Abstract
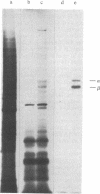
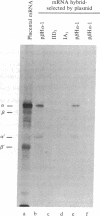
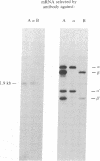
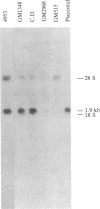
We have isolated a cDNA clone containing sequences complementary to mRNA encoding the alpha-chain of the lysosomal enzyme beta-hexosaminidase. RNA from a human lung fibroblast strain, IMR90, was enriched for beta-hexosaminidase messenger by polysome immunoselection with antiserum against beta-hexosaminidase A. This preparation was used to construct cDNA recombinant plasmids by the Okayama-Berg vector primer procedure. After transformation of Escherichia coli, 385 ampicillin-resistant colonies were obtained, 44 of which contained inserts in the plasmid DNA. Differential hybridization, with cDNA probes prepared from polysomal RNA enriched or depleted for beta-hexosaminidase messenger, was used to screen the recombinant plasmids for sequences encoding beta-hexosaminidase. One clone, p beta H alpha-1, containing a cDNA insert of approximately equal to 240 base pairs, was identified in this manner. The plasmid hybrid-selected a messenger from placental RNA that programed a translation system to synthesize the alpha-chain of beta-hexosaminidase. p beta H alpha-1 hybridized to an mRNA of approximately equal to 1.9 kilobases in preparations enriched separately in messenger for the alpha-chain or for both alpha- and beta-chains (by polysome immunoselection with antiserum against isolated alpha-chain or against beta-hexosaminidase A, respectively). It did not hybridize to an RNA preparation enriched for messenger of beta-chain by immunoselection with antiserum against beta-hexosaminidase B. The 1.9-kilobase mRNA was observed in poly(A)+ RNA preparations from control fibroblasts and from fibroblasts of a Tay-Sachs patient that synthesize an altered alpha-chain; however, it was not seen in similar preparations from fibroblasts of four Ashkenazi Tay-Sachs patients.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Kraus J. P., Rosenberg L. E. Purification of low-abundance messenger RNAs from rat liver by polysome immunoadsorption. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4015–4019. doi: 10.1073/pnas.79.13.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Proia R. L., Neufeld E. F. Synthesis of beta-hexosaminidase in cell-free translation and in intact fibroblasts: an insoluble precursor alpha chain in a rare form of Tay-Sachs disease. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6360–6364. doi: 10.1073/pnas.79.20.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proia R. L., d'Azzo A., Neufeld E. F. Association of alpha- and beta-subunits during the biosynthesis of beta-hexosaminidase in cultured human fibroblasts. J Biol Chem. 1984 Mar 10;259(5):3350–3354. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sando G. N., Neufeld E. F. Recognition and receptor-mediated uptake of a lysosomal enzyme, alpha-l-iduronidase, by cultured human fibroblasts. Cell. 1977 Nov;12(3):619–627. doi: 10.1016/0092-8674(77)90262-8. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstoshev P., Berg R. A., Rennard S. I., Bradley K. H., Trapnell B. C., Crystal R. G. Procollagen production and procollagen messenger RNA levels and activity in human lung fibroblasts during periods of rapid and stationary growth. J Biol Chem. 1981 Mar 25;256(6):3135–3140. [PubMed] [Google Scholar]
- Williams J. G., Lloyd M. M., Devine J. M. Characterization and transcription analysis of a cloned sequence derived from a major developmentally regulated mRNA of D. discoideum. Cell. 1979 Aug;17(4):903–913. doi: 10.1016/0092-8674(79)90330-1. [DOI] [PubMed] [Google Scholar]