Abstract
There are trace amounts of heavy metals in cosmetics. Heavy metals such as mercury (Hg), which is added to skin-whitening cosmetics, may cause acute or chronic damage to human cells. The aim of this study was to investigate the cytotoxicity of mercury chloride (HgCl2) to human keratinocytes. The keratinocytes were treated with various concentrations of HgCl2 and the cell survival fractions were found to be 38.08, 17.59, 12.76, 3.29 and 0.77% when the cells were treated with 0.25, 0.5, 0.75, 1 and 1.5 μM of HgCl2, respectively. Moreover, we observed that the greatest damage was to the cell membrane. The metallothionein (MT) protein expression was also investigated. MT expression levels increased with increasing concentrations of HgCl2. The results indicated that MT protects the keratinocytes against HgCl2-induced toxicity.
Keywords: cytotoxicity, mercury chloride, keratinocytes, metallothionein
Introduction
There are trace amounts of heavy metals in cosmetics. Heavy metals such as mercury (Hg), which is added to skin-whitening cosmetics, may cause acute or chronic damage to human cells. Hg, a divalent metal with no known biological function, may cause several deleterious effects in adults (1,2), as well as in developing organisms (3,4), which primarily involve the central nervous system (5–7) and the kidneys (1,8,9). Young animals seem to be more sensitive to Hg toxicity than adults, particularly during the first days following birth. Hg is also a widespread environmental and industrial pollutant that induces severe adverse effects in humans as well as the environment (10). Its carcinogenic activity has been well-documented. Hg is also known to alter the intracellular redox homeostasis (11,12), which is recognized as a factor that determines cell fate (13). The outcome of cells exposed to Hg-containing compounds depends on the chemical characteristics of the compound, as well as on its dosage, accounting for the various results reported in the literature, ranging from improved cell survival to apoptosis and necrosis.
Keratinocytes have long been considered the structural backbone of the epidermis; however, there is increasing evidence that they play an active role in the pathogenesis of skin damage by heavy metals (14). Available histopathological (15) and cytotoxicological (16–18) studies describing keratinocyte damage by mercury chloride (HgCl2) are currently limited. This underlines the importance of investigating the direct cytotoxic effects of the metals on keratinocytes, as well as intracellular damage, for which available data are limited.
Metallothioneins (MTs) are ubiquitous, low-molecular weight proteins, rich in cysteine residues. Their high content of sulfhydrilic amino acids (∼30%) gives these proteins unique metal-binding properties (19,20). Factors such as exposure to toxic or essential metals (3,21–23), stress (24,25), radiation (26) and other agents (27,28), promote the synthesis of these molecules (29). With respect to their biological functions and due to the metal affinity of their sulfhydryl groups, it is believed that MTs possess antioxidant properties (26,30), are involved in the homeostasis of essential metals such as zinc (Zn) and copper (Cu) (20,29) and act as detoxifying agents from metal ions (20,31,32).
In this study, we investigated the cytotoxicity of HgCl2 to human keratinocytes, using human keratinocyte-derived HaCaT cells as an experimental model. In addition, we focused on HgCl2-induced HaCaT cell damage and examined the expression of MTs.
Materials and methods
Materials
Human keratinocyte-derived (HaCaT) cells were obtained from the Food Industry Research and Development Institute (Hsinchu, Taiwan). Dulbecco’s modified Eagle’s medium (DMEM), heat-inactivated fetal calf serum (FCS), penicillin-streptomycin solution and trypsin-EDTA solution were purchased from Life Technologies Corporation (Carlsbad, CA, USA). Sterile dimethylsulfoxide (DMSO) 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and HgCl2 were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
HaCaT cells were grown in DMEM supplemented with heat-inactivated FCS (10%; v/v), streptomycin (100 U/ml) and penicillin (0.1 mg/ml), in a humidified atmosphere of 5% CO2 at 37°C. The culture medium was changed three times a week. The cells were subcultured following trypsinization and seeded in 6-well plate at a density of 1×105 cells per cm2.
Cells treated with HgCl2
The keratinocytes were treated with HgCl2 (0.25–1.5 μM) at 37°C for 24 h. When the non-treated control cells were grown confluently, the cell groups were prepared for cell viability assay or MT western blot analysis.
MTT assay
The cell viability was monitored following treatment with various concentrations of HgCl2. MTT was used to quantify the metabolically active living cells. Mitochondrial dehydrogenases metabolize MTT to a purple formazan dye, which was measured photometrically at 570 nm using a spectrophotometer (33).
Western blot analysis for MT protein expression
Cell homogenates were prepared by sonication of cells in 600 μl of ice-cold lysis buffer, containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.02% sodium azide, 100 μg/ml PMSF, 1 μg/ml aprotinin and 1% NP-40. Homogenates were clarified by centrifugation at 20,000 × g for 45 min at 4°C. Total protein concentration was determined using the BCA (Bio-Rad, Hercules, CA, USA) assay. Samples (50 μg of total protein) from HaCaT cells treated for 24 h with various concentrations of HgCl2 were analyzed for human MT proteins, using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Laemmli, 1970) in 10–20% gradient gels. Proteins were electrophoretically transferred to nitrocellulose membranes. The resulting membranes were incubated in 2.5% glutaraldehyde for 1 h and then washed 3 times for 5 min in phosphate buffer (8.1 mM Na2HPO4, 1.2 mM KH2PO4, 2.7 mM KCl, pH 7.4). Monoethanolamine (50 mM) was added to the third wash solution to quench residual glutaraldehyde reactivity. MT proteins were detected by Immun-Star Chemiluminescent Protein Detection Systems (Bio-Rad). A monoclonal antibody to polymerized equine renal MT (Dako, Carpinteria, CA, USA) was used for immunodetection.
Statistical analysis
Means ± standard error (SE) were calculated in triplicate. A statistical significance between the groups was determined by the Student’s t-test. P<0.05 was considered to indicate a statistically significant difference between the two groups.
Results
Cell survival fractions of HaCaT cells treated with HgCl2 at various concentrations
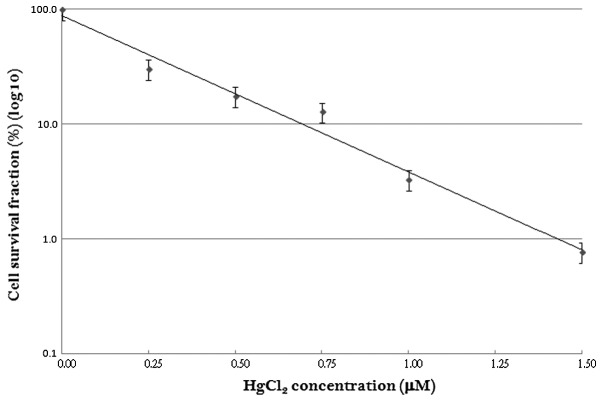
Comparison of cell survival fractions in HaCaT cells treated with HgCl2 at various concentrations from 0.25 to 1.5 μM is shown in Fig. 1. The cell survival fraction was 38.08% when the keratinocytes were treated with 0.25 μM of HgCl2. The cell survival fractions were 17.59, 12.76, 3.29 and 0.77%, when the keratinocytes were treated with 0.5, 0.75, 1 and 1.5 μM of HgCl2, respectively. For each concentration investigated, a linear characteristic concentration-response curve was observed, with decreased cell survival at increasing concentrations of HgCl2 on a semi-log scale.
Figure 1.
Cell survival fractions of HaCaT cells treated with HgCl2 at different concentrations. The cell survival fractions were 38.08, 17.59, 12.76, 3.29 and 0.77% when the keratinocytes were treated with 0.25, 0.5, 0.75, 1 and 1.5 μM of HgCl2, respectively.
Effect of HgCl2 on HaCaT cell morphology
Keratinocytes were treated with HgCl2 for 24 h or left untreated. The HaCaT cell morphology is shown in Fig. 2. The cell membrane of untreated cells is clear and intact (Fig. 2A), whereas that of HgCl2-treated cells is unclear and interrupted (Fig. 2B).
Figure 2.
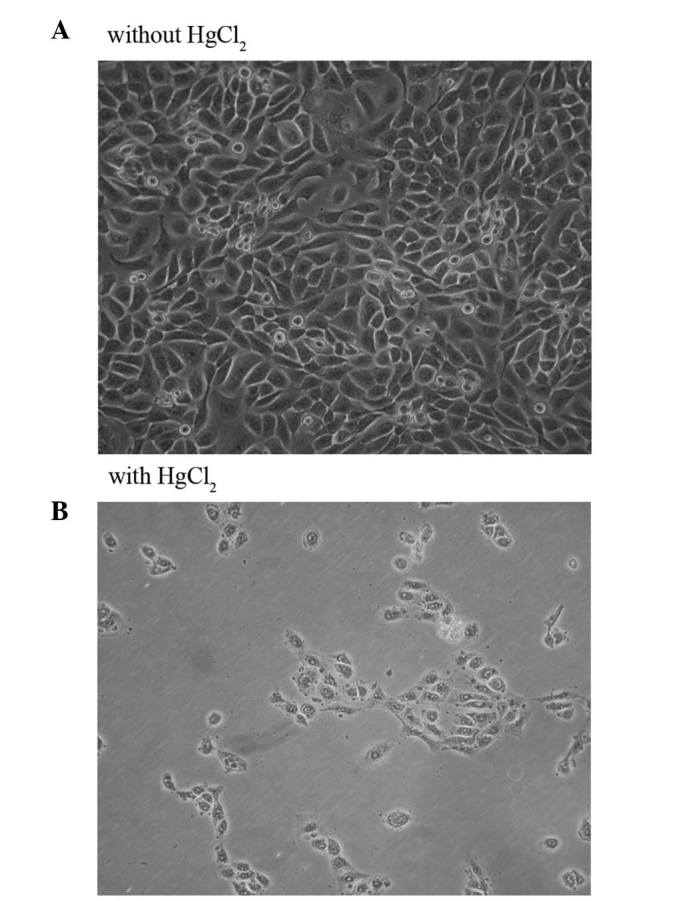
Morphology of HaCaT cells. (A) Control group of HaCaT cells (not treated with HgCl2). (B) HaCaT cells treated with 1.5 μM of HgCl2 for 24 h (magnification, ×40).
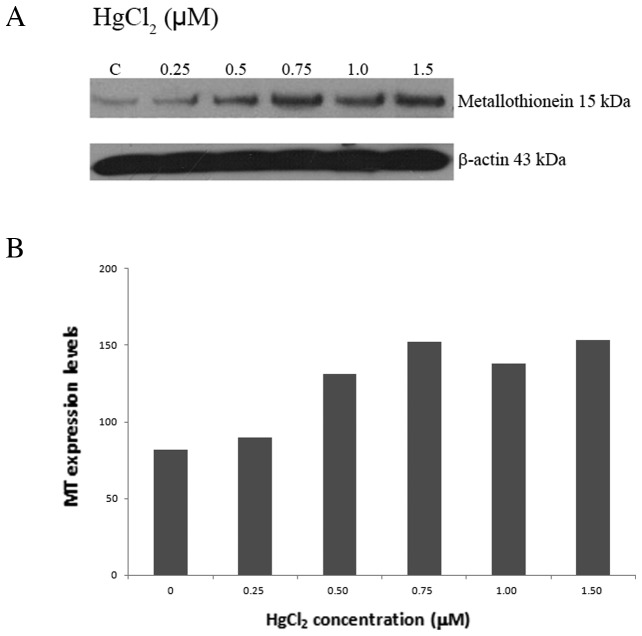
Effect of HgCl2 on MT expression
MT expression levels in HaCaT cells treated with various concentrations of HgCl2 are presented in Fig. 3. MT expression levels increased significantly with the increase in the concentrations of HgCl2.
Figure 3.
Metallothionein (MT) expression in HaCaT cells treated with HgCl2. (A) Total cellular protein (50 μg) from HaCaT cells treated with 0.25–1.5 μM of HgCl2 was assessed by western blot analysis for MT expression. C, untreated cells; 0.25, 0.5, 0.75, 1.0 and 1.5, cells treated with 0.25, 0.5, 0.75, 1.0 and 1.5 μM of HgCl2, respectively. Constitutive expression of β-actin in HaCaT cells demonstrated the overall protein and quality in cell lysates. (B) Quantification of the expression levels of MT in HaCaT cells treated with 0.25–1.5 μM of HgCl2 and in untreated cells.
Discussion
The purpose of this study was to assess the cytotoxicity of HgCl2 to the keratinocytes, as well as MT expression in HgCl2-treated keratinocytes. The results demonstrated that exposure of HaCaT cells to HgCl2 resulted in dose-dependent cell death and distinct cell membrane damage. Reports of Hg poisoning due to exposure to skin-whitening creams, ointments and soaps have increased significantly over the past few years. Furthermore, since people with lighter skin tone may represent a higher status in certain cultures, skin-whitening cosmetics are widely used by women to enhance their appeal (34–36). Otto et al(36) detected high Hg concentrations in the blood and urine of Balkan refugees of varying ages who had been exposed to a Hg-based skin-bleaching ointment.
We have demonstrated that exposure of keratinocytes to HgCl2 resulted in cell membrane damage. Picoli et al(37) also investigated the effect of HgCl2 on gap junction intercellular communication (GJIC) in cultured human keratinocytes. They demonstrated that subcytotoxic concentrations of HgCl2, as low as 10 nM, may cause inhibition of the GJIC. In addition, they demonstrated that HgCl2-treated keratinocytes exhibited a decrease in free thiols and accumulation of mitochondria-derived reactive oxygen species, albeit no effect on the respiratory chain activity was observed.
This study has demonstrated that MT expression may be induced by HgCl2 in HaCaT cells. Kramer et al(38) demonstrated that MT may be induced by Hg+2 in neuronal cells and induced MT decreases the rate of metal binding to other structures, providing protection against metal toxicity (39). Apart from Hg, MT also plays a role in the homeostasis of essential metals such as Zn and Cu, the detoxication of toxic metals such as Cadmium (Cd) and protection against oxidative stress (40–42). Richards et al(43) and McCormick et al(44) demonstrated that plasma zinc concentrations were related to MT expression, further suggesting an association with cellular zinc homeostasis. Ogra et al(41) demonstrated that cell viability was significantly decreased in MT-null cells compared to wild-type cells by Cu(I)-specific chelator treatment (41). They also showed that MT expression levels were increased by Cu(I)-specific chelator treatment in wild-type cells. Thus, MT was induced under Cu-deficient conditions, in order to maintain the activities of intracellular cuproenzymes, such as cytochrome c oxidase and Cu/Zinc superoxidase dismutase. Urani et al(42) showed that MT expression was upregulated following exposure to CdCl2, with a saturation curve at 48 as well as 72 h. High levels of MT possibly confer an acquired tolerance to stress and protection against cell injury, as demonstrated by the low cytotoxicity values.
In conclusion, our results demonstrated that exposure of HaCaT cells to HgCl2 resulted in significant dose-dependent cell death and cell membrane damage. Moreover, MT expression may be induced by HgCl2 in HaCaT cells. This suggests that MT protects the keratinocytes against HgCl2-induced toxicity.
Acknowledgments
This study was supported by grant no. NSC 98-2314-B-238-001 from the National Science Council and grant no. VIT-98-CM-01 from Vanung University, Taiwan.
References
- 1.Emanuelli T, Rocha JB, Pereira ME, Porciúncula LO, Morsch VM, Martins AF, Souza DO. Effect of mercuric chloride intoxicationand dimercaprol treatment on δ-aminolevulinate dehydratase from brain, liver and kidney of adult mice. Pharmacol Toxicol. 1996;79:136–143. doi: 10.1111/j.1600-0773.1996.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 2.Shigematsu J, Yasuda T, Goto Y, Tanaka K, Tobimatsu S, Kato M. Recovery of brain dysfunction after methylmercury exposure in rats. J Neurol Sci. 2000;182:61–68. doi: 10.1016/s0022-510x(00)00450-0. [DOI] [PubMed] [Google Scholar]
- 3.Peixoto NC, Roza T, Flores EM, Pereira ME. Effects of zinc and cadmium on HgCl2-δ-ALA-D inhibition and Hg levels in tissues of suckling rats. Toxicol Lett. 2003;146:17–25. doi: 10.1016/j.toxlet.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Roza T, Peixoto NC, Welter A, Flores EM, Pereira ME. 2,3-Dimercapto-1-propanol does not alter the porphobilinogen synthase inhibition but decreases the mercury content in liver and kidney of suckling rats exposed to HgCl2. Basic Clin Pharmacol Toxicol. 2005;96:302–308. doi: 10.1111/j.1742-7843.2005.pto960405.x. [DOI] [PubMed] [Google Scholar]
- 5.Pereira ME, Morsch VM, Christofari RS, Rocha JB. Methyl mercury exposure during post-natal brain growth alters behavioral response to SCH 23390 in young rats. Bull Environ Contam Toxicol. 1999;63:256–262. doi: 10.1007/s001289900974. [DOI] [PubMed] [Google Scholar]
- 6.Rocha JB, Rocha LK, Emanuelli T, Pereira ME. Effect of mercuric chloride and lead acetate treatment during the second stage of rapid post-natal brain growth on the behavioral response to chlorpromazine and on δ-ALA-D activity in weaning rats. Toxicol Lett. 2001;125:143–150. doi: 10.1016/s0378-4274(01)00435-0. [DOI] [PubMed] [Google Scholar]
- 7.Peixoto NC, Roza T, Morsch VM, Pereira ME. Behavioral alterations induced by HgCl2 depend on the postnatal period of exposure. Int J Devl Neurosci. 2007;25:39–46. doi: 10.1016/j.ijdevneu.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Magos L, Webb M, Butler WH. The effect of cadmium pretreatment on the nephrotoxic action and kidney uptake of mercury in male and female rats. Br J Exp Pathol. 1974;55:589–594. [PMC free article] [PubMed] [Google Scholar]
- 9.Peixoto NC, Pereira ME. Effectiveness of ZnCl2in protecting against nephrotoxicity induced by HgCl2in newborn rats. Ecotoxicol Environ Saf. 2007;66:441–446. doi: 10.1016/j.ecoenv.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 11.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 12.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 13.D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 14.Luger TA, Schwarz T, Krutmann J, Kirnbauer R, Neuner P, Kock A, Urbanski A, Borth W, Schauer E. Interleukin-6 is produced by epidermal cells and plays an important role in the activation of human T-lymphocytes and natural killer cells. Ann NY Acad Sci. 1989;557:405–414. doi: 10.1111/j.1749-6632.1989.tb24033.x. [DOI] [PubMed] [Google Scholar]
- 15.Willis CM, Stephens CJ, Wilkinson JD. Epidermal damage induced by irritants in man: a light and electron microscopic study. J Invest Dermatol. 1989;93:695–699. doi: 10.1111/1523-1747.ep12319895. [DOI] [PubMed] [Google Scholar]
- 16.Picardo M, Zompetta C, De Luca C, Cristaudo A, Cannistraci C, Faggioni A, Santucci B. Nickel-keratinocyte interaction: a possible role in sensitization. Br J Dermatol. 1990;122:729–735. doi: 10.1111/j.1365-2133.1990.tb06259.x. [DOI] [PubMed] [Google Scholar]
- 17.Little MC, Gawkrodger DJ, MacNeil S. Chromium- and nickel-induced cytotoxicity in normal and transformed human keratinocytes: an investigation of pharmacological approaches to the prevention of Cr(VI)-induced cytotoxicity. Br J Dermatol. 1996;134:199–207. [PubMed] [Google Scholar]
- 18.Brosin A, Wolf V, Mattheus A, Heise H. Use of XTT-assay to assess the cytotoxicity of different surfactants and metal salts in human keratinocytes (HaCaT): Afeasible method for in vitro testing of skin irritants. Acta Derm Venereol. 1997;77:26–28. doi: 10.2340/0001555577026028. [DOI] [PubMed] [Google Scholar]
- 19.Chan J, Huang Z, Merrifield ME, Salgado MT, Stillman MJ. Studies of metal binding reactions in metallothioneins by spectroscopic, molecular biology, and molecular modelling techniques. Coord Chem Rev. 2002;233–234:319–339. [Google Scholar]
- 20.Dabrio M, Rodríguez AR, Bordin G, Bebianno MJ, De Ley M, Sestáková I, Vasák M, Nordberg M. Recent developments in quantification methods for metallothionein. J Inorg Biochem. 2002;88:123–134. doi: 10.1016/s0162-0134(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 21.Goering PL, Fowler BA. Metal constitution of metallothionein influences inhibition of δ-aminolaevulinic acid dehydratase (porphobilinogen synthase) by lead. Biochem J. 1987;245:339–345. doi: 10.1042/bj2450339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedersen SN, Pedersen KL, Hojrup P, Knudsen J, Depledge MH. Induction and identification of cadmium-, zinc- and copper-metallothioneins in the shore crab Carcinus maenas (L.) Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;120:251–259. doi: 10.1016/s0742-8413(98)10003-8. [DOI] [PubMed] [Google Scholar]
- 23.Bebianno MJ, Langston WJ. Metallothionein induction in mussels exposed to a metal mixture. In: Klaassen CD, editor. Metallothionein IV (Advances in Life Sciences) Birkhäuser Verlag; Basel, Switzerland: 2009. pp. 187–194. [Google Scholar]
- 24.Kondoh M, Tsukahara R, Kuronaga M, Higashimoto M, Takiguchi M, Sato M. Enhancement of MT synthesis by leptin in fasted mice. Life Sci. 2002;71:2425–2433. doi: 10.1016/s0024-3205(02)02022-2. [DOI] [PubMed] [Google Scholar]
- 25.Kondoh M, Kamada K, Kuronaga M, Higashimoto M, Takiguchi M, Watanabe Y, Sato M. Antioxidant property of metallothionein in fasted mice. Toxicol Lett. 2003;143:301–306. doi: 10.1016/s0378-4274(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 26.Cai L, Satoh M, Tohyama C, Cherian MG. Metallothionein in radiation exposure: its induction and protective role. Toxicology. 1999;132:85–98. doi: 10.1016/s0300-483x(98)00150-4. [DOI] [PubMed] [Google Scholar]
- 27.Rojas P, Cerutis DR, Happe HK, Murrin LC, Hao R, Pfeiffer RF, Ebadi M. 6-Hydroxydopamine-mediated induction of rat brain metallothionein I mRNA. Neurotoxicology. 1996;17:323–334. [PubMed] [Google Scholar]
- 28.Theocharis SE, Margeli AP, Skaltsas SD, Spiliopoulou CA, Koutselinis AS. Induction of metallothionein in the liver of carbon tetrachloride intoxicated rats: an immunohistochemical study. Toxicology. 2001;161:129–138. doi: 10.1016/s0300-483x(01)00340-7. [DOI] [PubMed] [Google Scholar]
- 29.Dunn MA, Blalock TL, Cousins RJ. Metallothionein. Proc Soc Exp Biol Med. 1987;185:107–119. doi: 10.3181/00379727-185-42525a. [DOI] [PubMed] [Google Scholar]
- 30.Cai L, Cherian MG. Zinc-metallothionein protects from DNA damage induced by radiation better than glutathione and copper- or cadmium-metallothioneins. Toxicol Lett. 2003;136:193–198. doi: 10.1016/s0378-4274(02)00359-4. [DOI] [PubMed] [Google Scholar]
- 31.Stillman MJ. Metallothioneins. Coord Chem Rev. 1995;144:461–511. [Google Scholar]
- 32.Yoshida M, Satoh M, Shimada A, Yasutake A, Sumi Y, Tohyama C. Pulmonary toxicity caused by acute exposure to mercury vapor is enhanced in metallothionein-null mice. Life Sci. 1999;64:1861–1867. doi: 10.1016/s0024-3205(99)00129-0. [DOI] [PubMed] [Google Scholar]
- 33.Green LM, Reade JL, Ware CF. Rapid colorimetric assay for cell viability: application to the quantitation of cytotoxic and growth inhibitory lymphokines. J Immunol Methods. 1984;70:257–268. doi: 10.1016/0022-1759(84)90190-x. [DOI] [PubMed] [Google Scholar]
- 34.Jovanovic S, Maisner V, Horras-Hun G, Gabrio T, Schwenk M. Poisoning of a family by a mercury-containing ointment. Gesundheitswesen. 1997;59:405–408. (In German). [PubMed] [Google Scholar]
- 35.Weldon MM, Smolinski MS, Maroufi A, Hasty BW, Gilliss DL, Boulanger LL, Balluz LS, Dutton RJ. Mercury poisoning associated with a Mexican beauty cream. West J Med. 2000;173:15–18. doi: 10.1136/ewjm.173.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otto M, Ahlemeyer C, Tasche H, von Mehlendahl KE. Endemic mercury burden caused by a bleaching ointment in Balkan refugees. Gesundheitswesen. 1994;56:686–689. (In German). [PubMed] [Google Scholar]
- 37.Piccoli C, D’Aprile A, Scrima R, Ambrosi L, Zefferino R, Capitanio N. Subcytoxic mercury chloride inhibits gap junction intercellular communication by a redox- and phosphorylation-mediated mechanism. Free Radical Bio Med. 2012;52:916–927. doi: 10.1016/j.freeradbiomed.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 38.Kramer KK, Zoelle JT, Klaassen CD. Induction of metallothionein mRNA and protein in primary murine neuron cultures. Toxicol Appl Pharmacol. 1996;141:1–7. doi: 10.1006/taap.1996.0253. [DOI] [PubMed] [Google Scholar]
- 39.Cherian MG, Nordberg M. Cellular adaptation in metal toxicology and metallothionein. Toxicology. 1983;28:1–15. doi: 10.1016/0300-483x(83)90101-4. [DOI] [PubMed] [Google Scholar]
- 40.Davis SR, Cousins RJ. Metallothionein expression in animals: a physiological perspective on function. J Nutr. 2000;130:1085–1088. doi: 10.1093/jn/130.5.1085. [DOI] [PubMed] [Google Scholar]
- 41.Ogra Y, Aoyama M, Suzuki KT. Protective role of metallothionein against copper depletion. Arch Biochem Biophys. 2006;451:112–118. doi: 10.1016/j.abb.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Urani CM, Melchioretto P, Canevali C, Morazzoni F, Gribaldo L. Metallothionein and hsp70 expression in HepG2 cells after prolonged cadmium exposure. Toxicol In Vitro. 2007;21:314–319. doi: 10.1016/j.tiv.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Richards MP, Cousins RJ. Mammalian zinc homeostasis: requirement for RNA and metallothionein synthesis. Biochem Biophys Res Commun. 1975;64:1215–1223. doi: 10.1016/0006-291x(75)90822-0. [DOI] [PubMed] [Google Scholar]
- 44.McCormick CC, Menard MP, Cousins RJ. Induction of hepatic metallothionein by feeding zinc to rats of depleted zinc status. Am J Physiol. 1981;240:E414–E421. doi: 10.1152/ajpendo.1981.240.4.E414. [DOI] [PubMed] [Google Scholar]