Abstract
The present study investigated the role of hydrogen sulfide (H2S), a novel gaseous transmitter, in chronic heart failure (CHF) induced by left-to-right shunt, leading to volume overload. Thirty male Sprague-Dawley rats were randomly divided into four groups: the shunt group, the sham group, the shunt + sodium hydrosulfide (NaHS) group and the sham + NaHS group. CHF was induced in the rats by abdominal aorta-inferior vena cava shunt operation. Rats in the shunt + NaHS and sham + NaHS groups were injected intraperitoneally with NaHS (H2S donor). Haemodynamic parameters were measured 8 weeks after surgery. In addition, left ventricular heme oxygenase (HO)-1 mRNA expression was measured by real-time PCR. Protein expression of HO-1 was evaluated by western blot analysis. Eight weeks after surgery, compared to the sham group, the left ventricular systolic pressure (LVSP) and left ventricular peak rate of contraction and relaxation (LV±dp/dtmax) were significantly reduced; the left ventricular end-diastolic pressure (LVEDP) was significantly increased in the shunt group (all P<0.05). However, NaHS increased LVSP and LV±dp/dtmax (all P<0.05) and decreased LVEDP (P<0.05). Protein expression of HO-1 was significantly decreased in the shunt group compared to that in the sham group (P<0.05). NaHS increased protein expression of HO-1 compared to that in the shunt group (P<0.05). HO-1 mRNA expression was significantly increased in the shunt + NaHS group compared to that in the shunt group (P<0.01). The present study demonstrated that H2S may play a protective role in volume overload-induced CHF by upregulating protein and mRNA expression of HO-1.
Keywords: hydrogen sulfide, heme oxygenase-1, volume overload
Introduction
Congenital heart disease (CHD) is the most type of common cardiovascular disease of childhood. Left-to-right shunt CHD results in an increase in cardiac volume load. Sustained volume overload induces cardiac hypertrophy and ventricular remodeling, eventually leading to decreased cardiac function, which results in chronic heart failure (CHF); however, the pathogenesis of CHF has not been fully elucidated. Hydrogen sulfide (H2S) affects a wide range of physiological and pathological processes in the cardiovascular system (1). H2S plays an important role in the prevention of the development and occurrence of coronary heart disease and the protection against ischemic myocardial injury (2–4). Exogenous H2S opens KATP channels to reduce myocardial infarct size (5). H2S exerts a protective effect on ischemic myocardium by inhibiting vascular endothelial cell apoptosis and promoting the regeneration of endothelial cells (6). In a previous study (7), we reported that increased myocardial collagen content (particularly type I collagen) in rats with volume overload caused CHF and treatment with sodium hydrosulfide (NaHS), an exogenous H2S donor, resulted in a decrease of myocardial collagen content (particularly type I collagen) in the left-to-right shunt operation group. This suggested that H2S plays a protective role in volume overload-induced ventricular remodeling. However, the mechanism underlying these changes has not been fully elucidated. Carbon monoxide (CO) is another important endogenous signaling molecule. Mammalian tissues continually produce CO as a result of the breakdown of heme by heme oxygenase (HO). HO degrades the pro-oxidant heme to CO, biliverdin and ferrous iron. HO has been reported to exist as its isoenzyme forms, HO-1, -2 and -3. HO-3 is inactive and is not expressed in humans. HO-1 is expressed ubiquitously at low levels and its expression is rapidly induced by heme as well as other stresses, including hypoxia, hyperthermia, metals, oxidized low-density lipoprotein and inflammatory cytokines. By contrast, HO-2 is constitutively expressed and widely distributed in the body, with higher concentrations in the brain and testis (8). HO-1 is upregulated by a host of oxidative stress stimuli in the cardiovascular system (9). The HO-1/CO system is beneficial in the prevention of atherosclerotic lesion formation, protection of ischemic myocardial injury and regulation of blood pressure (10–15). Considering these findings, the issues that should be addressed include whether H2S affects the HO-1/CO system and whether the interaction between H2S and the HO-1/CO system is involved in the regulation of volume overload-induced heart failure. The present study was designed in order to elucidate these issues by investigating the expression of HO-1 in rats with left-to-right shunt and in shunted rats treated with NaHS.
Materials and methods
Animal model of left-to-right shunt
Experiments were conducted in accordance with the Guide to the Care and Use of Experimental Animals issued by the Ministry of Health, the People’s Republic of China. Male Sprague-Dawley rats were provided by the Animal Research Centre of Peking University First Hospital. The rats were housed in plastic cages in a room with a controlled humidity of 40%, a temperature of 22°C and a 12-h light cycle from 6:00 a.m. to 6:00 p.m. The rat model was established by an abdominal aorta-inferior vena cava shunt operation, as previously described by Ocampo et al(16). Briefly, 30 male Sprague-Dawley rats, weighing 120–140 g, were randomly divided into four groups: the shunt group (n=8), the shunt + NaHS group (n=8), the sham group (n=8) and the sham + NaHS group (n=6). Rats in the shunt and shunt + NaHS groups were anesthetized with 0.25% pentobarbital sodium (40 mg/kg, intraperitoneal injection). The abdominal aorta and inferior vena cava were exposed and a bulldog vascular clamp was placed across the aorta, caudal to the left renal artery. The aorta was punctured at the union of the segment, two-thirds caudal to the renal artery and one-third cephalic to the aortic bifurcation, with an 18-gauge disposable needle. The needle was slowly withdrawn and a 9-0 silk thread was used to suture the puncture of the abdominal aortic wall. In the sham and sham + NaHS groups, rats underwent the same experimental protocol as mentioned above, except for the shunt procedure. Rats in the shunt + NaHS and sham + NaHS groups were injected intraperitoneally with NaHS (H2S donor) at 56 μmol/kg/day for 8 weeks, as previously described (17), whereas rats in the shunt and sham groups were injected with the same volume of normal saline (NS).
Measurement of haemodynamic parameters
At 8 weeks after the operation, rats in each group were anesthetized with 25% urethane (0.5 ml/100 g, intraperitoneal injection). After anesthesia, a cannula with a heparinized PP10 in PP50 catheter was inserted into the left ventricle (LV) through the right common carotid artery. The catheter was connected to a pressure transducer. The pressure transducer was connected to a data recording system (BL-420F, BioData Acquisition & Analysis System; TME Technology Co., Ltd., Chengdu, China). The haemodynamic parameters, such as left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), left ventricular peak rate of contraction (LV+dp/dtmax) and left ventricular peak rate of relaxation (LV-dp/dtmax), were measured as previously described (18).
RNA extraction and cDNA synthesis
Total RNA was isolated from frozen LV tissue using the Total RNA Extraction kit (Tiangen Biotech, Co., Ltd., Beijing China; code no. DP419) according to the manufacturer’s protocol and quantified by measuring the absorbance at 260 nm. The quality of the isolated RNA was determined by measuring the 260:280 ratio. Subsequently, first-strand cDNA was synthesized using the First-Strand cDNA Synthesis kit (Tiangen Biotech, Co., Ltd.; code no. KR104) according to the manufacturer’s protocol.
Relative gene expression analysis by real-time PCR
PCR primers were designed using commercial software (Beacon Designer; Bio-Rad Laboratories, Hercules, CA, USA) to produce an amplicon 75–150 bp in length. Primers used in the PCR assays are presented in Table I. Real-time PCR was performed with the ABI Prism 7500 system (Applied Biosystems, Carlsbad, CA, USA), using the Ultra SYBR-Green PCR kit (Beijing CoWin Bioscience Co., Ltd., Beijing, China; code no. CW0956). The thermal cycling conditions included an initial denaturation step at 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec and at 60°C for 60 sec. A melting curve was determined at the end of each cycle to confirm the specificity of the primers and the purity of the PCR product. Results were analyzed using Applied Biosystems 7500 Real-Time PCR System Sequence Detection Software version 1.4 (Applied Biosystems) to obtain CT values (threshold cycles at which a statistically significant increase in detection of SYBR-Green emission intensity occurs). CT values were then normalized to a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) endogenous control to account for variability in RNA concentrations between samples to obtain ΔCT values. To obtain ΔΔCT values, we subtracted the ΔCT value for the control samples from that for the treated samples. The relative quantification value was then calculated as 2−ΔΔCT.
Table I.
Primers used in the study.
| Gene | Nucleotide sequence |
|---|---|
| HO-1 | |
| Sense | 5′-AGA GTT TCC GCC TCC AAC CA-3′ |
| Antisense | 5′-CGG GAC TGG GCT AGT TCA GG-3′ |
| GAPDH | |
| Sense | 5′-CAA GGT CAT CCA TGA CAA CTT TG-3′ |
| Antisense | 5′-GGG CCA TCC ACA GTC TTC TG-3′ |
HO-1, heme oxygenase-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western immunoblot analysis
LV tissue was lysed in a lysis buffer [0.2 ml 1 M Tris-HCl (pH 8.0), 0.3 ml 5 M NaCl, 10 μl 500 mM ethylenediaminetetraacetic acid, 0.1 ml 100 mM phenylmethanesulfonyl fluoride and 10 μl Triton X-100, with water added to 10 ml]. The extracts were clarified by centrifugation at 13,400 x g for 15 min at 4°C. Protein concentrations were determined with the BCA™ Protein Assay kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). Equal amounts of total protein (60 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 15% gel and transferred onto a polyvinylidene difluoride membrane (GE Healthcare UK Ltd., Little Chalfont, UK). The membrane was blocked with 5% (w/v) fat-free milk in TBST [0.05% (v/v) Tween in TBST] at room temperature for 1 h and then probed with rabbit polyclonal antibodies (Abcam Inc., Cambridge, UK) against HO-1 at a 1:500 dilution overnight at 4°C. The membrane was then probed with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2,500; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 2 h at room temperature. The reactions were developed with enhanced chemiluminescence reagents (Beijing TransGen Biotech Co., Ltd., Beijing, China) and the images were obtained by exposure to X-ray films (Kodak Life Science Imaging film, USA). The films were digitized with Bio-Rad Gel Doc XR (Bio-Rad Laboratories) and quantified with Quantity One software (Bio-Rad Laboratories). GAPDH blots were used as a loading control. The bands were normalized to GAPDH controls.
Statistical analysis
Data are expressed as the means ± standard error (SE) and were analyzed by SPSS software, version 16.0 (SPSS Inc., Chicago, IL, USA). For the homogeneity of variance values, a comparison among groups was performed with one-way analysis of variance (ANOVA), followed by the Least Significance Difference test. For some heterogeneity of variance values, comparison among groups was performed with one-way ANOVA followed by Tamhane’s T2 test. P<0.05 was considered to indicate a statistically significant difference.
Results
LV haemodynamic parameters
Eight weeks after the operation, the shunt group exhibited significantly increased LVEDP (24±7 vs. 14±5 mmHg; 1 mmHg= 0.133 kPa, P<0.05) and significantly decreased LVSP and LV±dp/dtmax (101±19 vs. 145±26 mmHg; 3768±321 vs. 5768±432 mmHg/sec; 3219±219 vs. 5312±418 mmHg/sec, all P<0.05), compared to the sham group. In addition, the shunt + NaHS group exhibited significantly decreased LVEDP (19±6 vs. 24±7 mmHg, P<0.05) and significantly increased LVSP and LV±dp/dtmax (121±16 vs. 101±19 mmHg; 4865±254 vs. 3768±321 mmHg/sec; 4138±207 vs. 3219±219 mmHg/sec, all P<0.05), compared to the shunt group. There was no significant difference between the sham and the sham + NaHS groups (Fig. 1).
Figure 1.
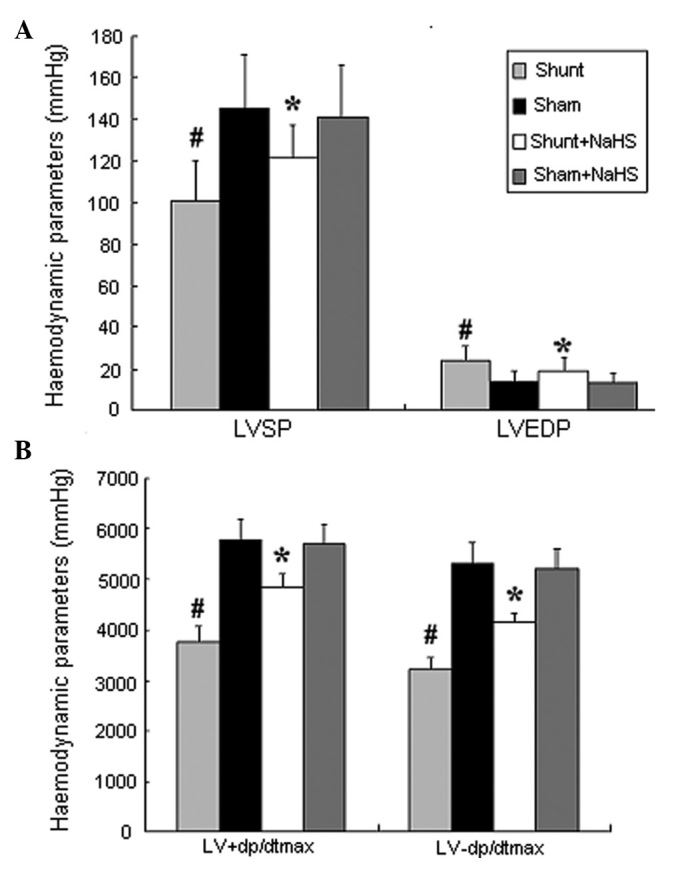
Haemodynamic parameters of rats in the four groups. Values are expressed as means ± standard deviation (SD). #P<0.05 vs. the sham group, *P<0.05 vs. the shunt group. LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; LV±dp/dtmax, left ventricular peak rate of contraction and relaxation; NaHS, sodium hydrosulfide.
HO-1 mRNA expression in the LV
Eight weeks after the operation, HO-1 mRNA expression tended to decrease in the shunt group compared to that in the sham operation group (P>0.05). The shunt + NaHS group exhibited significantly increased HO-1 mRNA expression compared to that in the shunt group (P<0.01). There was no significant difference in HO-1 mRNA expression between the sham and the sham + NaHS groups (Table II).
Table II.
Heme oxygenase-1 mRNA expression in the left ventricle (2−ΔΔCT)a.
| Shunt | Sham | Shunt + NaHS | Sham + NaHS | |
|---|---|---|---|---|
| No. | 8 | 8 | 8 | 6 |
| HO-1 | 1.86±0.29 | 2.05±0.24 | 5.86±0.61b | 2.94±0.63 |
Values are expressed as the mean ± standard error (SE).
P<0.01 compared to the shunt group. HO-1, Heme oxygenase-1; NaHS, sodium hydrosulfide.
Western blot analysis results of HO-1 in the LV
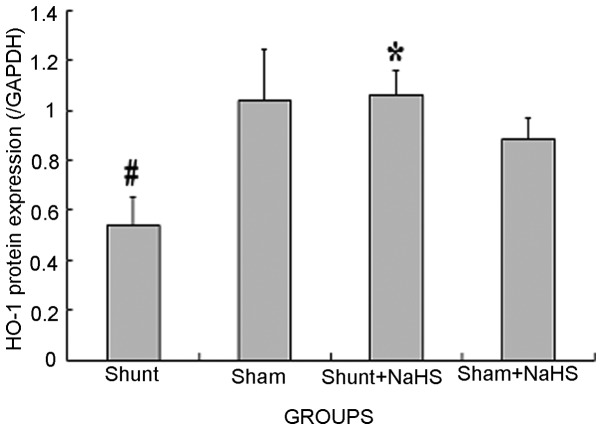
Eight weeks after the operation, HO-1 protein expression was significantly decreased in the shunt group compared to that in the sham operation group (0.54±0.11 vs. 1.04±0.20, P<0.05). HO-1 protein expression was significantly increased in the shunt + NaHS group compared to that in the shunt group (1.06±0.10 vs. 0.54±0.11, P<0.05). There was no significant difference in HO-1 protein expression between the sham and the sham + NaHS groups (Figs. 2 and 3).
Figure 2.
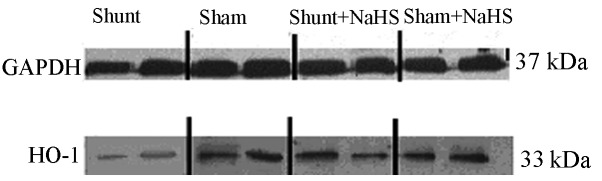
Representative western immunoblots for heme oxygenase-1 (HO-1) in left ventricular (LV) myocardial extracts. SDS-PAGE analysis shows HO-1 protein levels in the LV of rats. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NaHS, sodium hydrosulfide.
Figure 3.
Cardiac protein expression bar graph in the left ventricle (LV) using the western blot analysis method, demonstrating the change in HO-1 protein expression. Values are expressed as means ± standard error (SE). #P<0.05 vs. the sham group, *P<0.05 vs. the shunt group. HO-1, heme oxygenase-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NaHS, sodium hydrosulfide.
Discussion
CHD is one of the most common human birth defects, with an incidence of 6–8/1,000 live births (19). CHF is a common complication of left-to-right shunt CHD and ventricular remodeling is an important pathophysiological mechanism underlying volume overload-induced CHF. With ventricular structural remodeling, cardiac function is compromised, which results in irreversible heart failure. Therefore, it is critical to elucidate the mechanism behind volume overload-induced CHF and perform timely interventions.
Similar to nitric oxide (NO) and CO, which are considered as gaseous transmitters, H2S has also been shown to be a gaseous transmitter which plays an important role in normal physiological processes as well as in the process/progression of several diseases. The four most important mammalian enzymes involved in H2S synthesis are cystathionine-β-synthase (CBS), cystathionine-γ-lyase (cystathionase, CSE) and cysteine aminotransferase (CAT) in conjunction with 3-mercaptopyruvate sulfurtransferase (3-MST) (1). H2S is synthesized from the sulfur-containing amino acid L-cysteine by either CBS or CSE, with pyridoxal 5′-phosphate used as a cofactor. Along with CAT, 3-MST produces H2S using L-cysteine and α-ketoglutarate as substrates. Both enzymes contribute to H2S formation in the brain and vascular endothelium. Recent experimental studies demonstrated that endogenous H2S synthesis was lower in hearts of an arteriovenous fistula-induced CHF model (1). In the present study, 8 weeks after the shunt surgery, there were significant changes in LVSP, LVEDP and LV±dp/dtmax, indicating that H2S may improve cardiac function in volume overload-induced CHF. Furthermore, the results indicated that long-term treatment with NaHS may not affect the left ventricular haemodynamic parameters in sham-operated rats, although it exerted a significant pharmacological effect under pathological conditions (such as CHF). This finding indicated that H2S may protect the heart against CHF; however, its mechanism has not yet been elucidated. Endogenous H2S is produced through the metabolism of terminal waste of sulfur-containing amino acids in the body. H2S may occur as gaseous H2S or NaHS. NaHS in the body may dissociate to sodium ions (Na+) and sulfur hydrogen ions (HS−). HS− combines with internal hydrogen ions (H+) to generate H2S. Therefore, H2S and NaHS are in a type of dynamic balance. NaHS may ensure the stability of H2S concentrations in solution and the majority of intervention experiments on H2S used NaHS solution (17). Therefore, in this study, we used NaHS solution as the H2S donor.
CO is an endogenously derived gas formed from the breakdown of heme by the HO enzyme. Although long considered an insignificant and potentially toxic waste product of heme catabolism, CO is now recognized as an important signaling molecule that regulates numerous cardiovascular functions. Of note, alterations in CO synthesis are associated with several cardiovascular disorders, including atherosclerosis, septic shock, hypertension, metabolic syndrome and ischemia-reperfusion injury; restoration of physiological CO levels exerts a beneficial effect on several of these conditions, suggesting a crucial role for CO in the maintenance of cardiovascular homeostasis. CO causes relaxation of numerous vascular tissues and regulates blood pressure by activating soluble guanylate cyclase in vascular smooth muscle, leading to the production of cyclic guanine monophosphate (20). Considerable evidence supports a protective role for the HO-1/CO system against coronary artery ischemia-reperfusion injury. Pharmacological induction of HO-1 expression significantly reduces infarct size and the incidence of reperfusion arrhythmia following myocardial ischemia-reperfusion, whereas cardiac tissue damage is exacerbated by HO inhibitors (21–25). The induction of HO-1 may also have therapeutic benefits during CHF. Upregulation of HO-1 expression during heart failure serves to mitigate pathological LV remodeling and reduce myocardial hypertrophy, oxidative stress and inflammatory activation. HO-1 overexpression promotes neovascularization and ameliorates apoptosis in the heart failure model (26).
H2S and CO are involved in the regulation of several physiological as well as pathological processes. They have similar biological functions, as well as competitive and antagonist actions. For example, H2S administered to rats with experimentally induced hypoxic pulmonary hypertension leads to an increase in plasma CO concentrations and an increase in pulmonary artery expression of HO-1 protein and mRNA (27). In the present study, 8 weeks after the shunt surgery, the expression of LV HO-1 mRNA and protein was significantly increased in the shunt + NaHS group compared to those in the shunt group. No difference in HO-1 expression was observed between the sham group and the sham + NaHS group. These findings demonstrated that H2S may play a protective role in volume overload-induced heart failure by upregulating protein and mRNA expression of HO-1.
The regulatory effect of NaHS on the HO-1/CO system pathway has not been fully elucidated. A previous study by Oh et al(28) demonstrated that an H2S solution, prepared by bubbling pure H2S gas and NaHS, dose-dependently induced HO-1 expression through the activation of the extracellular signal-regulated kinase. However, it has also been shown that increased expression of HO-1 is not necessarily accompanied by increased HO activity, which should also be measured (8). Therefore, HO-1 activity requires further investigation.
In conclusion, the present study demonstrated that H2S upregulates mRNA and protein expression of HO-1 in rats with volume overload-induced CHF caused by left-to-right shunt, which may be one of the mechanisms by which H2S attenuates volume overload-induced heart failure. However, the specific underlying mechanisms and interaction with NO require further investigation.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (no. 30872787), Beijing outstanding talents training program (20081D0303200107) and Science Foundation for High-Level Medical Talents of Beijing Health System (2011).
References
- 1.Liu YH, Lu M, Hu LF, Wong PT, Webb GD, Bian JS. Hydrogen sulfide in the mammalian cardiovascular system. Antioxid Redox Signal. 2012;17:141–185. doi: 10.1089/ars.2011.4005. [DOI] [PubMed] [Google Scholar]
- 2.Ji Y, Pang QF, Xu G, Wang L, Wang JK, Zeng YM. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur J Pharmacol. 2008;587:1–7. doi: 10.1016/j.ejphar.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 3.Bliksoen M, Kaljusto ML, Vaage J, Stenslokken KO. Effects of hydrogen sulphide on ischaemia-reperfusion injury and ischaemic preconditioning in the isolated, perfused rat heart. Eur J Cardiothorac Surg. 2008;34:344–349. doi: 10.1016/j.ejcts.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Muellner MK, Schreier SM, Laggner H, et al. Hydrogen sulfide destroys lipid hydroperoxides in oxidized LDL. Biochem J. 2009;420:277–281. doi: 10.1042/BJ20082421. [DOI] [PubMed] [Google Scholar]
- 5.Johansen D, Ytrehus K, Baxter GF. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury - Evidence for a role of KATP channels. Basic Res Cardiol. 2006;101:53–60. doi: 10.1007/s00395-005-0569-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhu YZ, Wang ZJ, Ho P, et al. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol. 2007;102:261–268. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]
- 7.Li XH, Zhang CY, Zhang T. Sodium hydrosulfide improves cardiac functions and structures in rats with chronic heart failure. Zhonghua Yi Xue Za Zhi. 2011;91:3044–3049. (In Chinese). [PubMed] [Google Scholar]
- 8.Peterson SJ, Frishman WH, Abraham NG. Targeting heme oxygenase: therapeutic implications for diseases of the cardiovascular system. Cardiol Rev. 2009;17:99–111. doi: 10.1097/CRD.0b013e31819d813a. [DOI] [PubMed] [Google Scholar]
- 9.Immenschuh S, Schroder H. Heme oxygenase-1 and cardiovascular disease. Histol Histopathol. 2006;21:679–685. doi: 10.14670/HH-21.679. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RA, Colombari E, Columbari DS, Lavesa M, Talman WT, Nasjletti A. Role of endogenous carbon monoxide in central regulation of arterial pressure. Hypertension. 1997;30:962–967. doi: 10.1161/01.hyp.30.4.962. [DOI] [PubMed] [Google Scholar]
- 11.Ndisang JF, Zhao W, Wang R. Selective regulation of blood pressure by heme oxygenase-1 in hypertension. Hypertension. 2002;40:315–321. doi: 10.1161/01.hyp.0000028488.71068.16. [DOI] [PubMed] [Google Scholar]
- 12.Otterbein LE, Zuckerbraun BS, Haga M, et al. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 13.Yet SF, Layne MD, Liu X, Chen YH, Ith B, Sibinga NE, Perrella MA. Absence of heme oxygenase-1 exacerbates atherosclerosis lesion formation and vascular remodeling. FASEB J. 2003;17:1759–1761. doi: 10.1096/fj.03-0187fje. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto H, Ohno M, Ayabe S, et al. Carbon monoxide protects against cardiac ischemia - reperfusion injury in vivo via MAPK and Akt - eNOS pathways. Arterioscler Thromb Vasc Biol. 2004;24:1848–1853. doi: 10.1161/01.ATV.0000142364.85911.0e. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Pachori AS, Ward CA, et al. Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. FASEB J. 2006;20:207–216. doi: 10.1096/fj.05-4435com. [DOI] [PubMed] [Google Scholar]
- 16.Ocampo C, Ingram P, Ilbawi M, Arcilla R, Gupta M. Revisiting the surgical creation of volume load by aorto-caval shunt in rats. Mol Cell Biochem. 2003;251:139–143. [PubMed] [Google Scholar]
- 17.Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–27. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Wang Q, Guo W, Zhu YZ. Hydrogen sulfide attenuates cardiac dysfunction in a rat model of heart failure: a mechanism through cardiac mitochondrial protection. Biosci Rep. 2011;31:87–98. doi: 10.1042/BSR20100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadowski SL. Congenital cardiac disease in the newborn infant: past, present, and future. Crit Care Nurs Clin North Am. 2009;21:37–48. doi: 10.1016/j.ccell.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Ndisang JF, Tabien HE, Wang R. Carbon monoxide and hypertension. J Hypertens. 2004;22:1057–1074. doi: 10.1097/00004872-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Hangaishi M, Ishizaka N, Aizawa T, et al. Induction of heme oxygenase-1 can act protectively against cardiac ischemia/reperfusion in vivo. Biochem Biophys Res Commun. 2000;279:582–588. doi: 10.1006/bbrc.2000.3973. [DOI] [PubMed] [Google Scholar]
- 22.Masini E, Vannacci A, Marzocca C, et al. Heme oxygenase-1 and the ischemia-reperfusion injury in the rat heart. Exp Biol Med (Maywood) 2003;228:546–549. doi: 10.1177/15353702-0322805-25. [DOI] [PubMed] [Google Scholar]
- 23.Guo Y, Stein AB, Wu WJ, et al. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1649–H1653. doi: 10.1152/ajpheart.00971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.L’Abbate A, Neglia D, Vecoli C, et al. Beneficial effect of heme oxygenase-1 expression on myocardial ischemia-reperfusion involves an increase in adiponectin in mildly diabetic rats. Am J Physiol Heart Circ Physiol. 2007;293:H3532–H3541. doi: 10.1152/ajpheart.00826.2007. [DOI] [PubMed] [Google Scholar]
- 25.Varadi J, Lekli I, Juhasz B, et al. Beneficial effects of carbon monoxide-releasing molecules on post-ischemic myocardial recovery. Life Sci. 2007;80:1619–1626. doi: 10.1016/j.lfs.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Hamid T, Keith RJ, et al. Cardioprotective and anti-apoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121:1912–1925. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qingyou Z, Junbao D, Weijin Z, Hui Y, Chaoshu T, Chunyu Z. Impact of hydrogen sulfide on carbon monoxide/heme oxygenase pathway in the pathogenesis of hypoxic pulmonary hypertension. Biochem Biophys Res Commun. 2004;317:30–37. doi: 10.1016/j.bbrc.2004.02.176. [DOI] [PubMed] [Google Scholar]
- 28.Oh GS, Pae HO, Lee BS, et al. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]