Abstract
Pools of mosquitoes were tested for insect-specific viruses using cytopathic effect (CPE) assays on Aedes albopictus (C6/36) cells. Illumina sequencing of RNA from pool TR7094, which produced extensive CPE 2 days post-infection, yielded the complete genome sequences of a previously unknown Bunyavirus, designated Cumuto virus (CUMV), and a second virus designated Wallerfield virus (WALV). WALV shared highest amino acid identity (60.1 %) with Dezidougou virus from Côte d’Ivoire, a positive-sense, single-strand RNA, insect-specific virus belonging to the newly proposed genus Negevirus associated with mosquitoes and phlebotomine sandflies. The S, M and L segments of CUMV were most closely related to those of Gouleako virus, also from Côte d’Ivoire (amino acid identities of 36 %, 38 % and 54 % respectively). Neither virus produced CPE on vertebrate cells, or illness in newborn mice. Isolation and characterization of these viruses increase our knowledge of the geographical distribution, diversity and host range of mosquito-specific bunyaviruses and negeviruses.
Mosquitoes are vectors for many viruses of public and veterinary health importance. Mosquito-borne viruses consist primarily of members of the families Flaviviridae, Togaviridae and Bunyaviridae that are generally maintained in transmission cycles involving replication in both mosquito vectors and vertebrate hosts. In addition to these arthropod-borne viruses (arboviruses) of vertebrates, a number of novel mosquito-specific viruses have been identified recently. Most are members of the genus Flavivirus and are associated with Aedes and Culex spp. mosquitoes (reviewed by Cook et al., 2012). However, more recently, the repertoire of mosquito-specific viruses has expanded (reviewed by Junglen & Drosten, 2013) to include flaviviruses from other mosquito genera such as Nakiwogo virus from Mansonia sp. (Cook et al., 2009), Nounane virus from Uranotaenia sp. (Junglen et al., 2009) and Aripo virus from Psorophora albipes (A. J. Auguste, M. Woodson, C. V. Carrington, B. G. Bolling, M. B. Sherman, H. Guzman, V. L. Popov, A. P. T. da Rosa, S. G. Widen, T. G. Wood, R. B. Tesh & S. C. Weaver; unpublished data), as well as other virus families including Bunyaviridae, Mesoniviridae, Reoviridae, Togaviridae and the newly proposed taxon, Negevirus (Attoui et al., 2005; Kuwata et al., 2013; Lauber et al., 2012; Marklewitz et al., 2011; Nasar et al., 2012; Vasilakis et al., 2013; Zirkel et al., 2013). This indicates that insect-specific viruses are more abundant and widely distributed than previously realized. However, little is yet known about their ecological niche, their place in the evolutionary history of their respective viral taxa, or to what extent they affect transmission of their vertebrate-pathogenic relatives. In particular, there is interest in their potential for use as vaccines, or for biological control of pathogenic virus vectors.
We screened a random sample of 300 Culex mosquito pools collected in Trinidad (Auguste et al., 2010) for viral cytopathic effects (CPE) in cultures of C6/36 (Aedes albopictus) mosquito cells. One pool, TR7094, produced extensive CPE 2 days after inoculation. Electron microscopic examination of ultrathin sections of infected cells showed that the most prominent cytopathology was a tremendous expansion of perinuclear space and granular endoplasmic reticulum (Fig. 1a). These expansions were filled with vesicles approximately 20–40 nm in diameter (Fig. 1b) and tubular elements (Fig. 1a), suggesting some vesicles might represent cross-sections of the tubules. Additionally, large vacuoles were found containing spherical structures 40–60 nm in diameter at their inner periphery. These structures morphologically resembled alphavirus spherules but were also characteristic of the cytopathology observed with negeviruses (Vasilakis et al., 2013). Smaller vacuoles, which appeared to be Golgi derived, contained spherical enveloped particles ~75 nm in diameter with a dense core (Fig. 1c). These particles were similar in size and morphology to bunyavirus virions.
Fig. 1.
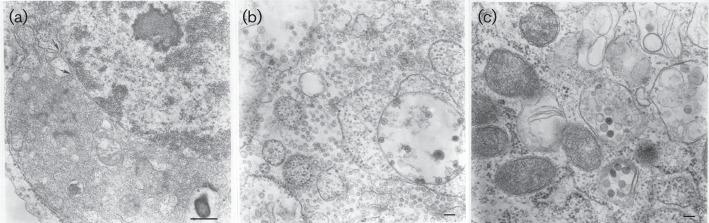
Ultrastructure of C6/36 cells infected with Wallerfield and Cumuto viruses. (a) Expansions of perinuclear space and granular endoplasmic reticulum filled with tubular and vesicular structures. Arrows show points of perinuclear membrane expansion. Bar, 0.5 µm. (b) Large vacuoles with spherules of Wallerfield virus at their periphery and expansions of granular endoplasmic reticulum filled with vesicles 20–40 nm in diameter. Bar, 100 nm. (c) Two vacuoles with spherical enveloped particles ~75 nm in diameter possibly representing Cumuto virus. Bar, 100 nm.
The isolates were subsequently cultured in larger quantities of C6/36 cells, precipitated with polyethylene glycol, and the pellet was resuspended in 250 µl of TEN buffer (10 mM Tris/HCl, pH 7.5; 1 mM EDTA, pH 8.0; and 0.1M NaCl). Total RNA was extracted and next-generation sequencing using the Illumina platform (Illumina) was used to determine the complete genome sequence. blastx searches (E-value cut off of 1E−10) indicated the presence of two viruses: (1) a bunyavirus designated Cumuto virus (CUMV; GenBank accession numbers KF543244–KF543246); and (2) a second virus, designated Wallerfield virus (WALV; GenBank accession number KF042857), which belongs to a newly described group of insect-specific viruses known as negeviruses.
Neither virus subsequently produced CPE in Vero E6 (African green monkey kidney) or BHK-S (baby hamster kidney) cells or caused overt disease in intracerebrally inoculated, 2 day old CD1 mice, suggesting that they both were insect-specific viruses. The absence of cytopathology does not, however, rule out the possibility that these viruses can replicate in mammalian cells.
CUMV
CUMV was named after the town of Cumuto, which lies on the south-west boundary of the Aripo Savannahs Scientific Reserve, close to the area where mosquito pool TR7094 was collected. As indicated above, CUMV belongs to the family Bunyaviridae, and is most closely related to viruses in the genus Phlebovirus, which, like other bunyaviruses, have tripartite-segmented, negative (−) sense RNA genomes consisting of: (1) a large segment (L), which encodes an RNA-dependent RNA polymerase (RdRp) necessary for replication and transcription of genomic RNA; (2) a medium segment (M) encoding the viral surface glycoproteins Gn and Gc, and a non-structural protein NSm that occurs in the sandfly fever group of phleboviruses but not in the Uukuniemi group; and (3) a small segment (S) that also exhibits an ambisense coding strategy, which encodes the nucleocapsid (N) protein and a non-structural protein, NSs, found in some members of the family (Plyusnin et al., 2011). The L, M and S segments of CUMV were found to be 6367 nt (GC content, 34.9 %), 3269 nt (GC content, 39.2 %) and 1176 nt (GC content, 41.5 %) in length respectively, and were most similar to Gouleako virus (Marklewitz et al., 2011) from Côte d’Ivoire in West Africa (amino acid identities of 54 %, 38 % and 36 % respectively). Together with Gouleako virus, CUMV may represent a novel genus of insect-specific bunyaviruses that are evolutionarily related to phleboviruses.
The ORF for the RdRp gene within the CUMV L segment was 6216 nt in length (GC content, 31 %), beginning at position 41. The 2071 amino acid RdRp gene product was estimated to be 240 kDa. The M segment ORF (2955 nt beginning at position 97) encoded the glycoprotein precursor (984 aa; 109.8 kDa) that is subsequently cleaved into Gn and Gc, with the predicted cleavage site between amino acids 491 and 492 (http://www.ebi.ac.uk/Tools/pfa/iprscan/). Four potential N-linked glycosylation sites were identified in Gn and three in Gc, which is similar to Uukuniemi group viruses that contain 4 N-linked glycosylation sites in both proteins (Kakach et al., 1989; Rönnholm & Pettersson, 1987). In phleboviruses, the M segment ORF can have multiple in-frame AUG start codons, ranging from 1 in the Uukuniemi group to 13 in the sandfly borne Punta Toro virus (Gerrard et al., 2007; Kakach et al., 1989; Matsuoka et al., 1988; Moureau et al., 2010). Similar to Gouleako virus, three were evident in CUMV, at nucleotide positions 97, 145 and 157. The NSm protein that occurs in the sandfly fever group is absent in Gouleako virus and the Uukuniemi group viruses (Marklewitz et al., 2011), and was also not detected in CUMV.
The S segment of CUMV (which is ambisense in phleboviruses) was 1176 nt long (GC content, 41.5 %). It contained 5 ORFs (>100 bp) including an N gene of 804 bp in length (267 aa; 29.3 kDa protein). The other four ORFs (two in reverse and two in forward orientation) were deemed too small to encode relevant proteins (all less than 60 aa) and none showed detectable homology to the putative 11.6kD protein identified in Gouleako virus (which has a molecular mass similar to the NSs protein of orthobunyaviruses). The absence of this 11.6 kDa protein from CUMV gives further credence to the suggestion that this protein may not exist in Gouleako virus (Marklewitz et al., 2011). The NSs protein in phleboviruses is relevant for mammalian cell infection, because it contributes to the inhibition of type-I interferon synthesis (Bird et al., 2008; Bouloy et al., 2001; Ikegami et al., 2006; Weber et al., 2002). Previous work has shown that although the NSm and NSs proteins are dispensable for growth in cell culture, they play an important role in Rift valley fever virus pathogenesis (Gerrard et al., 2007; Ikegami et al., 2006). Absence of both NSm and NSs proteins in CUMV may therefore be consistent with the apparent inability of CUMV to replicate in mammalian cells, although it should be noted that Rift valley fever virus can replicate in cell culture in the absence of a NSs protein.
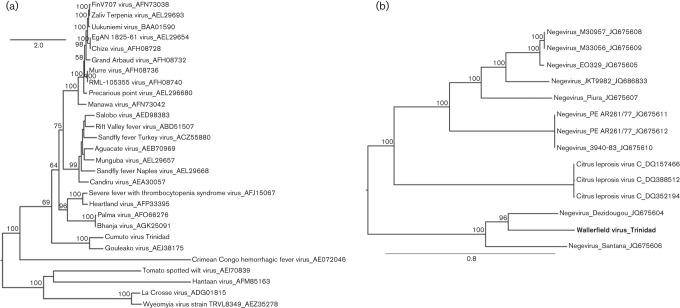
Fig. 2(a) shows a maximum-likelihood (ML) phylogeny estimated from an alignment of CUMV amino acid sequences for the L segment together with corresponding sequences from other selected phleboviruses, as well as representatives of other Bunyavirus genera. Sequence alignment was performed using clustal w v2.0 (Larkin et al., 2007) and the alignment trimmed to 3406 positions (including gaps) such that CUMV RdRp amino acid positions 25–2069 were represented. The ML phylogeny was estimated using the Blosum62 substitution model with six gamma-distributed rate categories and the proportion of invariant sites and gamma shape parameter estimated from the data (available from the authors on request). A BioNJ tree was used as the starting tree and the SPR search algorithm was employed. Bootstrap support was estimated from 100 replicates.
Fig. 2.
(a) Midpoint rooted ML phylogeny estimated from amino acid sequences for phlebovirus L segment; (b) midpoint rooted ML phylogeny estimated from nucleotide sequences of negevirus RdRp genes. Bootstrap support values >50 % indicated at relevant nodes. Taxon names include virus name, strain and corresponding accession numbers used for analysis. Scale bars represent percentage divergence.
CUMV clustered with its closest relative, Gouleako virus, with 100 % bootstrap support. Phylogenies generated based on the M segment and N gene also support this grouping with Gouleako virus with high bootstrap support (data not shown). These results together with the low sequence identity between CUMV and the other bunyaviruses suggest that CUMV and Gouleako viruses may represent a new genus in the family Bunyaviridae that is evolutionarily related to but distinct from phleboviruses. Consequently, we propose that the tentative name for this genus should be Gouleakovirus, after the first virus to be detected in this group.
WALV
The WALV complete genome shared highest nucleotide (67 %) and amino acid identity (60 %) with the negevirus, Dezidougou virus from Côte d’Ivoire, followed by Santana virus (also a negevirus) from Brazil (65 % and 49 % respectively). The negeviruses are a recently described taxon of insect-specific viruses that have been isolated from mosquitoes and phlebotomine sandflies (Vasilakis et al., 2013). Their closest but still distant genetic relationship is with viruses included in the unassigned genus Cilevirus (Vasilakis et al., 2013), mite-transmitted viruses that cause disease in citrus plants (Locali-Fabris et al., 2006; Pascon et al., 2006). Negeviruses appear to have a wide host range among the Diptera, having been isolated from Culex, Aedes, and Anopheles mosquitoes, as well as Lutzomyia sandflies. They are also widely distributed geographically, having been isolated from insects in Israel, Indonesia, Ivory Coast, United States, Peru, Portugal and Brazil (Ferreira et al., 2013; Vasilakis et al., 2013).
The WALV genome is a 9023 nt long single-stranded, positive-sense RNA with a GC content of 35 %, typical of negeviruses whose genomes are approximately 9–10 kb in length (Vasilakis et al., 2013). Similar to other negeviruses, the WALV genome contains three ORFs flanked by non-coding regions, of which the 3′ end is polyadenylated. Each ORF is one frame-shift downstream of the preceding one. ORF1 begins at nucleotide position 19 and is 6789 nt long. It encodes the putative non-structural proteins (2262 aa) with four functional domains identified by using Geneious v6.1 to search the Pfam database. These include a viral methyltransferase (amino acid positions 114–488), an S-adenosylmethionine-dependent methyltransferase (positions 795–978), a viral helicase (positions 1331–1602), and an RNA-dependent RNA polymerase (positions 1808–2240).
A signal peptide and potential cytoplasmic domain were identified in ORF2 (ORF2 positions are 6837–8105), which is 1269 nt (423 codons) long. In contrast to other negeviruses (Vasilakis et al., 2013), no transmembrane regions were predicted in ORF2 of WALV, but three transmembrane domains were predicted in ORF3 transmembrane helix prediction based on hidden Markov models (TMHMM) (Krogh et al., 2001). ORF3 (positions 8227–8826) is 600 nt (200 codons) long and thought to encode the negevirus envelope glycoproteins.
The 5′ WALV UTR is 18 nt long and the 3′ UTR is 197 nt. We inferred glycosylation sites using the NetNGlyc server 1.0 (http://www.cbs.dtu.dk/services/NetNGlyc/) with a threshold value of 0.5 across the entire WALV genome and found 11 potential sites in ORF1, four in ORF2 and two in ORF3. These data suggest that the ORF3-encoded structural protein may undergo post-translational modification at two amino acid positions in the structural protein.
Fig. 2(b) shows a ML phylogeny estimated from alignments of the RdRp genes of all previously reported negeviruses and citrus leprosis virus C. WALV clusters with high bootstrap support with Santana and Dezidougou viruses (Fig. 2b); however, another analysis based on the helicase gene showed that its position in the two phylogenies was not distinct, such that the helicase phylogeny resulted in WALV grouping more closely with Santana virus with low bootstrap support (data not shown), and the RdRp phylogeny resulted in WALV grouping with Dezidougou virus, with high bootstrap support. These results suggest recombination, but further analyses with additional negevirus sequences are needed to obtain more robust evidence. Analysis of sequence identities suggested that WALV is more closely related to Dezidougou than Santana virus, supporting the phylogeny based on the RdRp sequences.
Conclusions
In summary, the complete genome sequences of two novel insect-specific viruses were derived from a single pool of Culex declarator mosquitoes collected in Trinidad. CUMV and WALV are novel insect-specific viruses belonging to the family Bunyaviridae and the genus Negevirus, respectively. Both are sufficiently genetically distinct to be considered new member species in their respective taxa. Their genetic characterization increases our knowledge of the known geographical distribution, diversity and host range of insect-specific bunyaviruses and negeviruses. Additionally, their close genetic relationship with West African viruses provides evidence that, like many other arboviruses, the progenitors of these viruses may have originated in the Old World. The complete genome sequences provided should be helpful in the development of specific detection methods for studying the distribution, patterns of global spread, and prevalence of these viruses in Diptera, as well as to better understand the evolutionary histories of these genera by using phylogenetic methods.
Acknowledgements
This work was supported by National Institutes of Health contract HHSN272201000040I / HHSN27200004 / D04 and the Robert E. Shope International Fellowship in Infectious Diseases from the American Society of Tropical Medicine and Hygiene. A. J. A. is supported by the James W. McLaughlin Endowment fund. The authors do hereby declare that they have no competing interests in this scientific work.
References
- Attoui H., Mohd Jaafar F., Belhouchet M., Biagini P., Cantaloube J. F., de Micco P., de Lamballerie X. (2005). Expansion of family Reoviridae to include nine-segmented dsRNA viruses: isolation and characterization of a new virus designated Aedes pseudoscutellaris reovirus assigned to a proposed genus (Dinovernavirus). Virology 343, 212–223 10.1016/j.virol.2005.08.028 [DOI] [PubMed] [Google Scholar]
- Auguste A. J., Adams A. P., Arrigo N. C., Martinez R., Travassos da Rosa A. P., Adesiyun A. A., Chadee D. D., Tesh R. B., Carrington C. V., Weaver S. C. (2010). Isolation and characterization of sylvatic mosquito-borne viruses in Trinidad: enzootic transmission and a new potential vector of Mucambo virus. Am J Trop Med Hyg 83, 1262–1265 10.4269/ajtmh.2010.10-0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird B. H., Albariño C. G., Hartman A. L., Erickson B. R., Ksiazek T. G., Nichol S. T. (2008). Rift valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J Virol 82, 2681–2691 10.1128/JVI.02501-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Janzen C., Vialat P., Khun H., Pavlovic J., Huerre M., Haller O. (2001). Genetic evidence for an interferon-antagonistic function of rift valley fever virus nonstructural protein NSs. J Virol 75, 1371–1377 10.1128/JVI.75.3.1371-1377.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S., Moureau G., Harbach R. E., Mukwaya L., Goodger K., Ssenfuka F., Gould E., Holmes E. C., de Lamballerie X. (2009). Isolation of a novel species of flavivirus and a new strain of Culex flavivirus (Flaviviridae) from a natural mosquito population in Uganda. J Gen Virol 90, 2669–2678 10.1099/vir.0.014183-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S., Moureau G., Kitchen A., Gould E. A., de Lamballerie X., Holmes E. C., Harbach R. E. (2012). Molecular evolution of the insect-specific flaviviruses. J Gen Virol 93, 223–234 10.1099/vir.0.036525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira D. D., Cook S., Lopes A., de Matos A. P., Esteves A., Abecasis A., de Almeida A. P., Piedade J., Parreira R. (2013). Characterization of an insect-specific flavivirus (OCFVPT) co-isolated from Ochlerotatus caspius collected in southern Portugal along with a putative new Negev-like virus. Virus Genes 47, 532–545 10.1007/s11262-013-0960-9 [DOI] [PubMed] [Google Scholar]
- Gerrard S. R., Bird B. H., Albariño C. G., Nichol S. T. (2007). The NSm proteins of Rift Valley fever virus are dispensable for maturation, replication and infection. Virology 359, 459–465 10.1016/j.virol.2006.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T., Won S., Peters C. J., Makino S. (2006). Rescue of infectious rift valley fever virus entirely from cDNA, analysis of virus lacking the NSs gene, and expression of a foreign gene. J Virol 80, 2933–2940 10.1128/JVI.80.6.2933-2940.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junglen S., Drosten C. (2013). Virus discovery and recent insights into virus diversity in arthropods. Curr Opin Microbiol 16, 507–513 10.1016/j.mib.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junglen S., Kopp A., Kurth A., Pauli G., Ellerbrok H., Leendertz F. H. (2009). A new flavivirus and a new vector: characterization of a novel flavivirus isolated from uranotaenia mosquitoes from a tropical rain forest. J Virol 83, 4462–4468 10.1128/JVI.00014-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakach L. T., Suzich J. A., Collett M. S. (1989). Rift Valley fever virus M segment: phlebovirus expression strategy and protein glycosylation. Virology 170, 505–510 10.1016/0042-6822(89)90442-X [DOI] [PubMed] [Google Scholar]
- Krogh A., Larsson B., von Heijne G., Sonnhammer E. L. (2001). Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 305, 567–580 10.1093/bioinformatics/bts199 [DOI] [PubMed] [Google Scholar]
- Kuwata R., Satho T., Isawa H., Yen N. T., Phong T. V., Nga P. T., Kurashige T., Hiramatsu Y., Fukumitsu Y. & other authors (2013). Characterization of Dak Nong virus, an insect nidovirus isolated from Culex mosquitoes in Vietnam. Arch Virol 158, 2273–2284 10.1007/s00705-013-1741-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A. & other authors (2007). clustal w and clustal_x version 2.0. Bioinformatics 23, 2947–2948 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Lauber C., Ziebuhr J., Junglen S., Drosten C., Zirkel F., Nga P. T., Morita K., Snijder E. J., Gorbalenya A. E. (2012). Mesoniviridae: a proposed new family in the order Nidovirales formed by a single species of mosquito-borne viruses. Arch Virol 157, 1623–1628 10.1007/s00705-012-1295-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locali-Fabris E. C., Freitas-Astúa J., Souza A. A., Takita M. A., Astúa-Monge G., Antonioli-Luizon R., Rodrigues V., Targon M. L., Machado M. A. (2006). Complete nucleotide sequence, genomic organization and phylogenetic analysis of Citrus leprosis virus cytoplasmic type. J Gen Virol 87, 2721–2729 10.1099/vir.0.82038-0 [DOI] [PubMed] [Google Scholar]
- Marklewitz M., Handrick S., Grasse W., Kurth A., Lukashev A., Drosten C., Ellerbrok H., Leendertz F. H., Pauli G., Junglen S. (2011). Gouleako virus isolated from West African mosquitoes constitutes a proposed novel genus in the family Bunyaviridae. J Virol 85, 9227–9234 10.1128/JVI.00230-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y., Ihara T., Bishop D. H., Compans R. W. (1988). Intracellular accumulation of Punta Toro virus glycoproteins expressed from cloned cDNA. Virology 167, 251–260 10.1016/0042-6822(88)90075-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moureau G., Bichaud L., Salez N., Ninove L., Hamrioui B., Belazzoug S., de Lamballerie X., Izri A., Charrel R. N. (2010). Molecular and serological evidence for the presence of novel phleboviruses in sandflies from northern Algeria. Open Virol J 4, 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasar F., Palacios G., Gorchakov R. V., Guzman H., Da Rosa A. P. T., Savji N., Popov V. L., Sherman M. B., Lipkin W. I. & other authors (2012). Eilat virus, a unique alphavirus with host range restricted to insects by RNA replication. Proc Natl Acad Sci U S A 109, 14622–14627 10.1073/pnas.1204787109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascon R. C., Kitajima J. P., Breton M. C., Assumpção L., Greggio C., Zanca A. S., Okura V. K., Alegria M. C., Camargo M. E. & other authors (2006). The complete nucleotide sequence and genomic organization of Citrus Leprosis associated Virus, Cytoplasmatic type (CiLV-C). Virus Genes 32, 289–298 10.1007/s11262-005-6913-1 [DOI] [PubMed] [Google Scholar]
- Plyusnin A., Beaty B. J., Elliott R. M., Goldbach R., Kormelink R., Lundkvist A., Schmaljohn C. S., Tesh R. B. (2011). Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses, vol. 9, pp 735–741 Edited by King A.M.Q., Adams J., Lefkowitz E. Amsterdam: Elsevier [Google Scholar]
- Rönnholm R., Pettersson R. F. (1987). Complete nucleotide sequence of the M RNA segment of Uukuniemi virus encoding the membrane glycoproteins G1 and G2. Virology 160, 191–202 10.1016/0042-6822(87)90060-2 [DOI] [PubMed] [Google Scholar]
- Vasilakis N., Forrester N. L., Palacios G., Nasar F., Savji N., Rossi S. L., Guzman H., Wood T. G., Popov V. & other authors (2013). Negevirus: a proposed new taxon of insect-specific viruses with wide geographic distribution. J Virol 87, 2475–2488 10.1128/JVI.00776-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., Bridgen A., Fazakerley J. K., Streitenfeld H., Kessler N., Randall R. E., Elliott R. M. (2002). Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon. J Virol 76, 7949–7955 10.1128/JVI.76.16.7949-7955.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirkel F., Roth H., Kurth A., Drosten C., Ziebuhr J., Junglen S. (2013). Identification and characterization of genetically divergent members of the newly established family Mesoniviridae. J Virol 87, 6346–6358 10.1128/JVI.00416-13 [DOI] [PMC free article] [PubMed] [Google Scholar]