Abstract
Coronary artery disease (CAD) is one of the leading causes of mortality in developed countries. Adenosine triphosphate (ATP)-binding cassette A1 (ABCA1) belongs to the superfamily of membrane proteins that function as a key factor in the regulation of plasma high-density lipoprotein cholesterol (HDL-C) and the metabolism of apolipoprotein A-I (Apo AI). The role of this gene in CAD remains controversial. The aim of this study was to investigate the frequency of single-nucleotide polymorphism (SNP) rs2230806 in the ABCA1 gene of 120 CAD patients and 100 age-matched, healthy controls using restriction fragment length polymorphism and direct sequencing. Total serum cholesterol, HDL-C and serum triglyceride levels were also assayed. Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald formula. When compared, the G allele occurred significantly more frequently in CAD patients compared to the control subjects. The odds ratio (OR) for CAD conferred by carrying the ABCA1 G allele was 2.362 [95% confidence interval (CI) 0.9055–6.161] (P<0.08). The K variant of SNP rs2230806 in the G allele was associated with a decrease in HDL-C levels, but an increased frequency of CAD. In conclusion, the results showed that SNP rs2230806 in the ABCA1 gene is significantly associated with the incidence of CAD. Homozygosity for the G allelic variant in CAD patients may be associated with an increased risk of CAD/MI.
Keywords: adenosine triphosphate-binding cassette transporter 1, polymorphism, coronary artery disease, genotyping
Introduction
Coronary artery disease (CAD) is a complex disease that develops due to environmental, lifestyle and genetic factors. Clinical and epidemiological studies have demonstrated an inverse association between high-density lipoprotein cholesterol (HDL-C) concentrations and cardiovascular disease risk (1,2). This association is supported by the anti-atherogenic properties of HDL-C; in particular, HDL-C functions in reverse cholesterol transport (RCT) (3). The initial step in RCT is regulated by adenosine triphosphate (ATP)-binding cassette A1 (ABCA1), which participates in the apolipoprotein-mediated efflux of cholesterol and phospholipid from peripheral cells (3). The identification of mutations in the ABCA1 gene in patients with Tangier disease, a rare disorder characterized by the absence of HDL (4), suggests a significant role for ABCA1 in regulating HDL-C levels. ABCA1 is a transmembrane protein that mediates the efflux of cholesterol and phospholipid to apolipoprotein A-I (Apo AI). This is the first step in RCT, the process by which cholesterol from peripheral macrophages is transferred back to the liver. Thus, ABCA1 availability is considered the rate-limiting step in HDL production (5).
Although HDL is involved in numerous atheroprotective mechanisms, the relative activity of ABCA1 plays a significant role (6). Evaluation of common genetic variations in the ABCA1 gene may be critical to understanding the contribution of ABCA1 to inter-individual variability in plasma lipid levels and susceptibility to CHD in the general population. Several common polymorphisms of the ABCA1 gene may differentially affect HDL-C levels and may be of clinical importance (7–12). The common rs2230806 single-nucleotide polymorphism (SNP) of the ABCA1 gene has been associated with an effect on HDL-C concentrations in some populations, suggesting that this SNP is of clinical importance (13–16). However, the presence of this polymorphism did not result in a consistent effect on HDL-C and other circulating lipids. The aim of the present study was to assess the impact of a common SNP of the ABCA1 gene (rs2230806) on the HDL-C concentration and the related plasma lipid profile in the Saudi Arabian population.
Materials and methods
Patient population
This study was approved by the Clinical Research Ethics Committee of the Institutional Review Board of King Saud University. In total, 120 Saudi patients with CAD (68 male, 52 female; mean age ± standard deviation, 39.8±15.2 years) and 100 ethnically matched, healthy controls from Riyadh, Saudi Arabia (60 males, 40 females; age-matched) were included. At least 95% of the patients were previously diagnosed with CAD based on their electrocardiographic profiles. None of the subjects had a history of diabetes, hypertension or hypercholesterolemia. None of the subjects recruited for the study were ingesting lipid-lowering drugs, and those with triglyceride levels ≥400 mg/dl were excluded from the analysis. All of the study subjects provided informed consent as per the protocol approved by the Ethics Review Board.
Determination of lipid parameters
Serum total cholesterol, HDL-C and triglyceride levels were measured by standard enzymatic methods, including the cholesterol oxidase-peroxidase-amidopyrine method (Roche Diagnostics, Mannheim, Germany) and the glycerol phosphate oxidase-peroxidase-amidopyrine method (Roche Diagnostics). Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald formula if total cholesterol, triglyceride and HDL-C values were available. Serum samples were separated by centrifugation and stored at −70°C until determination of the lipid parameters.
Genotyping the rs2230806 polymorphism by restriction fragment length polymorphism (RFLP)
DNA was extracted using a Qiagen kit according to the manufacturer’s instructions. The presence of SNP rs2230806 (R219K variant) in exon 7 of the ABCA1 gene (c.969A→G) was determined for each sample. The presence of the missense mutation at nucleotide 969 (AGG to AAG), which leads to the replacement of arginine with lysine at codon 219, was evaluated in genomic DNA by polymerase chain reaction (PCR)-RFLP analysis, according to methods described by Cook et al(17). A 333-bp PCR product was generated using forward 5′-TCC AAAAGACTTCAAGGACCCAGCT-3′ and reverse 5′-AAGT CATGCTGTCCAAGGAAAA-3′ primers. PCR was performed in a 96-well microtiter plate in a 25-μl volume containing 50 ng of genomic DNA, 5 μM of each primer, 2.5 μl of 10X buffer, 20 nM each of dATP, dCTP, dGTP, dTTP and 1 unit of Taq polymerase (HotStarTaq; Applied Biosystems, Carlsbad, CA, USA). Initial denaturation at 94°C for 2 min was followed by 40 cycles of denaturation at 94°C for 15 sec, annealing at 55°C for 30 sec and extension at 72°C for 1 min, with a final extension at 72°C for 2 min.
The PCR products were digested with Cac81 (New England Biolabs, Ipswich, MA, USA) for 4 h and separated by electrophoresis on a 2% agarose gel containing ethidium bromide. The 333-bp PCR product was not digested in homozygous wild-type samples (AA), whereas samples from individuals heterozygous for the missense mutation caused by rs2230806 (AG) demonstrated 333-, 189- and 144-bp bands. Samples from individuals homozygous for the missense mutation (GG) were cleaved into two fragments of 189 and 144 bp. Subsets of the samples were also directly sequenced.
Direct sequencing
Briefly, purified PCR products were directly sequenced using the dideoxynucleotide chain-termination method with an ABI PRISM Big Dye Terminator v3.1 Cycle Sequencing kit, following the manufacturer’s instructions and processed on an ABI 3730×l capillary sequencer (Applied Biosystems). Sequence analysis was performed using the SeqMan 6.1 module of the Lasergene software package (DNAStar Inc., Madison, WI, USA), and the sequences were compared with the reference GenBank sequence.
Statistical analysis
Data are presented as means ± SD. The association between CAD occurrence and the SNP genotype and allelic frequencies were measured by the odds ratio (OR) with its confidence interval (CI). The degree of significance was calculated using the Chi-square method. P≤0.05 was considered to indicate a statistically significant difference. The Hardy-Weinberg equilibrium (HWE) and deviation from HWE for genotype distribution were tested for each SNP for statistically significant association with CAD in order to examine the effects of genotype frequencies in the population by comparing the observed to the expected genotype frequencies.
Results
The characteristics of the patients and control subjects, including the presence of traditional risk factors for CAD, are provided in Table I. The mean age of the control subjects was 39.8±15.2 years. There was a significant difference in the mean age between the two groups. To assess the important genotype-phenotype associations, plasma lipid profile determination and genotyping were performed for the control (n=100) and patient (n=120) samples. A comparison of the plasma lipid parameters and the genotypic data revealed significant differences between the control and patient groups. The lipid profiles for CAD patients and healthy control subjects are provided in Table I. The results indicated that the mean levels of TC and LDL-C in the CAD group were significantly higher than those observed in the control group. CAD patients had significantly higher TG levels (P<0.001), and HDL-C levels were significantly lower in CAD patients as compared to the controls. All of the subjects selected for the study were non-smokers.
Table I.
Clinical and biochemical characteristics in coronary artery disease (CAD) patients and control subjects.
| Characteristics | Controlsa (n=100) | CAD groupa (n=120) | P-valueb |
|---|---|---|---|
| Age, years | 39.8±15.2 | 38.8±16.2 | <0.001c |
| Gender | |||
| Male (%) | 60 (60) | 68 (56.66) | <0.001c |
| Female, (%) | 40 (40) | 52 (43.33) | |
| TG, mmol/l | |||
| Mean ± SD | 1.06±0.28 | 1.56±0.70 | <0.0001c |
| Range | (0.53–1.72) | (0.57–3.77) | |
| TC, mmol/l | |||
| Mean ± SD | 3.80±0.48 | 4.24±1.09 | 0.0002c |
| Range | (3.01–5.11) | (0.77–7.5) | |
| HDL-C, mmol/l | |||
| Mean ± SD | 1.70±0.39 | 1.11±0.88 | <0.0001c |
| Range | (0.76–2.11) | (0.53–5.1) | |
| LDL-C, mmol/l | |||
| Mean ± SD | 1.65±0.33 | 2.53±0.91 | <0.0001c |
| Range | (1.0–2.5) | (1.01–4.89) | |
Data are presented as mean ± SD.
The Student’s t-test was used to compare the values of each group.
P-value indicates significance.
SD, standard deviation; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein-C cholesterol.
Distribution of the rs2230806 genotypes in the CAD and control groups
The observed genotype frequencies in each case demonstrated Hardy-Weinberg equilibrium, as indicated by the P-values (Table II). The genotype distribution and allelic frequency of exon 7 rs2230806 variants of ABCA1 are provided in Table III for CAD patients and control subjects. The frequencies of the rs2230806-associated genotypes GG, GA and AA were 84, 33 and 3% for CAD patients and 60, 35 and 5% for control subjects, respectively. Moreover, the allelic frequency of the G allele was significantly higher in CAD patients compared to the controls, suggesting that the variant confers a higher risk for developing disease. The crude OR for CAD conferred by carrying the ABCA1 GG genotype was 2.333 (95% CI 0.537–10.140), P<0.05 (Table III).
Table II.
Genotype distributions and allelic frequency of rs2230806 in the ATP-binding cassette A1 (ABCA1) gene.
| rs2230806 | Genotypes | In HWE chi-squarea | P-valueb | Deviation from HWE chi-squarea | P-valueb | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Wild-type | Heterozygote | Homozygote | |||||
| CAD patients (n=120) | |||||||
| Observed (n) | 84 | 33 | 3 | 0.90 | NSc | 0.16 | NSb |
| Expected (n) | 84.17 | 32.66 | 3.17 | ||||
| Control subjects (n=100) | |||||||
| Observed (n) | 60 | 35 | 5 | 0.97 | NSb | 0.23 | NSb |
| Expected (n) | 60.06 | 34.88 | 5.06 | ||||
Pearson’s Chi-square at 1 degree of freedom.
Significant.
NS, not significant (the 5% significance level for 1 degree of freedom is = 3.84).
HWE, Hardy-Weinberg equilibrium. Test for Hardy-Weinberg Equilibrium and deviation from Hardy-Weinberg Equilibrium for single-nucleotide polymorphism significantly associated with coronary artery disease (CAD) occurrence.
Table III.
Genotype and allelic distributions with odds ratios (ORs) of the assessed polymorphisms.
| Genotype and allele | CAD (120) | Control (100) | OR (95% CI) | P-valuea |
|---|---|---|---|---|
| G/G | 84 | 60 | 2.333 (0.537–10.140) | 0.05 |
| G/A | 33 | 35 | 2.053 (0.478–8.810) | 0.24 |
| A/A | 3 | 5 | ||
| G | 201 | 155 | 1.496 (0.928–2.411) | 0.32 |
| A | 39 | 45 |
Significant.
Total number of samples, 220.
CI, confidence interval.
Association of the rs2230806 polymorphism with CAD and plasma lipid profiles
Controls and patients were divided into two subgroups with normal and high observed values for serum lipids (Table IV). In each of the subgroups, individuals carrying the K allele presented higher triglyceride levels; however, there was no evidence of a more pronounced phenotype in the older patients. There was no significant difference in the correlation between age and HDL and triglyceride levels in individuals carrying different rs2230806 (R219K) genotypes (data not shown). An analysis of the relationship between plasma lipid profiles and genotypic background demonstrated an association between the G allele and decreased HDL levels. The K variant of the G allele was detected at a higher frequency in CAD patients compared to the controls, suggesting that the K variant of the G allele is likely a genetic risk factor for CAD. The multiple logistic regression analysis of the association of the ABCA1 rs2230806 G allele with HDL yielded an OR of 1.73 (95% CI, 1.026–2.864) (Table IV), suggesting that individuals carrying the G allele may have a low HDL-C profile and thus, an increased relative risk of developing CAD.
Table IV.
Odds ratios (ORs) and confidence intervals (CIs) for carriers of rs2230806.
| Alleles | |||||
|---|---|---|---|---|---|
|
|
|||||
| Metabolic profile | AA | AG | GG | A | G |
| Controls, TG (mmol/l) | |||||
| Normal (%) | 30 (30) | 17 (17) | 10 (10) | 0.385 | 0.185 |
| High (%) | 23 (23) | 18 (18) | 2 (2) | 0.32 | 0.11 |
| Patients, TG (mmol/l) | |||||
| Normal | 32 (26.6) | 51 (42.5) | 9 (0.075) | 0.479 | 0.287 |
| High | 17 (14.1) | 11 (9.1) | 0.187 | 0.045 | |
| OR of GG genotypes vs AA+AG, (95% CI), P-value | 2.362 (0.9055–6.161), 0.08 | ||||
| Controls, TC (mmol/l) | |||||
| Normal (%) | 14 (14) | 35 (35) | 26 (26) | 0.216 | 0.381 |
| High (%) | 8 (8) | 9 (9) | 8 (8) | 0.125 | 0.125 |
| Patients, TC (mmol/l) | |||||
| Normal (%) | 53 (44.1) | 21 (17.5) | 22 (18.2) | 0.528 | 0.27 |
| High (%) | 13 (10.8) | 3 (2.5) | 8 (6.6) | 0.12 | 0.079 |
| OR of GG genotypes vs. AA+AG, (95% CI), P-value | 0.6182 (0.3073–1.244), 0.2158 | ||||
| Controls, HDL-C (mmol/l) | |||||
| Normal (%) | 53 (53) | 26 (26) | 7 (7) | 0.66 | 0.2 |
| High (%) | 5 (5) | 5 (5) | 4 (4) | 0.075 | 0.065 |
| Patients, HDL-C (mmol/l) | |||||
| Normal (%) | 20 (16.6) | 73 (60.8) | 13 (10.7) | 0.48 | 0.411 |
| High (%) | 6 (5) | 5 (4.1) | 3 (2.5) | 0.07 | 0.045 |
| OR of GG genotypes vs. AA+AG, (95%CI), P-value | 1.730 (0.7222–4.143), 0.0247 | ||||
| Controls, LDL-C (mmol/) | |||||
| Normal (%) | 23 (23) | 60 (60) | 5 (5) | 0.475 | 0.455 |
| High (%) | 8 (8) | 4 (4) | 0 (0) | 0.10 | 0.04 |
| Patients, LDL-C (mmol/l) | |||||
| Normal (%) | 65 (45.1) | 19 (15.7) | 9 (7.5) | 0.62 | 0.153 |
| High (%) | 8 (6.6) | 15 (12.5) | 4 (3.3) | 0.129 | 0.095 |
| OR of GG genotypes vs. AA+AG, (95% CI), P-value | 0.2043 (0.06718–0.6215), 0.0039 | ||||
Data are presented as percentages, unless otherwise specified. The odds ratios were calculated by using the wild-type genotype as the reference group. The odds ratios for coronary artery disease (CAD)-associated genotypes with lipid parameters for CAD patient samples versus controls demonstrated a significant association of lower high-density lipoprotein-C (HDL-C) levels with GG genotypes. LDL-C, low-density lipoprotein-C.
The results of this study suggest that the GG genotype for the ABCA1 gene is associated with CAD in the Saudi population. However, when gender was analyzed separately, the K allele was present less frequently in females compared to males (data not shown). In a subset of the samples, sequencing was used to validate the GG, AA and GA genotypes of the R219K polymorphism that were identified by RFLP (Fig. 1).
Figure 1.
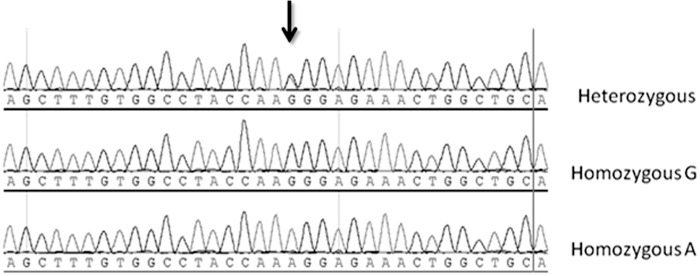
Sequence chromatogram of rs2230806 showing the sequence of the heterozygous allelic variant (AG), the homozygous allelic variant containing the missense mutation (GG), and the homozygous wild-type allelic variant (AA). The single-nucleotide polymorphism (SNP) rs2230806 is indicated by the black arrow.
Discussion
ABCA1 mediates the cellular efflux of cholesterol via the transfer of cholesterol from the inner to the outer layer of the cell membrane, thereby regulating extracellular cholesterol levels in the heart and central nervous system. Attention has been focused on the association between ABCA1 gene polymorphisms and different phenotypes, including lipid variables and clinical endpoints (18–21). Our results showed a significant association of rs2230806 with CAD, which is consistent with studies conducted on other populations and ethnicities (9,20,21). We also observed an association between triglyceride levels and the R219K polymorphism, but no significant associations were observed for other lipid parameters. The most common missense polymorphism in the coding region of the ABCA1 gene is R219K, with an allelic frequency of 25–46% in the Caucasian population. Two large studies including 2,028 and 794 individuals resulted in contradictory conclusions regarding the possible role of rs2230806 (R219K) in arteriosclerosis. One study reported an elevated R219K allelic frequency in patients with CHD and a low HDL level compared to disease-free individuals, suggesting that the mutant allele is likely associated with decreased HDL levels, the promotion of arteriosclerosis and the subsequent development of CHD (11). The other study reported a decreased R219K allelic frequency in patients with CHD in conjunction with high observed levels of HDL, suggesting that the mutant allele conferred a protective effect (22). The reason for the inconsistency between the various studies remains to be determined. However, the frequency of ABCA1 G allele is variable between different races and ethnic groups. This polymorphism has been shown to be associated with triglyceride levels (22), but not with HDL-C levels (9,21). Our results are consistent with the observations concerning triglyceride levels reported in those studies. However, to the best of our knowledge, the present study provides the first evidence for an association between an ABCA1 gene polymorphism and decreased HDL-C levels.
In this study, we observed a correlation between the R219K allele and lipid parameters, although the observed associations were not very significant, except for the associations with HDL-C levels (P=0.02). Total HDL-C levels were low in the CAD patient group and the presence of the rs2230806 polymorphism was correlated with these lower levels, suggesting that this polymorphism is a genetic risk factor for CAD in the Saudi population. Lutucuta et al(23) reported the effects of polymorphisms in the promoter of the ABCA1 gene on coronary atherosclerosis. However, findings of this study provide evidence for the importance of rs2230806 in the ABCA1 gene in the development of CAD with low levels of HDL-C within a specific population. Thus, this SNP is a potential genetic biomarker that may be used to screen individuals at high risk of CAD.
In conclusion, we identified significant allelic frequency increases in the K allele in the patient group, suggesting that theR219K polymorphism is a potential risk factor for CAD in the Saudi Arabian population. The K allele was highly associated with high triglyceride and low HDL-C levels in the CAD patient group, suggesting a possible role for the ABCA1 gene in the promotion of CAD via its function in decreasing HDL-C levels. However, future investigations should focus on the association between the K allele and CAD in other populations, as the disease association of this polymorphism may be population-dependent.
Acknowledgements
This study was supported by a grant from the ‘Research Center of the Center for Female Scientific and Medical Colleges’, Deanship of Scientific Research, King Saud University.
References
- 1.Bruckert E, Baccara-Dinet M, Eschwege E. Low HDL-cholesterol is common in European Type 2 diabetic patients receiving treatment for dyslipidaemia: data from a pan-European survey. Diabet Med. 2007;24:388–391. doi: 10.1111/j.1464-5491.2007.02111.x. [DOI] [PubMed] [Google Scholar]
- 2.Goldbourt U, Yaari S, Medalie JH. Isolated low HDL cholesterol as a risk factor for coronary heart disease mortality. A 21-year follow-up of 8000 men. Arterioscler Thromb Vasc Biol. 1997;17:107–113. doi: 10.1161/01.atv.17.1.107. [DOI] [PubMed] [Google Scholar]
- 3.Fredenrich A, Bayer P. Reverse cholesterol transport, high density lipoproteins and HDL cholesterol: recent data. Diabetes Metab. 2003;29:201–205. doi: 10.1016/s1262-3636(07)70029-0. [DOI] [PubMed] [Google Scholar]
- 4.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr, Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 5.Benton JL, Ding J, Tsai MY, Shea S, Rotter JI, Burke GL, Post W. Associations between two common polymorphisms in the ABCA1 gene and subclinical atherosclerosis: Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;193:352–360. doi: 10.1016/j.atherosclerosis.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Doosti M, Najafi M, Reza JZ, Nikzamir A. The role of ATP-binding-cassette-transporter-A1 (ABCA1) gene polymorphism on coronary artery disease risk. Transl Res. 2010;155:185–190. doi: 10.1016/j.trsl.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Clee SM, Kastelein JJ, van Dam M, Marcil M, Roomp K, Zwarts KY, Collins JA, Roelants R, Tamasawa N, Stulc T, Suda T, Ceska R, Boucher B, Rondeau C, DeSouich C, Brooks-Wilson A, Molhuizen HO, Frohlich J, Genest J, Jr, Hayden MR. Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J Clin Invest. 2000;106:1263–1270. doi: 10.1172/JCI10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pullinger CR, Hakamata H, Duchateau PN, Eng C, Aouizerat BE, Cho MH, Fielding CJ, Kane JP. Analysis of hABC1 gene 5′ end: additional peptide sequence, promoter region, and four polymorphisms. Biochem Bibiophys Res Comm. 2000;271:451–455. doi: 10.1006/bbrc.2000.2652. [DOI] [PubMed] [Google Scholar]
- 9.Brousseau ME, Bodzioch M, Schaefer EJ, Goldkamp AL, Kielar D, Probst M, Ordovas JM, Aslanidis C, Lackner KJ, Bloomfield Rubins H, Collins D, Robins SJ, Wilson PW, Schmitz G. Common variants in the gene encoding ATP-binding cassette transporter 1 in men with low HDL cholesterol levels and coronary heart disease. Atherosclerosis. 2001;154:607–611. doi: 10.1016/s0021-9150(00)00722-x. [DOI] [PubMed] [Google Scholar]
- 10.Singaraja RR, Brunham LR, Visscher H, Kastelein JJ, Hayden MR. Efflux and atherosclerosis: the clinical and biochemical impact of variations in the ABCA1 gene. Arterioscler Thromb Vasc Biol. 2003;23:1322–1332. doi: 10.1161/01.ATV.0000078520.89539.77. [DOI] [PubMed] [Google Scholar]
- 11.Frikke-Schmidt R, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A. Genetic variation in ABC transporter A1 contributes to HDL cholesterol in the general population. J Clin Invest. 2004;114:1343–1353. doi: 10.1172/JCI20361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard BV, Ruotolo G, Robbins DC. Obesity and dyslipidemia. Endocrinol Metab Clin North Am. 2003;32:855–867. doi: 10.1016/s0889-8529(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 13.Qi LP, Yan XW, Ye P, Dang AM. Association between two common polymorphisms in ATP-binding cassette A1 gene and coronary heart disease complicated with diabetes in Chinese Han people. Chin Med. 2010;5:295–297. (In Chinese) [Google Scholar]
- 14.Shi WY, Zhao ZZ, Xiao DM, Tang CK, Xu GZ, Yang YZ. Study on single nucleotide polymorphisms of ABCA1R219K in Han Population. Pract Prev Med. 2009;16:1057–1060. (In Chinese) [Google Scholar]
- 15.Frikke-Schmidt R, Nordestgaard BG, Jensen GB, Steffensen R, Tybjaerg-Hansen A. Genetic variation in ABCA1 predicts ischemic heart disease in the general population. Arterioscler Thromb Vasc Biol. 2008;28:180–186. doi: 10.1161/ATVBAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 16.Tregouet DA, Ricard S, Nicaud V, Arnould I, Soubigou S, Rosier M, Duverger N, Poirier O, Mace S, Kee F, Morrison C, Denefle P, Tiret L, Evans A, Deleuze JF, Cambien F. In-depth haplotype analysis of ABCA1 gene polymorphisms in relation to plasma ApoA1 levels and myocardial infarction. Arterioscler Thromb Vasc Biol. 2004;24:775–781. doi: 10.1161/01.ATV.0000121573.29550.1a. [DOI] [PubMed] [Google Scholar]
- 17.Cook LJ, Ho LW, Wang L, Terrenoire E, Brayne C, Evans JG, Xuereb J, Cairns NJ, Turic D, Hollingworth P, Moore PJ, Jehu L, Archer N, Walter S, Foy C, Edmondson A, Powell J, Lovestone S, Williams J, Rubinsztein DC. Candidate gene association studies of genes involved in neuronal cholinergic transmission in Alzheimer’s disease suggests choline acetyltransferase as a candidate deserving further study. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:5–8. doi: 10.1002/ajmg.b.30068. [DOI] [PubMed] [Google Scholar]
- 18.Tan JH, Low PS, Tan YS, Tong MC, Saha N, Yang H, Heng CK. ABCA1 gene polymorphisms and their associations with coronary artery disease and plasma lipids in males from three ethnic populations in Singapore. Hum Genet. 2003;113:106–117. doi: 10.1007/s00439-003-0943-3. [DOI] [PubMed] [Google Scholar]
- 19.Clee SM, Zwinderman AH, Engert JC, Zwarts KY, Molhuizen HO, Roomp K, Jukema JW, van Wijland M, van Dam M, Hudson TJ, Brooks-Wilson A, Genest J, Jr, Kastelein JJ, Hayden MR. Common genetic variation in ABCA1 is associated with altered lipoprotein levels and a modified risk for coronary artery disease. Circulation. 2001;103:1198–1205. doi: 10.1161/01.cir.103.9.1198. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Burnett JR, Near S, Young K, Zinman B, Hanley AJ, Connelly PW, Harris SB, Hegele RA. Common and rare ABCA1 variants affecting plasma HDL cholesterol. Arterioscler Thromb Vasc Biol. 2000;20:1983–1989. doi: 10.1161/01.atv.20.8.1983. [DOI] [PubMed] [Google Scholar]
- 21.Cenarro A, Artieda M, Castillo S, Mozas P, Reyes G, Tejedor D, Alonso R, Mata P, Pocovi M, Civeira F. A common variant in the ABCA1 gene is associated with a lower risk for premature coronary heart disease in familial hypercholesterolaemia. J Med Genet. 2003;40:163–168. doi: 10.1136/jmg.40.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talmud PJ. Genetic determinants of plasma triglycerides: impact of rare and common mutations. Curr Atheroscler Rep. 2001;3:191–199. doi: 10.1007/s11883-001-0061-4. [DOI] [PubMed] [Google Scholar]
- 23.Lutucuta S, Ballantyne CM, Elghannam H, Gotto AM, Jr, Marian AJ. Novel polymorphisms in promoter region of atp binding cassette transporter gene and plasma lipids, severity, progression, and regression of coronary atherosclerosis and response to therapy. Circ Res. 2001;88:969–973. doi: 10.1161/hh0901.090301. [DOI] [PubMed] [Google Scholar]


