. Impact of smoking and smokeless tobacco on the risk of cancer of the head and neck. Head & Neck Surgery 1986;9(2):104‐10. [DOI] [PubMed] [Google Scholar]
- Tomar SL, Winn DM. Chewing tobacco use and dental caries among U.S. men. Journal of the American Dental Association 1999;130(11):1601‐10. [DOI] [PubMed] [Google Scholar]
- Tomar SL. Dentistry's role in tobacco control. Journal of the American Dental Association 2001;132(Suppl):30S‐35S. [DOI] [PubMed] [Google Scholar]
- Walsh PM, Epstein JB. The oral effects of smokeless tobacco. Journal of the Canadian Dental Association 2000;66(1):22‐5. [PubMed] [Google Scholar]
- Warnakulasuriya S. Effectiveness of tobacco counseling in the dental office. Journal of Dental Education 2002;66(9):1079‐87. [PubMed] [Google Scholar]
- Warnakulasuriya S, Dietrich T, Bornstein MM, Peidro EC, Preshaw PM, Walter C, et al. Oral health risks of tobacco use and effects of cessation. International Dental Journal 2010;60(1):7‐30. [PubMed] [Google Scholar]
- Williams RR, Horm JW. Association of cancer sites with tobacco and alcohol consumption and socioeconomic status of patients: interview study from the Third National Cancer Survey. Journal of the National Cancer Institute 1977;58(3):525‐47. [DOI] [PubMed] [Google Scholar]
- Winn DM, Blot WJ, Shy CM, Pickle LW, Toledo A, Fraumeni JF Jr. Snuff dipping and oral cancer among women in the southern United States. New England Journal of Medicine 1981;304(13):745‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Carr A, Ebbert J. Interventions for tobacco cessation in the dental setting. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005084.pub2] [DOI] [PubMed] [Google Scholar]


