Abstract
Rhubarb is often used in Chinese herbal medicine for the treatment of systemic inflammatory response syndrome (SIRS). Emodin is the main active constituent of rhubarb. This study was performed to investigate the in vitro effects of emodin and dexamethasone on peritoneal macrophage (pMΦ) phagocytosis and the expression of intercellular adhesion molecule-3 (ICAM-3). A total of 40 Sprague-Dawley (SD) rats were randomly divided into sham surgery (n=10) and model groups (n=30). After 24 h, pMΦs were harvested and the model group was randomly divided into three subgroups (n=10 rats/subgroup): the 5 μg/ml emodin, 0.1 μmol/ml dexamethasone and control groups. The drugs were administered following macrophage (MΦ) adhesion for 24 h. pMΦ phagocytosis was significantly increased in the emodin group compared to that in the control group. Moreover, pMΦ phagocytosis was significantly increased in the emodin group compared to that in the dexamethasone group. The expression of ICAM-3 was significantly increased in the emodin group compared to that in the control group. The expression of ICAM-3 was significantly increased in the emodin group compared to that in the dexamethasone group. The expression of ICAM-3 was significantly increased in the emodin and dexamethasone groups compared to that in the control group. pMΦ phagocytosis and ICAM-3 expression were significantly increased following emodin treatment compared to those in the control and dexamethasone groups, indicating that emodin may enhance pMΦ phagocytosis and apoptotic cell clearance by altering ICAM-3 expression.
Keywords: emodin, severe acute pancreatitis, macrophage, intercellular adhesion molecule-3
Introduction
Acute pancreatitis is a common cause of clinical acute abdomen. Approximately 20–25% of patients with acute pancreatitis develop severe acute pancreatitis (SAP), which tends to be complicated by systemic inflammatory response syndrome (SIRS), with a high mortality rate >30% (1–3). SIRS is an early manifestation of multiple organ dysfunction syndrome and multiple organ failure.
It was previously reported that the polymorphonuclear neutrophil (PMN) life cycle is prolonged and apoptosis is delayed during infection, trauma and other types of stress, which promotes inflammatory reactions leading to organ injury (4–9). Inflammatory reactions may become more severe, unless apoptotic PMNs are cleared. Therefore, delayed PMN apoptosis and insufficient phagocytosis of apoptotic cells may enhance inflammation. The majority of apoptotic cells in vivo are cleared by macrophages (MΦs). MΦs identify, adhere to and phagocytize apoptotic PMNs to inhibit inflammatory reactions and promote inflammation absorption (10).
Intercellular adhesion molecule-3 (ICAM-3) is involved in cell adhesion and signal transduction (11,12). ICAM-3 is mainly expressed by leukocytes and highly expressed by lymphocytes, monocytes and neutrophilic granulocytes. ICAM-3 on apoptotic cells binds MΦ CD14 via bridging molecules to induce Ca2+ flow and phosphatidylserine externalization and promote the clearance of apoptotic cells (13).
The present study established a rat model of SAP/SIRS to investigate the in vitro effects of emodin (1,3,8-trihydroxy-6-methylanthraquinone; Fig. 1) compared to those of dexamethasone on peritoneal macrophage (pMΦ) ICAM-3 protein expression and phagocytosis.
Figure 1.
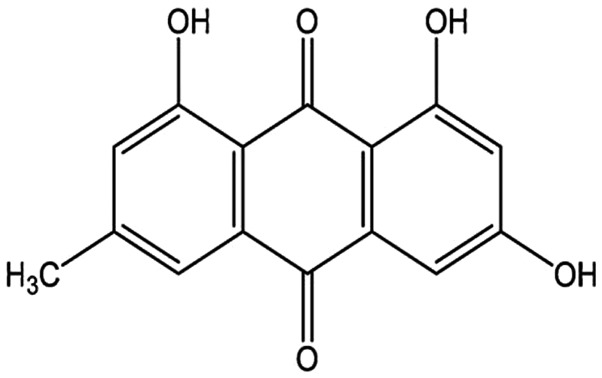
Chemical structure of emodin.
Materials and methods
Animals
A total of 40 healthy male Sprague-Dawley (SD) rats, weighing 220–250 g, were provided by the Laboratory Animal Center of Dalian Medical University. The SD rats were randomly divided into sham surgery (n=10) and model (SAP/SIRS) groups (n=30). pMΦs were harvested from the model group and the rats were randomly divided into three subgroups (n=10/subgroup): the emodin (5 μg/ml), dexamethasone (0.1 μmol/ml) and control groups. The drugs were administered following MΦ adhesion for 24 h.
Equipment
A high-speed refrigerated 5840R centrifuge was obtained from Eppendorf, Hamburg, Germany, a flow cytometer (FACSAsia) was purchased from BD Biosciences, Franklin Lakes, NJ, USA and an immunofluorescence microscope (CX31-32RFL) was purchased from Olympus Corporation, Tokyo, Japan.
Reagents and drugs
RPMI-1640 medium, fetal bovine serum (FBS), rabbit anti-ICAM-3 antibody, fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antibody and emodin and dianisidine were purchased from Sigma, St. Louis, MO, USA; Dextran T500 was purchased from Sigma-Aldrich (St. Louis, MO, USA).
SAP/SIRS model establishment (14)
The rats were fasted with free access to water for 12 h prior to surgery. The rats were then anesthetized with an intraperitoneal injection of 10% chloral hydrate at a dose of 0.3 ml/100 g. To expose the duodenum, a midline laparotomy was performed. An 1-ml syringe needle was inserted through the intestinal wall contralateral to the duodenal papilla into the bile and pancreatic ducts and clamped using a non-invasive bulldog clamp, followed by slow retrograde perfusion of 1.5% sodium deoxycholate (0.1 ml/100 mg) for 60 sec. The duodenal papilla was pinched to prevent backflow. The sham-surgery group was only subjected to a celiotomy.
Isolation, purification, culture and administration of pMΦs
Trypan blue staining revealed that the pMΦ survival rate and purity were >98 and >95%, respectively. The majority of cells exhibited the morphological characteristics of MΦs. pMΦs from each group were seeded in 6-well culture plates and treated with 5 μg/ml emodin and 0.1 μmol/ml dexamethasone. The control and sham-surgery groups were untreated. The cells were then incubated at 37°C with 5% CO2 for 24 h.
Detection of pMΦ ICAM-3 expression using flow cytometry
After a 24-h culture, the cells were washed three times in pre-warmed Hanks’ balanced salt solution and 0.25% trypsinized at 37°C for 5–6 min. After 90% of the adhered MΦs were round and transparent, as observed under an inverted microscope, digestion was terminated by addition of 10–20 ml RPMI-1640, followed by trituration. The cells were centrifuged at 111.8 × g for 10 min at 4°C. The supernatant was discarded and the cells were incubated with 1 μl anti-ICAM-3 antibody at room temperature for 1 h, washed with phosphate-buffered saline (PBS) three times, centrifuged at 111.8 × g (radius=10 cm) for 10 min at 4°C and incubated with a FITC-conjugated goat anti-rabbit antibody at room temperature for 15 min. Subsequently, the cells were washed with PBS three times and centrifuged at 111.8 × g for 10 min at 4°C. The cells were resuspended in 1 ml PBS and ICAM-3 expression was determined by flow cytometry.
PMN isolation and culture
The PMNs were isolated according to the method of Percoll density gradient centrifugation. The cells were suspended in RPMI-1640 medium supplemented with 100 U/ml streptomycin, 100 U/ml penicillin and 10% heat-inactivated FBS. The cell density was adjusted to 5×106 cells/ml. Trypan blue staining revealed that the PMN survival rate and purity were >95%.
Isolated PMNs were seeded at 5×106 cells in 2 ml medium per well in 24-well culture plates and incubated at 37°C in a humidified atmosphere with 5% CO2 for 24 h. PMN apoptosis was observed and the cells were collected.
Determination of rate and index of pMΦ phagocytosis
PMNs (200 μl) were co-cultured with pMΦs (pMΦ:PMN = 1:5) at 37°C with 5% CO2 for 30 min. Non-phagocytized PMNs were washed with pre-cooled PBS, fixed with 4% paraformaldehyde overnight and mixed with equal quantities of 1.25 mg/ml dianisidine and 0.05% H2O2 for myeloperoxidase (MPO) staining. MPO-positive PMNs exhibited brown staining, whereas MΦs were negative. The positively stained pMΦs were considered to be those that phagocytized PMNs and were quantified by microscopy (magnification, ×40). Three regions were randomly selected, 100 cells were quantified and the mean was calculated. The phagocytosis rate and phagocytic index were calculated to indicate phagocytosis ability as follows:
Detection of MΦ ICAM-3 protein expression using immunofluorescence
The glass slides were placed in 24-well culture plates and the MΦ concentration was adjusted to 5×106 cells/ml. After 30 min, cells in 2 ml RPMI-1640 containing serum were added and incubated at 37°C with 5% CO2 for 24 h. Emodin (5 μg/ml) and dexamethasone (0.1 μmol/ml) were added to the emodin and dexamethasone groups, respectively, followed by incubation at 37°C with 5% CO2 overnight.
The cells were harvested, washed with PBS three times, dried, fixed with 4% paraformaldehyde for 30 min and washed with PBS another three times. One drop of non-immune animal serum was added to each slide and incubated at room temperature for 30 min.
One drop of primary antibody (dilution 1:100, 100 μl) was then added to each slide and incubated at room temperature for 30 min in the dark, washed with PBS for 3×5 min and dried.
FITC-conjugated goat anti-rabbit antibody (50 μl) was added to each slide and incubated at room temperature for 10 min, washed with PBS for 3×5 min, dried and observed under a fluorescence microscope.
Specific fluorescence intensities were classified as follows: −, no fluorescence; ±, weak fluorescence; +, clear fluorescence; ++, low fluorescence; and +++/++++, strong fluorescence.
Statistical analysis
Data were analyzed using SPSS software, version 11.5 (SPSS Inc., Chicago, IL, USA) and expressed as means ± standard deviation. Enumeration data were analyzed using exact probability of 4-fold table and measurement data with completely random analysis of variance. A paired comparison was performed using a q-test. P<0.05 was considered to indicate a statistically significant difference at an α level of 0.05.
Results
Clinical manifestations
Following injection of 1.5% sodium deoxycholate, the rats exhibited rapid breathing and the symptoms were aggravated with time, followed by discoloration of the skin and mucosae (cyanosis), unconsciousness and occasionally death (the death rate was 20% in the model group).
Gross observation
Immediately following injection of 1.5% sodium deoxycholate, the pancreatic gland presented with evident regional or diffuse hyperemia and edema, with increased pancreatic envelope tension. After 24 h, pancreatic hemorrhage and necrosis and bloody ascites were observed in the surviving rats of the model group. In addition to the appearance of yellow saponaceous spots on the greater omentum and common bile duct, pulmonary hyperemia, edema and hemorrhage, gastric edema and paralytic expansion, hepatic swelling and renal augmentation were observed. However, in the rats in the sham surgery group, only a mild edema of the gastrointestinal mucosa and exudation in the abdominal cavity were observed.
Pathological changes in pancreatic tissues observed under a light microscope
The sham surgery group exhibited a distinct pancreatic lobular structure. The model group presented with necrosis in the pancreatic glandular parenchyma, bleeding and fatty degeneration, erythrocyte stasis, angiectasis and PMN infiltration of the interstitial space and parenchyma.
MΦ ICAM-3 expression
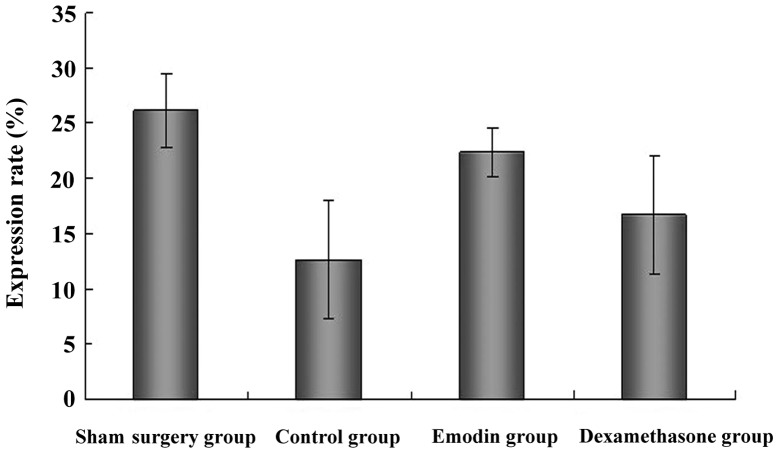
The ICAM-3 expression in each group as detected by flow cytometry (mean ± standard deviation) is presented in Table I and Fig. 2. The expression of ICAM-3 was significantly decreased in the control group, whereas the expression of ICAM-3 was significantly increased in the emodin and dexamethasone groups. Of note, the expression of ICAM-3 was significantly increased in the emodin group.
Table I.
Intercellular adhesion molecule-3 expression in each group as detected by flow cytometry (mean ± standard deviation).
| Groups | n | Expression rate (%) |
|---|---|---|
| Sham surgery | 10 | 26.12±3.32 |
| Control | 8 | 12.57±5.37a |
| Emodin | 8 | 22.40±2.25b |
| Dexamethasone | 8 | 16.64±5.36b,c |
P<0.05 vs. sham surgery group;
P<0.05 vs. control group;
P<0.05, vs. emodin group.
Figure 2.
Intercellular adhesion molecule-3 (ICAM-3) expression in each group as detected by flow cytometry. ICAM-3 expression was significantly decreased in the control group and increased in the emodin and dexamethasone groups. Of note, ICAM-3 expression was significantly increased in the emodin group.
pMΦ phagocytosis rate and phagocytic index
The positively stained pMΦs were considered to be those that phagocytized PMNs and were quantified by microscopy (magnification, 40). Three regions were randomly selected, 100 cells were quantified and the mean was calculated. The phagocytosis rate and phagocytic index were calculated to evaluate phagocytotic ability.
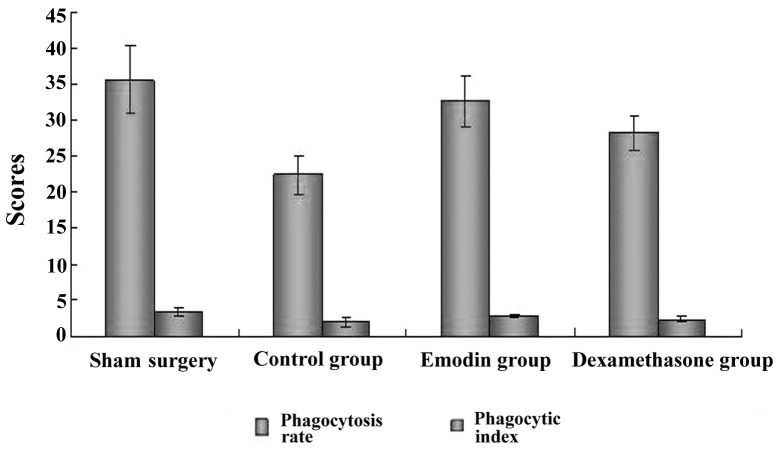
The phagocytosis rate and phagocytic index were significantly lower in the control group, alhtough they were increased in the emodin and dexamethasone groups. Of note, the phagocytosis rate and phagocytic index were significantly increased in the emodin group (Table II and Fig. 3).
Table II.
Peritoneal macrophage phagocytosis rate and phagocytic index in each group (mean ± standard deviation).
| Groups | n | Phagocytosis rate (%) | Phagocytic index |
|---|---|---|---|
| Sham surgery | 10 | 35.6±4.8 | 3.4±0.6 |
| Control | 8 | 22.4±2.7a | 2.0±0.5a |
| Emodin | 8 | 32.6±3.5b | 2.8±0.2b |
| Dexamethasone | 8 | 28.2±2.4c | 2.4±0.3c |
P<0.05 vs. sham surgery group;
P<0.05 vs. control group;
P<0.05 vs. emodin group.
Figure 3.
Peritoneal macrophage phagocytosis rate and phagocytic index in each group. The phagocytosis rate and phagocytic index were significantly lower in the control group, whereas they were increased in the emodin and dexamethasone groups. Of note, the phagocytosis rate and phagocytic index were significantly increased in the emodin group.
MΦ ICAM-3 protein expression
Immunofluorescence staining demonstrated that ICAM-3 was mainly expressed on the cell membrane. There were statistically significant differences between the control and sham surgery groups, between the emodin and control groups and between the dexamethasone and control groups (P<0.05; Table III).
Table III.
Expression of intercellular adhesion molecule-3 (ICAM-3) in the sham surgery, emodin and dexamethasone groups compared to the control group.
| ICAM-3 expression | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Groups | n | − | + | ++ | +++ | Positive rate (%) | P-value |
| Sham surgery | 10 | 2 | 3 | 4 | 1 | 80 | 0.016 |
| Emodin | 8 | 1 | 3 | 4 | 0 | 87.5 | 0.008 |
| Dexamethasone | 8 | 2 | 2 | 4 | 0 | 75 | 0.03 |
| Control | 8 | 5 | 3 | 0 | 0 | 37.5 | − |
−, no fluorescence; +, clear fluorescence; ++, low fluorescence; +++, strong fluorescence.
Discussion
PMNs are crucial for the control of infection. However, the pathogenesis of inflammation is associated with PMNs and their toxic content release. Following necrosis or disaggregation, PMNs may release a large amount of toxins, which induce tissue damage, a cascade reaction and SIRS (15). Under normal conditions, the half-life of PMNs is 6–10 h; however, after PMNs enter inflammatory sites, their half-life is significantly prolonged (16,17). Spontaneous apoptosis occurs in senescent PMNs, even in the absence of cytokines or under pro-inflammatory conditions. Apoptotic PMNs may be completely phagocytized, thereby preventing toxin release that may induce tissue injury. Moreover, MΦ phagocytosis of apoptotic PMNs does not stimulate the release of inflammatory mediators (18,19). Therefore, apoptosis is considered to be an effective physiological adjustment for the clearance of PMNs (20). Following acute inflammation, PMNs migrate from the circulation to inflammatory sites, significantly increasing the numbers of PMNs in the tissues. Moreover, inflammation reduces PMN apoptosis, resulting in PMN activation and SIRS.
MΦs exhibit a strong phagocytotic capacity. Recent evidence indicated that after MΦs phagocytize apoptotic PMNs, transforming growth factor (TGF)-β1 is released, which inhibits the production of inflammatory factors interleukin (IL)-1β, tumour necrosis factor α (TNF-α) and granulocyte-macrophage colony-stimulating factor (19,21–23). Following apoptosis, the phagocytes recognize and phagocytize apoptotic cells and bodies with an intact membrane structure. The process of MΦ phagocytosis of apoptotic cells is ‘quiet’ and is not accompanied by cytolysosome, mitochondrion and membrane breakage, preventing cell content release, inflammatory reaction and tissue injury. Therefore, the phagocytotic capacity of MΦs is crucial for the containment of inflammation. If the mechanism by which MΦs recognize and phagocytize apoptotic cells is damaged, apoptotic cells cannot be phagocytized, which leads to toxic content release and subsequent tissue and cell injury (18).
Biochemical and membrane changes in apoptotic cells may be the basis by which MΦs recognize and phagocytize apoptotic cells. MΦs express various receptors that identify apoptotic cells and apoptotic cells possess corresponding markers to exhibit their ‘edibility’. However, the interaction between selection and phagocytosis has not been fully elucidated.
In the present study, the phagocytotic capacity of pMΦs was shown to be reduced following SAP/SIRS, which may contribute to delayed PMN apoptosis and necrosis, as well as toxic content and chemotactic factor release, which inhibit ‘edibility’ signal expression. Moreover, the membrane structure and internal environment are altered by toxic content and chemotactic factor release, which may also attenuate the phagocytotic capacity of pMΦs.
ICAM-3 is a member of the immunoglobulin superfamily and is involved in cell adhesion and signal transduction. It is mainly expressed by leukocytes and highly expressed by lymphocytes, monocytes and neutrophilic granulocytes (24). ICAM-3 is also expressed on MΦs (24).
In the present study, pMΦ ICAM-3 protein expression was shown to be significantly decreased following SAP/SIRS compared to that in the control group. The likely mechanism is that SIRS induces organ and cell injury; thus, MΦs synthesize and release a large amount of TNF-α and other inflammatory mediators that trigger inflammatory reactions. TGF-β and IL-4 downregulate MΦ ICAM-3 expression, thereby inhibiting its interaction with CD14, which affects phosphatidylserine externalization and reduces the phagocytotic capacity of MΦs. Previous studies demonstrated that the delayed apoptosis of human neutrophils may be attributed to the disturbed identification of MΦs under pathological conditions (4,12).
Da Huang (rhubarb), a Chinese herb, was demonstrated to be clinically effective for the treatment of acute pancreatitis (25–28). Emodin is the main active component of Da Huang. Previous studies demonstrated that emodin affects bacteriostasis, catharsis, relieves Oddi sphincter spasm, inhibits abnormal metabolism of vasoactive substances (e.g., eicosenoic acid), improves the microcirculation and antagonizes coagulation and thrombus formation (25,29,30). The present study demonstrated that emodin significantly increases MΦ ICAM-3 protein expression in rats with SAP/SIRS (P<0.05), thereby promoting the interaction between ICAM-3 and CD14, enhancing identification and phagocytotic capacity of pMΦs and relieving inflammatory reactions.
Stephenson et al(31) first reported the application of glucocorticoids in the treatment of acute pancreatitis. However, the mechanism has not been fully elucidated. The effects of glucocorticoids on inflammation via receptor mediation may trigger anti-inflammatory processes. Glucocorticoids inhibit inflammatory exudation, leukocytic infiltration and inflammatory mediator production and release, improve the microcirculation, alleviate endotoxemia and induce apoptosis of pancreatic acinar cells, thereby reducing the degree of pancreatic necrosis in SAP (32,33). A previous study demonstrated that pancreatic cell apoptosis occurs during acute pancreatitis (34). Animal experiments also suggested that dexamethasone induces pancreatic cell apoptosis, stabilizes the internal environment and attenuates inflammation in pancreatic tissues (33,35). In the present study, dexamethasone enhanced the phagocytotic capacity of pMΦs, maintained the internal environment and increased MΦ ICAM-3 expression.
In conclusion, the capacity of pMΦs to phagocytize apoptotic PMNs is significantly reduced following SAP/SIRS. This effect may be associated with decreased pMΦ ICAM-3 expression. Emodin and dexamethasone were shown to enhance the phagocytotic capacity of pMΦs, possibly by increasing pMΦ ICAM-3 expression. Of note, emodin exerted more potent effects compared to dexamethasone.
Abbreviations
- ICAM-3
intercellular adhesion molecule-3
- SAP
severe acute pancreatitis
- SIRS
systemic inflammatory response syndrome
- MODS
multiple organ dysfunction syndrome
- MΦs
macrophages
- pMΦs
peritoneal macrophages
- PMNs
polymorphonuclear neutrophils
- MPO
myeloperoxidase
References
- 1.Brisinda G, Vanella S, Crocco A, et al. Severe acute pancreatitis: advances and insights in assessment of severity and management. Eur J Gastroenterol Hepatol. 2011;23:541–551. doi: 10.1097/MEG.0b013e328346e21e. [DOI] [PubMed] [Google Scholar]
- 2.Doctor N, Agarwal P, Gandhi V. Management of severe acute pancreatitis. Indian J Surg. 2012;74:40–46. doi: 10.1007/s12262-011-0384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 4.Blomgran R, Patcha Brodin V, Verma D, et al. Common genetic variations in the NALP3 inflammasome are associated with delayed apoptosis of human neutrophils. PLoS One. 2012;7:e31326. doi: 10.1371/journal.pone.0031326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paunel-Gorgulu A, Kirichevska T, Logters T, Windolf J, Flohe S. Molecular mechanisms underlying delayed apoptosis in neutrophils from multiple trauma patients with and without sepsis. Mol Med. 2012;18:325–335. doi: 10.2119/molmed.2011.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz JT, Bandyopadhyay S, Kobayashi SD, et al. Francisella tularensis alters human neutrophil gene expression: insights into the molecular basis of delayed neutrophil apoptosis. J Innate Immun. 2013;5:124–136. doi: 10.1159/000342430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terashima M, Aoyama-Ishikawa M, Ueda T, et al. The effects of n-3 polyunsaturated fatty acid-rich total parenteral nutrition on neutrophil apoptosis in a rat endotoxemia. J Clin Biochem Nutr. 2013;52:154–159. doi: 10.3164/jcbn.12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang D, Wang BZ, Bi W, Chen HL, Qi QH, Guan FL. Abnormal polymorphonuclear neutrophils apoptosis in systemic inflammatory response syndrome. Journal of Dalian Medical University. 2006;28:161–163. (In Chinese) [Google Scholar]
- 9.Zhang J, He J, Xia J, Chen Z, Chen X. Delayed apoptosis by neutrophils from COPD patients is associated with altered Bak, Bcl-xl, and Mcl-1 mRNA expression. Diagn Pathol. 2012;7:65. doi: 10.1186/1746-1596-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willems CH, Urlichs F, Seidenspinner S, Kunzmann S, Speer CP, Kramer BW. Poractant alfa (Curosurf®) increases phagocytosis of apoptotic neutrophils by alveolar macrophages in vivo. Respir Res. 2012;13:17. doi: 10.1186/1465-9921-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costantini C, Calzetti F, Perbellini O, et al. Human neutrophils interact with both 6-sulfo LacNAc+DC and NK cells to amplify NK-derived IFNγ: role of CD18, ICAM-1, and ICAM-3. Blood. 2011;117:1677–1686. doi: 10.1182/blood-2010-06-287243. [DOI] [PubMed] [Google Scholar]
- 12.Moffatt OD, Devitt A, Bell ED, Simmons DL, Gregory CD. Macrophage recognition of ICAM-3 on apoptotic leukocytes. J Immunol. 1999;162:6800–6810. [PubMed] [Google Scholar]
- 13.Gregory CD, Devitt A. CD14 and apoptosis. Apoptosis. 1999;4:11–20. doi: 10.1023/a:1009673914340. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol. 1995;269:C1295–C1304. doi: 10.1152/ajpcell.1995.269.5.C1295. [DOI] [PubMed] [Google Scholar]
- 15.Li F, Zhang H, Xu KY, Wei Q, Zhou GX. Role of the chemokine fractalkine in a rat model of acute necrotizing pancreatitis and the interventional effect of ulinastatin. Arch Iran Med. 2013;16:83–87. [PubMed] [Google Scholar]
- 16.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 17.Kolaczkowska E, Koziol A, Plytycz B, Arnold B. Inflammatory macrophages, and not only neutrophils, die by apoptosis during acute peritonitis. Immunobiology. 2010;215:492–504. doi: 10.1016/j.imbio.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Hart SP, Rossi AG, Haslett C, Dransfield I. Characterization of the effects of cross-linking of macrophage CD44 associated with increased phagocytosis of apoptotic PMN. PLoS One. 2012;7:e33142. doi: 10.1371/journal.pone.0033142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothlin CV, Lemke G. TAM receptor signaling and autoimmune disease. Curr Opin Immunol. 2010;22:740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chilvers ER, Cadwallader KA, Reed BJ, White JF, Condliffe AM. The function and fate of neutrophils at the inflamed site: prospects for therapeutic intervention. J R Coll Physicians Lond. 2000;34:68–74. [PMC free article] [PubMed] [Google Scholar]
- 21.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michlewska S, Dransfield I, Megson IL, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. FASEB J. 2009;23:844–854. doi: 10.1096/fj.08-121228. [DOI] [PubMed] [Google Scholar]
- 23.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 24.Estecha A, Aguilera-Montilla N, Sanchez-Mateos P, Puig-Kroger A. RUNX3 regulates intercellular adhesion molecule 3 (ICAM-3) expression during macrophage differentiation and monocyte extravasation. PLoS One. 2012;7:e33313. doi: 10.1371/journal.pone.0033313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Xia X, Zhang S, Zhang A, Bo W, Zhou R. Up-regulation of Toll-like receptor 4 was suppressed by emodin and baicalin in the setting of acute pancreatitis. Biomed Pharmacother. 2009;63:120–128. doi: 10.1016/j.biopha.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Sun B, Gao Y, Meng QH, Jiang HC. The effect of emodin-assisted early enteral nutrition on severe acute pancreatitis and secondary hepatic injury. Mediators Inflamm. 2007;2007:29638. doi: 10.1155/2007/29638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang CY, Shen L, Xie ZG, Jiang X, Liang N, Chen ZH. Experimental studies of therapeutic effect of Rheum officinale on acute pancreatitis. J Chin Med Mater. 2011;34:84–88. (In Chinese) [PubMed] [Google Scholar]
- 28.Zheng SH, Tong QY, Zhu ZY, Li ZY, You H. Effect of Rhubarb administered via different routes on blood inflammatory cytokines levels of patients with severe acute pancreatitis. Chin Crit Care Med. 2011;23:437–438. (In Chinese) [PubMed] [Google Scholar]
- 29.Kuo YC, Meng HC, Tsai WJ. Regulation of cell proliferation, inflammatory cytokine production and calcium mobilization in primary human T lymphocytes by emodin from Polygonum hypoleucum Ohwi. Inflamm Res. 2001;50:73–82. doi: 10.1007/s000110050727. [DOI] [PubMed] [Google Scholar]
- 30.Li A, Dong L, Duan ML, et al. Emodin improves lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Microcirculation. 2013 Apr 1; doi: 10.1111/micc.12061. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 31.Stephenson HE, Jr, Pfeffer RB, Saypol GM. Acute hemorrhagic pancreatitis; report of a case with cortisone treatment. AMA Arch Surg. 1952;65:307–308. [PubMed] [Google Scholar]
- 32.Jingmin O, Xiping Z, Chun W, Ping Y, Qian Y. Study of dexamethasone, baicalin and octreotide on brain injury of rats with severe acute pancreatitis. Inflamm Res. 2012;61:265–275. doi: 10.1007/s00011-011-0408-4. [DOI] [PubMed] [Google Scholar]
- 33.Zhang XP, Chen L, Hu QF, et al. Effects of large dose of dexamethasone on inflammatory mediators and pancreatic cell apoptosis of rats with severe acute pancreatitis. World J Gastroenterol. 2007;13:5506–5511. doi: 10.3748/wjg.v13.i41.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jha RK, Ma Q, Sha H, Palikhe M. Acute pancreatitis: a literature review. Med Sci Monit. 2009;15:RA147–RA156. [PubMed] [Google Scholar]
- 35.Ou JM, Zhang XP, Wu CJ, Wu DJ, Yan P. Effects of dexamethasone and Salvia miltiorrhiza on multiple organs in rats with severe acute pancreatitis. J Zhejiang Univ Sci B. 2012;13:919–931. doi: 10.1631/jzus.B1100351. [DOI] [PMC free article] [PubMed] [Google Scholar]