Summary
A replication study of a previous genome-wide association study (GWAS) suggested that a single nucleotide polymorphism (SNP) linked to the POLB gene is associated with systemic lupus erythematosus (SLE). This SNP is correlated with decreased POLB expression (Pol β). To determine if decreased Pol β activity results in SLE, we constructed a mouse model of POLB that encodes an enzyme with slow DNA polymerase activity. Pol β is a key enzyme in the base excision repair (BER) pathway.. We show that mice expressing this hypomorphic POLB allele develop autoimmune pathology strongly resembling SLE. Of note, the immunoglobulin heavy chain junctions from the POL BY265C/C mice have shorter lengths, and somatic hypermutation is dramatically increased. These results demonstrate that decreased Pol β activity during the generation of immune diversity leads to lupus-like disease in mice and suggest that decreased expression of Pol β in humans is an underlying cause of SLE.
INTRODUCTION
Base excision repair (BER) functions during class switch recombination (CSR) and somatic hypermutation (SHM) (for a review see (Alt et al., 2013)). Although BER is mainly known for its function in the repair of at least 20,000 endogenous base lesions per human cell per day (Barnes and Lindahl, 2004) it appears to have been co-opted from this role to act in the generation of antibody diversity (for a review see (Di Noia and Neuberger, 2007)). DNA polymerase beta (Pol β) is a key protein in the BER pathway where it repairs single-strand breaks. Deletion of the POLB gene from mice results in embryonic lethality (Gu et al., 1994).
In a large-scale replication study based upon a previous GWAS of SLE in the Han Chinese population association evidence for rs12676482 with SLE was replicated independently in two large cohorts (Sheng et al.). The significance of this lies in the fact that rs12676842 is a SNP in the noncoding region adjacent to the POLB gene on 8p11.21. Of note, the lupus-associated SNP, rs12676482, is in perfect linkage disequilibrium with rs2272733, which is highly correlated with decreased POLB expression (Zeller et al., 2010). This suggests that low Pol β activity is an underlying cause of SLE. We reasoned that mice expressing a slow Pol β mutant polymerase, such as the Y265C hypermorphic allele, would be an excellent model to test the hypothesis that limiting levels of active Pol β leads to SLE. The Y265C mutant of POL B encodes a protein that synthesizes DNA significantly more slowly than WT Pol β (Washington et al., 1997). Therefore, we constructed the POL BY265C/C mouse model using targeted gene disruption (Senejani et al., 2012). We demonstrate that these mice exhibit several pathologies associated with SLE. In addition, our strategy allowed us to define the contributions of Pol β during V(D)J recombination and SHM. Importantly, our studies suggest that an imbalance of error-prone and error-free break repair during V(D)J recombination and SHM results in autoimmune disease.
RESULTS
The POL BY265C/C mice have pathologies resembling SLE
We previously constructed the POL BY265C/C mouse model using targeted gene disruption (Senejani et al., 2012). Observation of the mice as they aged revealed an intriguing set pathologies resembling SLE. The POL BY265C/C and POL BY265C/+ mice exhibited an increased prevalence of dermatitis (Figure 1A and Figure S1). Dermatitis is a major manifestation of SLE in humans (Norris and Lee, 1985) and is also observed in the SLE-prone MRL/lpr lupus-like mouse model (Furukawa et al., 1984). The POL BY265C/+ and POL BY265C/C mice exhibited significantly increased levels of antinuclear antibodies (ANA) in their blood sera compared to that of WT mice, and the ANA levels continued to rise over the life of the mice (Figure 1B).
Figure 1. The POL B Y265c/c and Y265c/+ mice exhibit multi-organ symptoms of SLE.
A. The levels of lupus-like dermatitis are increased significantly in the POL B Y265c/c and Y265c/+ mice compare to WT mice as the mice age. Shown are curves for each genotype (POL B+/+, c/+, and c/c) representing the percentage of mice with lupus-like dermatitis. B. The POLBY265c/c and POLBY265c/+ mice have higher levels of ANA than WT mice. Shown above the graph is an example of immunofluorescence intensity in 12M old mutant mice compared to the WT littermates and the MRL/lpr mice. Note that the fluorescence pattern observed in the MRL/lpr mice differs from that of the POLBY265c/c and POLBY265c/+ mice. In the graph, levels of ANA were quantified from several mice (WT, blue squares; c/+, red circles; c/c, green triangles) at various ages. Briefly, ANA was tested by immunofluorescence using human epithelial (HEp-2) cells in 12-well slides (Diasorin Inc). For each run 1:50 diluted sera were used at a screening investigation. Samples from mice with high and low ANA scores were used on the same slide to confirm the sensitivity and specificity of the test during each scoring. C. The POLBY265c/c and POLBY265c/+ mice exhibit increased severity of glomerular nephritis compared to WT mice. The severity of kidney lesions was scored from 0 to 3 for normal, mild, moderate, or severe. For each mouse, more than 10 glomerular, tubular, or interstitial areas were evaluated and scored for glomerular cellularity, infiltrating leukocytes, severity of tubular lesions, mesangial matrix expansion, crescent formation, interstitial mononuclear cell infiltrates in the medulla and cortex. Lesion scores for each mouse were calculated as the mean of the summed individual scores from each evaluated factors. D. Examples of renal disease in the POLBY265c/c mice compared to WT. The black arrow points to mesangial hypercellularity. The white arrow denotes basement membrane thickening and white arrowheads are glomerular crescents. The gray arrow denotes deposits of antibodies and shrunken glomeruli in the outer cortex denoted by the black arrow. E. The kidneys of the POLBY265c/c exhibit staining of IgG that is of significantly higher intensity than what is observed in WT mice. F. The POLBY265c/c exhibit significantly increased levels of cervical lymphadenopathy compared to WT mice. G. Examples of enlarged cervical lymph nodes in the POLBY265c/c and c/+ mice compared to the normal nodes shown in the WT mouse. Arrows point to the cervical lymph nodes.
Besides ANA, another hallmark feature of SLE is glomerular nephritis (Radic et al., 2011), which results from the formation of immune complexes on the kidneys. The POL BY265C/+ and POL BY265C/C mice develop significantly increased levels of glomerular nephritis compared to WT mice, (Figure 1C). By 12 months of age we observe increased levels of IgG localized to the glomeruli of the POL BY265C/C mice versus WT controls (Figure 1E). Approximately 70% of the POL BY265C/+ and POL BY265c/c mice exhibit cervical lymphadenopathy (Figure 1F–G) with significant infiltration of T and B lymphocytes (Figure S2), which are recognized symptoms of SLE (Jonsson et al., 1987; Lavoie et al., 2011). In contrast, few of their WT siblings had enlarged cervical lymph nodes. Several of the mutant mice also exhibited enlarged salivary glands that had infiltrating lymphocytes, which were predominantly T and B cells (Fig. S3). In combination, our results are consistent with the interpretation that expression of a low activity Pol β variant leads to lupus-like disease in mice.
The CDR3 Junctions of the Heavy Chains are Short in the POL BY265c/c Mice
To determine if the process of V(D)J recombination was altered in our mice, we sequenced the V-D and D–J junctions of T and B-cell receptors from the WT and POL BY265C/C mice and found that the B-cells derived from mutant mice had shorter CDR3 junctions in the immunoglobulin heavy chain compared to WT (Figures 2A, 2B and Figure S4). The majority of CDR3 junctions in the POL BY265C/C mice were between 31–35 base pairs in length whereas they were 41–45 base pairs in WT mice (Figure 2A), with many more unidentifiable D regions in the mutant versus WT mice (Figure 2C) (Bertocci et al., 2006). The lengths of N/P additions between the rearranged V and D segments were significantly shorter in the POL BY265C/C mice versus WT controls (p<0.05) (Figure 2D). No significant differences in length of the immunoglobulin light chain junctions or the T cell receptors were observed (data not shown). Thus, the slow polymerase activity of the Y265C variant leads to fewer N/P additions between the rearranged V and D segments of the Ig heavy chain.
Figure 2. VDJ recombination is aberrant in the POLBY265c/c mice.
A. Polymerase chain reaction (PCR)-amplified products from B220+ IgM− bone marrow and splenic cell genomic DNA of 3–4 three week-old POLB+/+ and C/C mice were cloned and sequenced. The lengths of the CDR3 sequences as a function of the percent of sequences with particular lengths are plotted in the graph. Note that the majority of CDR3 sequences from the POLBY265c/c mice have a length between 31–35 bases whereas the CDR3 region of WT mice is 41–45 bases in length. At least three mice of each genotype were characterized. B. The table shows the ranges of lengths of the CDR3 regions from spleen and bone marrow found in WT and POLBY265c/c mice. C. The percentage of D regions in the POLBC/C mice that were unidentifiable were significantly greater than WT controls. We identified D-regions on the basis of six contiguous nucleotides. D. The average length of N/P-nucleotide addition was reduced in the POLBC/C mice. The allocation of N/P nucleotides was based on the known sequences of the germline elements. The average length of the N/P region between the rearranged V and D junction (N1) was significantly shorter in POLBC/C mice compared to WT controls. The N/P region between the rearranged D and J junction (N2) was not significantly different between the POLB+/+ and C/C mice.
Class Switch Recombination is Similar in the WT and POL BY265C/C Mice
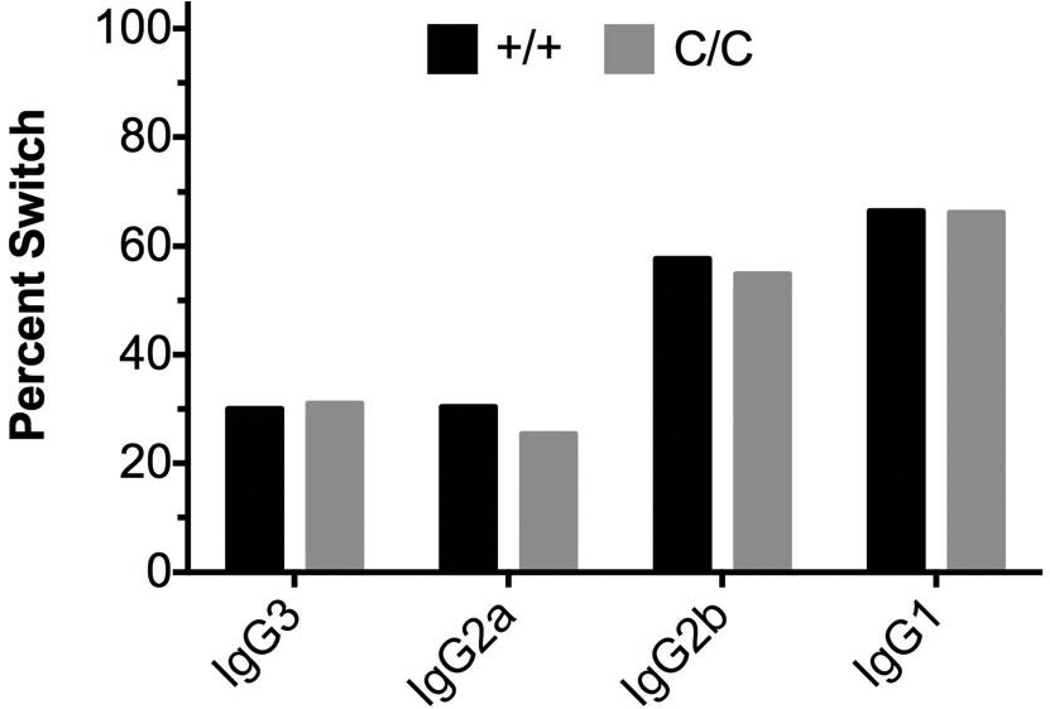
Previous work has suggested that Pol β functions in CSR. In these studies, fetal liver cells isolated from the WT or POL BΔ/Δ mice were transplanted into irradiated hosts and in vitro CSR assays showed slight increases in switching to IgG2a (Wu and Stavenezer, 2007). As shown in Figure 3, we observed no differences in the levels of IgG1, IgG2a, IgG2b and IgG3 in the POL BY265C/C versus WT mice, suggesting that CSR is not altered in the Pol β mutant mice.
Figure 3. CSR is normal in the POLBY265c/c mice.
Naïve splenic B cells were isolated from spleens of 3–5 POLBY265C/C and WT control mice and induced in vitro with either LPS to induce switching to IgG1, LPS with IL-4 to induce switching to IgG3, LPS with INFγ to induce switching to IgG2a, or LPS with TGFβ to induce switching to IgG2b. No significant change in CSR in the B cells was observed between the POL BY265C/C and WT mice.
The Rate of SHM is Significantly Higher in the POL BY265C/C Mice
SHM is a coopted form of BER and occurs in later stages of B cell development within the germinal center (Di Noia and Neuberger, 2007; Maul and Gearhart, 2010; Victora and Nussenzweig, 2012). SHM occurs primarily in the variable region of the immunoglobulin heavy chain, which results in the production of high-affinity antibodies (Di Noia and Neuberger, 2007; Liu and Schatz, 2009; Rajewsky et al., 1987; Weigert et al., 1970). Because Y265C Pol β is a very slow BER polymerase, gaps in DNA that are generated during SHM are unlikely to be filled efficiently during SHM in the POL BY265C/C mice. To determine if this was the case, we characterized SHM in the JH4 intron downstream of VH81xDJH4, using PCR followed by DNA sequencing. Our analysis revealed that the POL BY265C/C mice exhibit a significantly increased frequency of somatic hypermutation compared to WT (Figure 4A and B). The frequencies of transversions at GC base pairs are most significantly increased (Figure 4A and C) although increases in mutations at A:T base pairs are also elevated. The POL BY265C/C mice also display increased levels of mutation in the hotspot motifs that are targeted by AID (Figure 4D).
Figure 4. The rate of SHM is increased in the POLBY265c/c mice.
A. Products were amplified using PCR and genomic DNA isolated from B220+ PNAhigh Peyer’s patches of 3–5 month-old POLB+/+ and C/C mice. These products were cloned and sequenced. Somatic hypermutation results showing that the overall frequencies are increased in the POLBY265c/c mice compared to WT. B. For SHM the 344 nucleotides downstream of the JH4 gene region of rearranged VDJ segments on the heavy chain locus of the VHJ558 gene was PCR-amplified and sequenced. The numbers of mutations versus the total length of DNA sequences and the mutation frequency were analyzed in three mutant and three age-matched WT animals, using SHMTool, which is a webserver for comparative analysis of somatic hypermutation datasets. At the center of the pie chart is shown the numbers of clones analyzed for each genotype. Segments show the percentage of each clone that contained a defined number of mutations and these are indicated with different colors. C. All types of mutations are increased (p=3.7 × 10−18) in the JH4 intron from the POLBY265c/c, but the types of mutations that are most significantly increased are transversions at GC base pairs (p= 2.8 × 10−8), followed by transitions at GC (p = 7.0 × 10−7), transversions at AT (p= 3 × 10−5), and transitions at AT base pairs (p = 0.0029). At least three mice of each genotype were used for these experiments. D. Amino acid motifs where many of the SHM mutations occur in the POLBY265c/c mice compared to WT. Mutations arise predominantly in the GYW (p = 7.4 × 10−5), RGYW (p=0.0003), and DGYW (p = 7.4 × 10−5), which are the motifs in which deamination catalyzed by activation-induced cytidine deaminase (AID) occur.
Increased numbers of Germinal Centers in the POL BY265C/C Mice
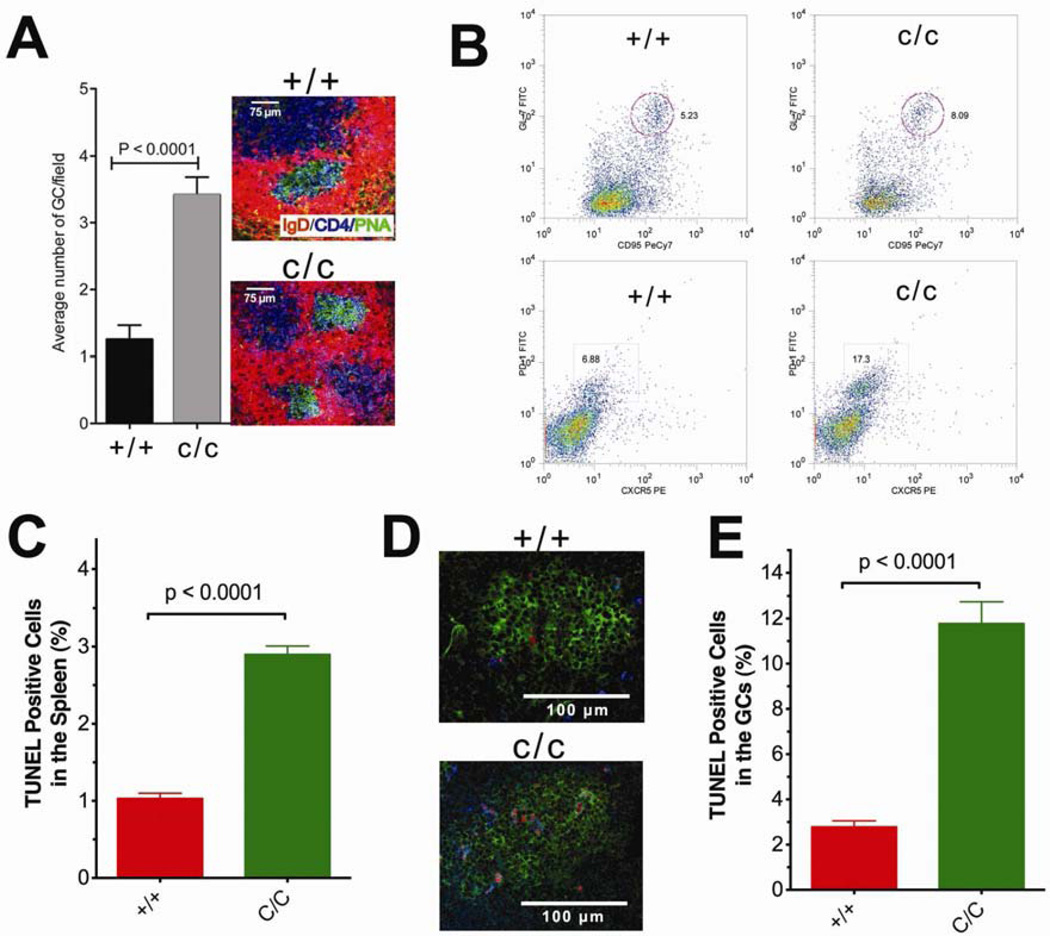
The increased frequencies of SHM in POL BY265C/C mice prompted us to determine if these mice possess increased numbers of germinal centers (GCs), as is often found in lupus-prone mice. The POL BY265C/C mice exhibit increased numbers of germinal centers (GCs) in spleen (Figure 5A). This observation is supported by FACs analysis showing that the elevated numbers of GC B-cells and follicular helper T-cells (TFH) in the spleens of the POL BY265C/C compared to WT mice (Figure 5B). However, there are significantly higher levels of apoptosis in the spleen of the POL BY265C/C versus WT mice (Figure 5C). To determine if cell death was occurring in the GCs, we performed TUNEL analysis. The POL BY265C/C exhibit significantly increased levels of TUNEL positive cells that mostly overlap with CD4 T-helper cells. (Figure 5D and E).
Figure 5. The POLBY265c/c mice have increased numbers of germinal centers compared to WT mice.
A. Spleens of 3–4 five month-old mice were frozen with OCT compound, prepared as a frozen section and stained overnight at 4°C for confocal analysis. PNA-positive germinal centers were counted per field (25×). The graph compares the numbers of germinal centers in POLBY265c/c and WT mice and the image depicts an example of multiple germinal centers in the spleen of a POLBY265c/c mouse. B. Examples of FACs sorted splenic cells. For flow cytometry, splenic cells from 3–5 five month-old mice were processed and stained with anti-mouse antibodies (eBioscience) as described in Supplementary Methods. Top. Germinal center B-cells from a 5 month-old mouse were CD19+IgD-lo gated followed by gating for CD95 Gl7 double-positive cells (WT (+/+) is on the left and c/c is on the right). Bottom. Follicular help T-cells (TFH) were CD4+ CD44-lo gated followed by gating for CXCR5 PD-1 double-positive cells. C. Graph showing percent TUNEL positive cells from the POLBY265c/c and WT mice spleen, which is representative of at least three mice of each genotype. D. Spleen frozen sections were stained overnight at 4°C for c onfocal analysis. The image represents an example of germinal centers in the spleen of POLBY265c/c and WT mice. PNA-positive germinal centers are shown green (FITC), the blue (αCD4-Cy5) shows the CD4 positive helper T cells, and the TUNEL positive cells are shown in red. E. Graph showing percent TUNEL positive cells in the germinal center of POLBY265c/c and WT mice, which is representative of 8–10 germinal centers of 2–3 mice of each genotype.
Discussion
Here we show that mice carrying the Y265C hypomorphic allele of POLB develop several SLE-associated pathologies, suggesting that low activity of Pol β leads to SLE. Our results suggest that this phenotype arises as a result of aberrant V(D)J recombination and a high frequency of SHM. Our findings strongly implicate Pol β as being a critical player in both V(D)J recombination and somatic hypermutation.
Processing by POL BY265C/C Leads to Short CDR3 Junctions During V(D)J Recombination
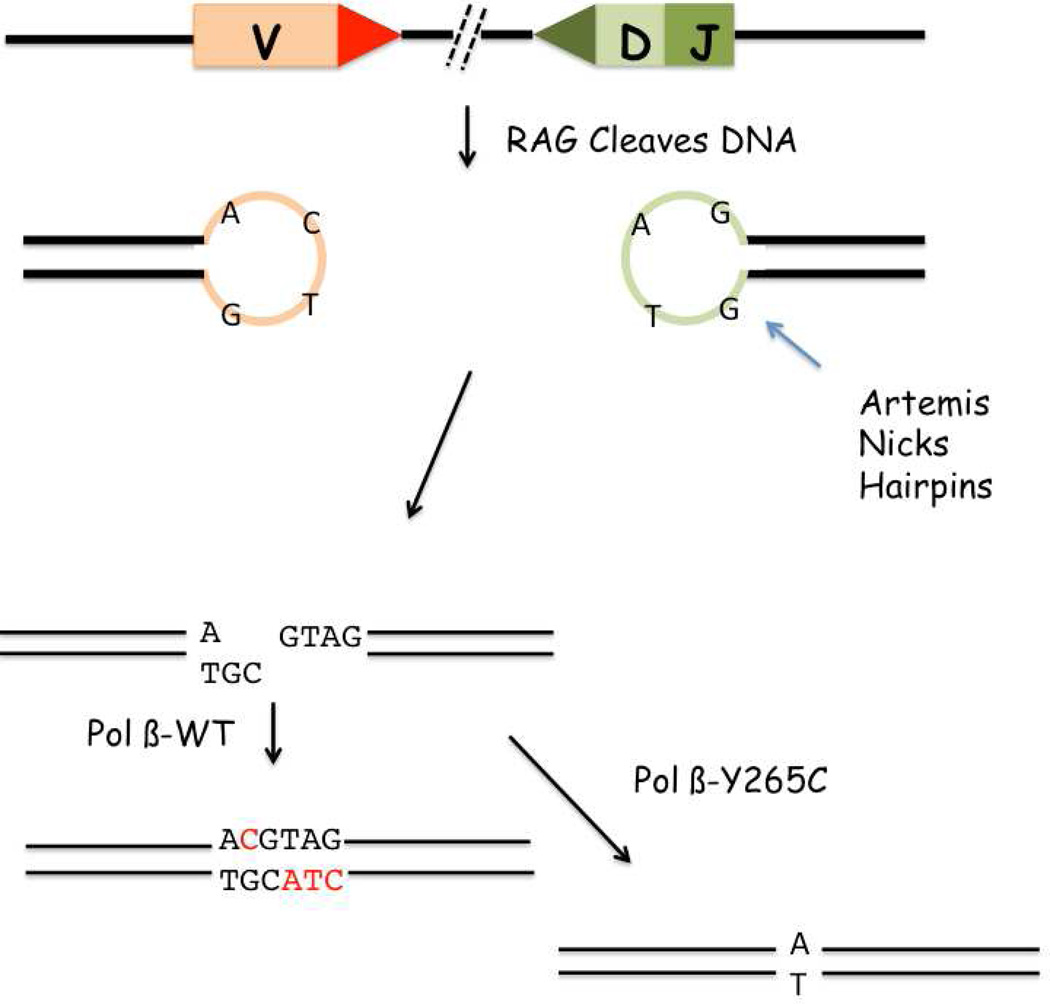
SLE is a classic autoimmune disease that is characterized by the production and circulation of antinuclear antibodies (ANA) that participate in tissue destruction. We have shown that the POL BY265C/C mice produce significantly higher levels of ANA than WT mice by six months of age, and that the levels of ANA continue to increase over the life of the mice, leading to glomerulonephritis, dermatitis, and cervical lymphadenopathy. Because the production of ANA is a major causative factor of SLE and because DNA repair proteins are central players in development of the lymphocyte repertoire, we investigated the potential role of Pol β Y265C in that process, starting with VDJ recombination. We showed that the CDR3 junctions of IgH, especially the N/P additions between the V and D fragments, are shorter in the POL BY265C/C mice versus WT. A model depicting the role of Pol β Y265C in joining the V and D fragments is shown in Figure 6. After cleavage by the RAG proteins, hairpins are formed and cut by the Artemis endonuclease. Should the incision by Artemis result in staggered ends as shown in Figure 6, a DNA polymerase fills the gap, followed by ligation. When Y265C is present, gap filling is slow and inefficient, leading to nuclease activity that results in a shorter CDR3 junction. We suggest that Y265C Pol β acts in a dominant negative manner by not permitting access of other DNA polymerases, including Pol λ and Pol μ, to the gapped DNA, eliminating functional redundancy. We point out that short gaps are the optimum DNA substrate for Pol β (Chagovetz et al., 1997). Previous work (Bertocci et al., 2006) has provided evidence that Pol λ processes this gap, using mice deleted of the POL L gene. Characterization of V(D)J recombination in the absence of Pol β was not possible because the POL BΔ/Δ mice do not survive past birth. In vitro reconstitution studies (Ma et al., 2004) did not demonstrate a role for Pol β. However, in these studies, Pol β was never assessed in combination with terminal transferase, Pol λ, and/or Pol μ, so its participation may have not been detected.
Figure 6. Model of aberrant V(D)J and SHM in the POLBY265c/c mice.
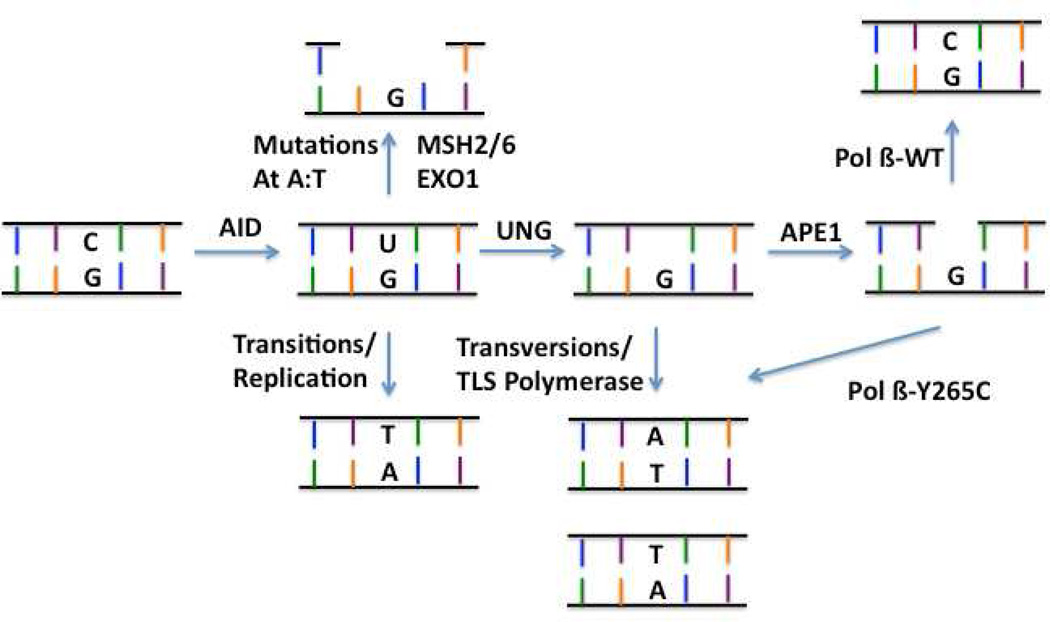
A. Y265C Pol β promotes deletion during joining of the V and D fragments. This cartoon depicts joining of the V to the D fragment. After the Rag proteins cleave the DNA, hairpins form. Artemis nicks the hairpins which sometime results in a substrate with DNA gaps. WT Pol β or other × family polymerases fill the gaps. However, in the presence of the slow Y265C Pol β polymerase, gaps are filled inefficiently, which can result in nuclease activity leading to deletions and shortening of the CDR3 junction. B. Y265C permits highly error-prone SHM resulting in autoimmunity. After deamination of cytosine by AID, replication can occur resulting in transitions. Alternatively, the mismatch repair pathway can bind to the U:G mismatch and recruit Exo 1, resulting in mutations at A:T base pairs. A third possibility is that APE1 incises the DNA at the AP site, resulting in a single nucleotide gap that is normally filled in by Pol β. However, in the presence of Y265C Pol β the gap is filled inefficiently or not at all, leading to translesion synthesis and increased mutagenesis.
The Presence of POL BY265C/C Does Not Alter CSR
CSR occurs by a process of intrachromasomal deletion that is initiated by the formation of double-strand breaks (DSBs). DSB formation is initiated when AID deaminates cytosine on both strands of the DNA in the switch regions. The resulting uracil residues are recognized and excised by UNG, followed by APE1 incision (for a review see (Stavnezer et al., 2008)). Should the uracils be clustered and on opposite strands of the DNA, incision by APE1 results in DSBs. Alternatively, the U:G mispair is recognized by mismatch repair proteins that recruit exonuclease I (Exo I) to the DNA. Exo 1 excision opposite a nick that results from incision by APE1 can also result in a DSB. Therefore, gap filling by Pol β can prevent formation of DSBs during CSR, as suggested previously when a slight increase in switching to IgG2a was observed in POL BΔ/Δ B cells (Wu and Stavnezer, 2007). In contrast, we observed no significant increases in CSR in the B cells from the POL BY265C/C mice induced to switch in vitro. One explanation for our results is that the presence of the Y265C Pol β polymerase prevents significant DSB formation through the APE1 pathway because it has the ability to bind to and slowly fill the DNA gap. This suggests that a major DSB formation pathway during CSR could be through mismatch repair. The binding of Y265C Pol β to the 3’OH of the gap could also prevent further processing by nucleases.
Lack of Gap Filling by Y265C Pol β Results in Increased SHM
We showed that the rate of SHM in the POL BY265C/C mice is significantly increased over that of WT mice, and we observe increases predominantly in transversions at AID hotspots. These findings are consistent with the idea that Pol β plays a critical role in maintaining a balance between error-free and error-prone repair during SHM. A model for the role of Pol β Y265C is presented in Figure 7. After AID deaminates cytosine to uracil, there are three pathway choices. If replication occurs, transitions are observed at AID deamination hotspots. If the U:G mismatch is recognized by the mismatch repair pathway, mutations at A:T base pairs are observed. Alternatively, UNG removes uracil. Bypass of the resulting AP site by a translesion polymerase leads to transversions at the AID hotspot. However, should APE1 incise the backbone, a single nucleotide gap could be filled in by a translesion polymerase, resulting in transversions or the gap could be filled in by Pol β in the canonical BER pathway, resulting in error-free repair. We suggest that gap filling is slow in the presence of Y265C Pol β, which would eventually lead to gap filling by TLS polymerases and the transversions we observe during SHM. Alternatively, Y265C could fill the gap in an error-prone manner. We do not favor this explanation because we do not observe increased levels of transversions at G:C base pairs in in vivo or in vitro studies with Y265C Pol β (Clairmont et al., 1999; Opresko et al., 1998; Washington et al., 1997). Another possibility is that many gaps remain unfilled, eventually resulting in cell death, consistent with our observation of increased TUNEL foci in the spleens of the POL BY265C/C mice. We note that in addition to increased levels of mutation at G:C base pairs, we also observe increases of A:T mutations. This could occur if the unfilled gap is bound by a nuclease, which would enlarge the gap. Filling of the larger gap by a translesion polymerase would lead to mutations at A:T base pairs.
The significance of our findings is that a balance between error-prone and error-free repair during SHM is critical, and that Pol β plays an important role in maintaining this balance. Our results suggest that too much mutagenesis during SHM has the potential to lead to autoimmune disease. In support of this suggestion, reversion of the mutations produced during SHM results in antibodies that no longer have antinuclear activity, suggesting that SHM itself is one mechanism of creating autoreactive antibodies (Guo et al., 2010; Wellmann et al., 2005).
Selection in the Germinal Center?
Not only do the POL BY265C/C mice have increased numbers of GCs but the GCs exhibit significantly increased TUNEL staining compare to WT mice. Gaps that arise during SHM or during the repair of oxidative damage that occurs during the proliferation of B cells in the germinal center may not be filled in efficiently by Y265C Pol β, leading to cell death. The presence of apoptotic or dying cells in the GCs could result in the release of antigen, resulting in positive selection of germinal center B cells that produce autoantibodies. This suggestion is supported by studies showing that mice with defective clearance of apoptotic cells develop lupus-like disease (Bickerstaff et al., 1999; Hanayama et al., 2004; Napirei et al., 2000). We suggest that for the POL BY265C/C the large amount of apoptotic cells in GCs could overwhelm the apoptotic clearance machinery, thereby escaping clearance and being used for positive selection of autoreactive B cells. It is also possible that the increased SHM generates autoreactivity by targeting IgG-expressing memory B cells re-entering the germinal center as a result of chronic exposure to self-antigen (Kohler et al., 2008; Meffre and Wardemann, 2008). Finally, it is possible that extrafollicular B cells could play a role in the generation of autoantibodies as described for the MRL/lpr mouse model of SLE (Teichmann et al., 2010).
Aberrant DNA Repair and Autoimmunity
Previous work has shown that mutations of the TREX1 DNA repair gene in humans are also associated with SLE (Stetson et al., 2008) but there is no evidence that these proteins act during the immunological processes of V(D)J, CSR, and SHM. Our findings demonstrate for the first time that a balance of hypermutation and error-free BER during SHM is critical for the prevention of autoimmune disease. Our results do not rule out the possibility of other mechanisms that are not B cell-intrinsic. For example, many cell types utilize Pol β Y265C during BER and the accumulation of BER intermediates in these cells could lead to alterations in a variety of tissues including alterations of the gut epithelial barrier, including stem cells. Any resulting mucosal alterations could drive expansion of autoreactive clones. The results of our study suggest that mutations in DNA repair genes associated with immunological processes could lead to the development of autoimmune disease, including SLE.
Experimental Procedures
Strain and genotyping of mice
Hybrid (129/Sv and C57BL/6) mice of both sexes were used for this study.
Skin histology
Skin tissues were fixed in histological 10% formalin solution fixative (Sigma-Aldrich), and embedded in paraffin. Skin sections were analyzed by a dermatopathologist.
Detection and scoring of antinuclear autoantibodies (ANA)
ANA was tested by immunofluorescence using human epithelial (Hep-2) cells on 12-well slides (Diasorin Inc).
Histology and scoring of kidney lesions
Tissues from mice were isolated and fixed in histological 10% formalin solution fixative (Sigma-Aldrich), and embedded in paraffin. H&E stained tissues were evaluated as described in Supplemental Information.
Immunohistochemistry
Details are described in Supplemental Information.
Analysis of Somatic Hypermutation (SHM)
Genomic DNA was extracted from B220+PNAhigh cells obtained from Peyer’s patches of two non-immunized mice that were 3.5–5 months of age and analyzed as described (Jolly et al., 1997; McDonald et al., 2003)(Maccarthy et al., 2009).
Preparation of genomic DNA, PCR amplification and analysis of VDJ recombination sequences
Genomic DNA was prepared from B220+ IgM− cells from spleen and bone marrow of 3–5 three week-old mice and analyzed as described in Supplemental Information (Gilfillan et al., 1993; Komori et al., 1993).
ELISA
ELISA 96 well plates were coated overnight at 4°C with appropriate antisera and analyzed as described in Supplemental Information.
Supplementary Material
Highlights.
Introduction of a hypermorphic mutation of the POL B gene in mice leads to SLE.
V(D)J recombination and SHM are aberrant in mice expressing the hypermorphic POL B mutation.
Our studies suggest that an imbalance of error-free and error-prone mutagenesis during development of the lymphocyte repertoire leads to autoimmune disease.
Our studies suggest that mutation of DNA repair genes lead to SLE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alt FW, Zhang Y, Meng FL, Guo C, Schwer B. Mechanisms of programmed DNA lesions and genomic instability in the immune system. Cell. 2013;152:417–429. doi: 10.1016/j.cell.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet. 2004;38:445–476. doi: 10.1146/annurev.genet.38.072902.092448. [DOI] [PubMed] [Google Scholar]
- Bertocci B, De Smet A, Weill J-C, Reynaud C-A. Nonoverlapping functions of DNA polymerases mu, lambda, and terminal deoxynucleotidyltransferase during immunoglobulin V(D)J recombination in vivo. Immunity. 2006;25:31–41. doi: 10.1016/j.immuni.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Bickerstaff MC, Botto M, Hutchinson WL, Herbert J, Tennent GA, Bybee A, Mitchell DA, Cook HT, Butler PJ, Walport MJ, Pepys MB. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nature medicine. 1999;5:694–697. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- Chagovetz AM, Sweasy JB, Preston BD. Increased activity and fidelity of DNA polymerase beta on single-nucleotide gapped DNA. J Biol Chem. 1997;272:27501–27504. doi: 10.1074/jbc.272.44.27501. [DOI] [PubMed] [Google Scholar]
- Clairmont CA, Narayanan L, Sun KW, Glazer PM, Sweasy JB. The Tyr-265-to-Cys mutator mutant of DNA polymerase beta induces a mutator phenotype in mouse LN12 cells. Proc Natl Acad Sci U S A. 1999;96:9580–9585. doi: 10.1073/pnas.96.17.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annual review of biochemistry. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- Furukawa F, Tanaka H, Sekita K, Nakamura T, Horiguchi Y, Hamashima Y. Dermatopathological studies on skin lesions of MRL mice. Archives of dermatological research. 1984;276:186–194. doi: 10.1007/BF00414018. [DOI] [PubMed] [Google Scholar]
- Gilfillan S, Dierich A, Lemeur M, Benoist C, Mathis D. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science. 1993;261:1175–1178. doi: 10.1126/science.8356452. [DOI] [PubMed] [Google Scholar]
- Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- Guo W, Smith D, Aviszus K, Detanico T, Heiser RA, Wysocki LJ. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. The Journal of experimental medicine. 2010;207:2225–2237. doi: 10.1084/jem.20092712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- Jolly CJ, Klix N, Neuberger MS. Rapid methods for the analysis of immunoglobulin gene hypermutation: application to transgenic and gene targeted mice. Nucleic Acids Res. 1997;25:1913–1919. doi: 10.1093/nar/25.10.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson R, Tarkowski A, Backman K, Holmdahl R, Klareskog L. Sialadenitis in the MRL-l mouse: morphological and immunohistochemical characterization of resident and infiltrating cells. Immunology. 1987;60:611–616. [PMC free article] [PubMed] [Google Scholar]
- Kohler F, Hug E, Eschbach C, Meixlsperger S, Hobeika E, Kofer J, Wardemann H, Jumaa H. Autoreactive B cell receptors mimic autonomous pre-B cell receptor signaling and induce proliferation of early B cells. Immunity. 2008;29:912–921. doi: 10.1016/j.immuni.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Komori T, Okada A, Stewart V, Alt FW. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science (New York, N Y) 1993;261:1171–1175. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]
- Lavoie TN, Lee BH, Nguyen CQ. Current concepts: mouse models of Sjogren's syndrome. Journal of biomedicine & biotechnology. 2011;2011:549107. doi: 10.1155/2011/549107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Schatz DG. Balancing AID and DNA repair during somatic hypermutation. Trends in immunology. 2009;30:173–181. doi: 10.1016/j.it.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Ma Y, Lu H, Tippin B, Goodman MF, Shimazaki N, Koiwai O, Hsieh CL, Schwarz K, Lieber MR. A biochemically defined system for mammalian nonhomologous DNA end joining. Molecular cell. 2004;16:701–713. doi: 10.1016/j.molcel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Maccarthy T, Roa S, Scharff MD, Bergman A. SHMTool: a webserver for comparative analysis of somatic hypermutation datasets. DNA Repair (Amst) 2009;8:137–141. doi: 10.1016/j.dnarep.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul RW, Gearhart PJ. AID and somatic hypermutation. Advances in immunology. 2010;105:159–191. doi: 10.1016/S0065-2776(10)05006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JP, Frank EG, Plosky BS, Rogozin IB, Masutani C, Hanaoka F, Woodgate R, Gearhart PJ. 129-derived strains of mice are deficient in DNA polymerase iota and have normal immunoglobulin hypermutation. The Journal of experimental medicine. 2003;198:635–643. doi: 10.1084/jem.20030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Current opinion in immunology. 2008;20:632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, Moroy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nature genetics. 2000;25:177–181. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- Norris DA, Lee LA. Pathogenesis of cutaneous lupus erythematosus. Clinics in dermatology. 1985;3:20–35. doi: 10.1016/0738-081x(85)90075-6. [DOI] [PubMed] [Google Scholar]
- Opresko PL, Sweasy JB, Eckert KA. The mutator form of polymerase beta with amino acid substitution at tyrosine 265 in the hinge region displays an increase in both base substitution and frame shift errors. Biochemistry. 1998;37:2111–2119. doi: 10.1021/bi9722711. [DOI] [PubMed] [Google Scholar]
- Radic M, Herrmann M, van der Vlag J, Rekvig OP. Regulatory and pathogenetic mechanisms of autoantibodies in SLE. Autoimmunity. 2011;44:349–356. doi: 10.3109/08916934.2010.536794. [DOI] [PubMed] [Google Scholar]
- Rajewsky K, Forster I, Cumano A. Evolutionary and somatic selection of the antibody repertoire in the mouse. Science. 1987;238:1088–1094. doi: 10.1126/science.3317826. [DOI] [PubMed] [Google Scholar]
- Senejani AG, Dalal S, Liu Y, Nottoli TP, McGrath JM, Clairmont CS, Sweasy JB. Y265C DNA polymerase beta knockin mice survive past birth and accumulate base excision repair intermediate substrates. Proc Natl Acad Sci U S A. 2012;109:6632–6637. doi: 10.1073/pnas.1200800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng YJ, Gao JP, Li J, Han JW, Xu Q, Hu WL, Pan TM, Cheng YL, Yu ZY, Ni C, et al. Follow-up study identifies two novel susceptibility loci PRKCB and 8p11.21 for systemic lupus erythematosus. Rheumatology. 2011;50:682–688. doi: 10.1093/rheumatology/keq313. [DOI] [PubMed] [Google Scholar]
- Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annual review of immunology. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33:967–978. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Nussenzweig MC. Germinal centers. Annual review of immunology. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- Washington SL, Yoon MS, Chagovetz AM, Li SX, Clairmont CA, Preston BD, Eckert KA, Sweasy JB. A genetic system to identify DNA polymerase beta mutator mutants. Proc Natl Acad Sci U S A. 1997;94:1321–1326. doi: 10.1073/pnas.94.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert MG, Cesari IM, Yonkovich SJ, Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970;228:1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Wellmann U, Letz M, Herrmann M, Angermuller S, Kalden JR, Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci U S A. 2005;102:9258–9263. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Stavnezer J. DNA polymerase beta is able to repair breaks in switch regions and plays an inhibitory role during immunoglobulin class switch recombination. The Journal of experimental medicine. 2007;204:1677–1689. doi: 10.1084/jem.20070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller T, Wild P, Szymczak S, Rotival M, Schillert A, Castagne R, Maouche S, Germain M, Lackner K, Rossmann H, et al. Genetics and beyond--the transcriptome of human monocytes and disease susceptibility. PloS one. 2010;5:e10693. doi: 10.1371/journal.pone.0010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.