Abstract
Background:
Pre-eclampsia (PE) is a pregnancy associated disorder characterized by hyper-tension and proteinuria. The first 2 stages of PE cause dysfunction in uteroplacental perfusion and oxidative stress while the third stage of PE is due to the release of inflammatory and angiogenic factors, which could lead to maternal endothelial damage and systemic inflammatory response. In this study, we compared the serum levels of tumor necrosis factor-α (TNF-α), interleukin-15 (IL-15), and interleukin-10 (IL-10) in PE and normotensive women.
Materials and Methods:
Serum samples of 84 pregnant women (44 PE and 40 normotensive) were evaluated for TNF-α, IL-15 and IL-10 by sandwich ELISA assay.
Results:
The women with PE showed significantly higher serum levels of TNF-α and IL-15 (P = 0.001 and 0.01 respectively) in comparison with normotensive pregnant women. Conversely, the serum levels of IL-10 in normotensive women were significantly higher compared to PE patients (P = 0.01).
Conclusion:
The results of this study demonstrated that inflammatory T helper 1-type responses are increased in PE women compared to normotensive pregnant women.
Keywords: Interleukin-10, interleukin-15, preeclampsia, tumor necrosis factor-α
INTRODUCTION
Pre-eclampsia (PE) is a pregnancy associated disorder known by hypertension and proteinuria. It occurs in 3-5% of pregnancies after 20th week of gestational age. The disorder affects both mother and neonate.[1,2] The etiology of PE is not fully understood and a great number of mechanisms are proposed as the disease pathophysiology; however, the only definite cure of this syndrome is delivery.[3] Placenta plays a major role in the pathogenesis of PE. It is a general consensus on the role of disruption between vascular generation and anti-angiogenic responses in creating PE.[4] In addition, immune mal-adaptation hypothesis suggests that PE is the result of an inappropriate regulation of the normally T helper 2 deviated response during the pregnancy.[5,6,7] In this way recent reports suggest that PE is associated with a Th1 predominant profile[8] while activation of the immune system leads to alteration in cytokine profile and defect in the acquisition of invasive phenotype by trophoblasts in PE patients.[6] PE develops in three stages. The first 2 stages of disease cause dysfunction in uteroplacental perfusion and oxidative stress while the 3rd stage of PE is due to the release of inflammatory and angiogenic factors, which could lead to maternal endothelial damage and systemic inflammatory response.[6,9]
Cytokines, growth factors, and adhesion molecules have been proposed as important mediators for successful placentation as well as endothelial function.[10,11] Over past decades, multiple analyses of cytokines provided substantial insight into the key players of the maternal immune system involved in PE.[5,12]
Tumor necrosis factor-α (TNF-α) is an inflammatory cytokine produced by human uterine and placental cells at the early and late stages of gestation and promotes the regulation of trophoblast growth and invasion. Furthermore, TNF-α could inhibit deeply integration and invasion of trophoblasts in maternal endometrium, which leads to PE.[11,13] Interleukin-15 (IL-15), which is also a proinflammatory cytokine, has profound effects on natural killer (NK) cells and their cytotoxicity activation. Regarding to the role of NK cells in the pathogenesis of PE, IL-15 could be a key player in development of disease.[14,15]
Interleukin-10 (IL-10) as an anti-inflammatory cytokine is produced by some subpopulations of T lymphocytes, monocytes, macrophages, and cytotrophoblasts.[16] Production of this cytokine is considered to play an important role in maternal immune tolerance of the fetus in normal pregnancy.[17]
In this study, we compared the serum levels of TNF-α, IL-15, and IL-10 in Iranian PE women with normotensive ones.
MATERIALS AND METHODS
The study was a case-control study, in which serum samples of 84 pregnant women were studied. 44 PE (cases) and 40 normotensive (controls) pregnant women were enrolled in this study. These 2 groups were selected among the women admitted to a university associated hospital of Tehran University of Medical Sciences. This study was approved by ethics committee of Tehran University of Medical Sciences. All participants signed informed consent before enrolling to the study.
PE patients were diagnosed by hypertension (blood pressure ≥ 140/90 mm Hg) and proteinuria (at least 2 + on dips teak examination or ≥ 300 mg/24 h on at least 2 occasions 6 h apart) after the 20th week of gestation. These patients were normotensive before pregnancy and during the first 20 weeks of gestation. The PE women were under the supervision and treatment of obstetrics and gynecology specialist during pregnancy.
The normotensive control group consisted of healthy women with uncomplicated pregnancy who referred for normal routine check-up examinations to a university associated hospital. These women had blood pressure < 140/90 mm Hg in all stages of pregnancy. Pregnant women with pre-gestational diabetes mellitus and chronic hypertension were excluded from the study. They did not receive any special drug during the pregnancy except routine supplements.
5 mL of venous blood sample were taken from both groups before beginning of the active phase of labor. Samples were centrifuged at 2500 G for 15 min at 4°C for serum separation. Serum specimens were aliquoted and stored at −70°C until they were assayed.
Serum levels of TNF-α and IL-10 were measured using a sandwich ELISA test due to manufacturer's instruction (Bender MedSystems, Austria). The levels of IL-15 were assayed by using IL-15 sandwich ELISA kit (R&D Systems, Minneapolis, MN). In above mentioned ELISA kits routine procedures were done in this order; coating the plate with capture antibody, blocking the plate, adding standards, and unknown samples, washing, adding the biotin conjugated specific antibody, washing, applying enzyme-linked streptavidin, and finally adding chemical substrate. Optical density of color chemicals that produced in the wells of the plate were read and the concentrations of cytokines were measured according to plots. The serums that their cytokine levels were beyond the upper limit of the kit were diluted for assessment. For all mentioned cytokines, standard curves were plotted and the concentrations were calculated according the manufacturer's instruction.
Student's t-test was used for comparison between serum levels of two groups. P value < 0.05 was considered statistically significant.
RESULTS
The characteristics of women included in the study are presented in Table 1. There was no statistically significant difference related to age and gestational age at the time of sampling between two groups; however, there were higher systolic and diastolic pressures in PE group compared to normotensive one (P values: 0.01 and 0.03 respectively for systolic and diastolic blood pressures). The women with PE showed significantly higher serum concentrations of TNF-α and IL-15 (P values: 0.001 and 0.01 respectively) relative to the control group. In comparison, levels of IL-10 in the serum of normotensive pregnant women were significantly higher than PE patients (P value = 0.01). The results are demonstrated in Figures 1–3.
Table 1.
Clinical characteristics of the preeclamptic and normotensive women
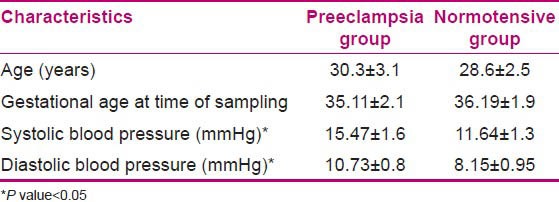
Figure 1.
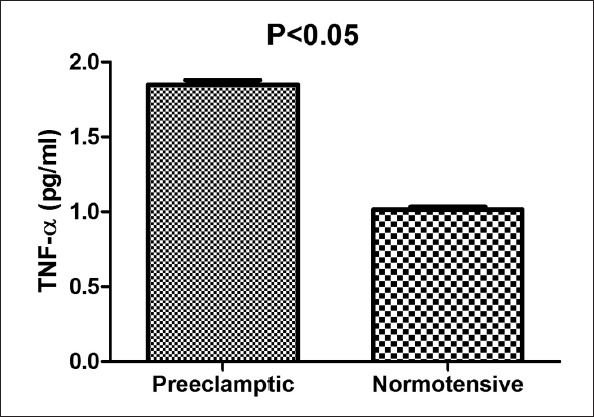
Serum levels of tumor necrosis factor-α in preeclamptic (PE) and normal subjects. Data are presented as mean ± SEM. Each bar represented 44 PE and 40 normotensive pregnant women
Figure 3.
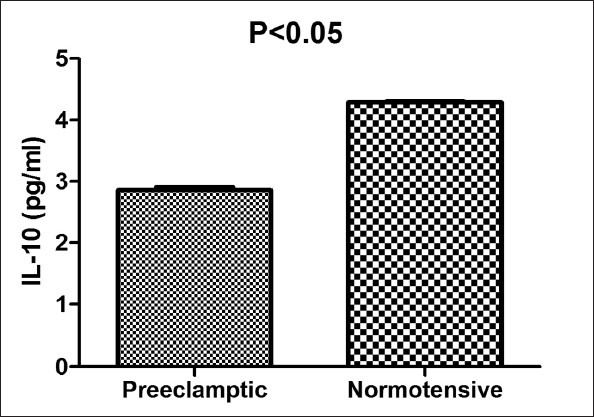
Serum levels of interleukin-10 in preeclamptic (PE) and normal subjects. Data are presented as mean ± SEM. Each bar represented 44 PE and 40 normotensive pregnant women
Figure 2.
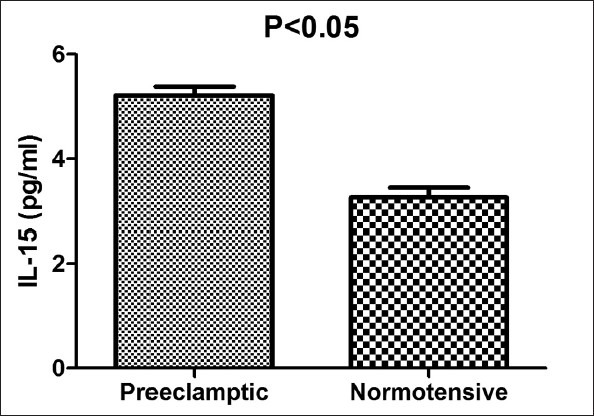
Serum levels of interleukin-15 in preeclamptic (PE) and normal subjects. Data are presented as mean ± SEM. Each bar represented 44 PE and 40 normotensive pregnant women
DISCUSSION
Results of this study demonstrated that in women having PE, compared to women with a normal pregnancy, inflammatory Th1-type responses are increased; while anti-inflammatory responses are decreased.
The higher levels of pro-inflammatory cytokines (TNF-α and IL-15) and lower levels of IL-10 in the PE group suggest an increased inflammatory status in PE during last months. Sharma et al. and Xie et al., in agree with our results, found that the level of inflammatory cytokines such as TNF-α increased significantly in preeclamptic patients compared with health controls.[18,19,20] Furthermore, Laskowska et al. in consistent with us, indicated that TNF-α increased in PE patients and suggested this cytokine may be play a role in pathogenesis and consequences of PE.[21] In general, these studies demonstrated that TNF-α is a potent pro-inflammatory cytokine, its primary biological activity includes inflammation and it may contribute to the abnormal placental invasion.[11,13,22] However, Afshari et al.[23] and Jonsson et al.[5] observed no significant changes of TNF-α in PE women compared to normal pregnant women. About the other inflammatory cytokine we analyzed in this study, Hu et al. showed upraised serum levels of IL-15 in PE cases compared to normotensive ones.[14] In line with this finding, El-Baradie et al. in their study indicated that serum levels of IL-15 and IL-16 were elevated in PE relative to normotensive pregnant women.[15] In contrast Lu et al. represented not elevated levels of IL-15 in PE subjects compared to normal women.[24]
In normal pregnancy, the trophoblasts invade the endovascular tissue and replace in the uterine arterioles.[9,25] It seems that in PE Th1-type cytokines such as TNF-α and IFN-γ induce trophoblastic apoptosis, inhibit differentiation and invasion of trophoblast, and finally, may be involved in the incomplete invasion of trophoblast to spiral arteries; pathologic processes that are integral parts of PE.[26]
Our findings showed higher levels of IL-10, which is an anti-inflammatory cytokine in sera of normal pregnant women. In concordance with our results, Sharma et al.[20] showed that IL-10 was decreased significantly in PE patients compared to normal pregnant women. These findings emphasize on the protective role of IL-10 in PE.[17] Inversely, Bakheit et al.[27] and Xie et al.[19] found higher serum levels of IL-10 in PE women and Jonsson et al.[5] observed no significant difference in IL-10 levels between normotensive and PE women.
These contradictory results of various studies could be related to sampling in different stages of pregnancy. PE as an inflammatory process starts from the first steps of placentation; however, this inflammatory grade lasts until parturition.
CONCLUSION
Consequently, upraised levels of pro-inflammatory cytokines in our study may be the perquisite of PE. Recognition of early detectable inflammatory markers could be a great help to predict and observe PE patients more accurately.
ACKNOWLEDGMENTS
The authors would like to acknowledge the kind cooperation of the colleagues, Immunology Department, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran. Project Research Number in Deputy of Research, TUMS: 85-02-30-3800.
Footnotes
Source of Support: Deputy of Research, TUMS
Conflict of Interest: None declared.
REFERENCES
- 1.Shah DM. Preeclampsia: New insights. Curr Opin Nephrol Hypertens. 2007;16:213–20. doi: 10.1097/MNH.0b013e3280d942e9. [DOI] [PubMed] [Google Scholar]
- 2.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–7. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Wagner LK. Diagnosis and management of preeclampsia. Am Fam Physician. 2004;70:2317–24. [PubMed] [Google Scholar]
- 4.Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: Multiple approaches for a multifactorial disease. Dis Model Mech. 2012;5:9–18. doi: 10.1242/dmm.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonsson Y, Rubèr M, Matthiesen L, Berg G, Nieminen K, Sharma S, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70:83–91. doi: 10.1016/j.jri.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Laresgoiti-Servitje E, Gómez-López N, Olson DM. An immunological insight into the origins of pre-eclampsia. Hum Reprod Update. 2010;16:510–24. doi: 10.1093/humupd/dmq007. [DOI] [PubMed] [Google Scholar]
- 7.Dekker G, Robillard PY. Pre-eclampsia: Is the immune maladaptation hypothesis still standing? An epidemiological update. J Reprod Immunol. 2007;76:8–16. doi: 10.1016/j.jri.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Borzychowski AM, Croy BA, Chan WL, Redman CW, Sargent IL. Changes in systemic type 1 and type 2 immunity in normal pregnancy and pre-eclampsia may be mediated by natural killer cells. Eur J Immunol. 2005;35:3054–63. doi: 10.1002/eji.200425929. [DOI] [PubMed] [Google Scholar]
- 9.Ahn H, Park J, Gilman-Sachs A, Kwak-Kim J. Immunologic characteristics of preeclampsia, a comprehensive review. Am J Reprod Immunol. 2011;65:377–94. doi: 10.1111/j.1600-0897.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- 10.Benian A, Madazli R, Aksu F, Uzun H, Aydin S. Plasma and placental levels of interleukin-10, transforming growth factor-beta1, and epithelial-cadherin in preeclampsia. Obstet Gynecol. 2002;100:327–31. doi: 10.1016/s0029-7844(02)02077-x. [DOI] [PubMed] [Google Scholar]
- 11.Szarka A, Rigó J, Jr, Lázár L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visser N, van Rijn BB, Rijkers GT, Franx A, Bruinse HW. Inflammatory changes in preeclampsia: Current understanding of the maternal innate and adaptive immune response. Obstet Gynecol Surv. 2007;62:191–201. doi: 10.1097/01.ogx.0000256779.06275.c4. [DOI] [PubMed] [Google Scholar]
- 13.Xu B, Nakhla S, Makris A, Hennessy A. TNF-α inhibits trophoblast integration into endothelial cellular networks. Placenta. 2011;32:241–6. doi: 10.1016/j.placenta.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Hu W, Wang H, Wang Z, Huang H, Dong M. Elevated serum levels of interleukin-15 and interleukin-16 in preeclampsia. J Reprod Immunol. 2007;73:166–71. doi: 10.1016/j.jri.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 15.El-Baradie SM, Mahmoud M, Makhlouf HH. Elevated serum levels of interleukin-15, interleukin-16, and human chorionic gonadotropin in women with preeclampsia. J Obstet Gynaecol Can. 2009;31:142–8. doi: 10.1016/s1701-2163(16)34098-1. [DOI] [PubMed] [Google Scholar]
- 16.Orange S, Rasko JE, Thompson JF, Vaughan J, Olive E, Pedler M, et al. Interleukin-10 regulates arterial pressure in early primate pregnancy. Cytokine. 2005;29:176–85. doi: 10.1016/j.cyto.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Kalkunte S, Nevers T, Norris WE, Sharma S. Vascular IL-10: A protective role in preeclampsia. J Reprod Immunol. 2011;88:165–9. doi: 10.1016/j.jri.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma D, Singh A, Trivedi SS, Bhattacharjee J. Intergenotypic variation of nitric oxide and inflammatory markers in preeclampsia: A pilot study in a North Indian population. Hum Immunol. 2011;72:436–9. doi: 10.1016/j.humimm.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Xie C, Yao MZ, Liu JB, Xiong LK. A meta-analysis of tumor necrosis factor-alpha, interleukin-6, and interleukin-10 in preeclampsia. Cytokine. 2011;56:550–9. doi: 10.1016/j.cyto.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol. 2007;58:21–30. doi: 10.1111/j.1600-0897.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 21.Laskowska M, Leszczyńska-Gorzelak B, Laskowska K, Oleszczuk J. Evaluation of maternal and umbilical serum TNFalpha levels in preeclamptic pregnancies in the intrauterine normal and growth-restricted fetus. J Matern Fetal Neonatal Med. 2006;19:347–51. doi: 10.1080/14767050600637937. [DOI] [PubMed] [Google Scholar]
- 22.Ouyang YQ, Li SJ, Zhang Q, Cai HB, Chen HP. Interactions between inflammatory and oxidative stress in preeclampsia. Hypertens Pregnancy. 2009;28:56–62. doi: 10.1080/10641950802233064. [DOI] [PubMed] [Google Scholar]
- 23.Afshari JT, Ghomian N, Shameli A, Shakeri MT, Fahmidehkar MA, Mahajer E, et al. Determination of Interleukin-6 and Tumor Necrosis Factor-alpha concentrations in Iranian-Khorasanian patients with preeclampsia. BMC Pregnancy Childbirth. 2005;5:14. doi: 10.1186/1471-2393-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu S, Wang H, Shi Y, Sun Y, Huang H, Dong M. Serum IL-16, not IL-15, was elevated in early second trimester of pregnancy in women who subsequently developed preeclampsia. Clin Chim Acta. 2007;383:175–7. doi: 10.1016/j.cca.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Poston L. Endothelial dysfunction in pre-eclampsia. Pharmacol Rep. 2006;58(Suppl):69–74. [PubMed] [Google Scholar]
- 26.Dong M, He J, Wang Z, Xie X, Wang H. Placental imbalance of Th1-and Th2-type cytokines in preeclampsia. Acta Obstet Gynecol Scand. 2005;84:788–93. doi: 10.1111/j.0001-6349.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- 27.Bakheit KH, Bayoumi NK, Eltom AM, Elbashir MI, Adam I. Cytokines profiles in Sudanese women with preeclampsia. Hypertens Pregnancy. 2009;28:224–9. doi: 10.1080/10641950802601245. [DOI] [PubMed] [Google Scholar]


