Abstract
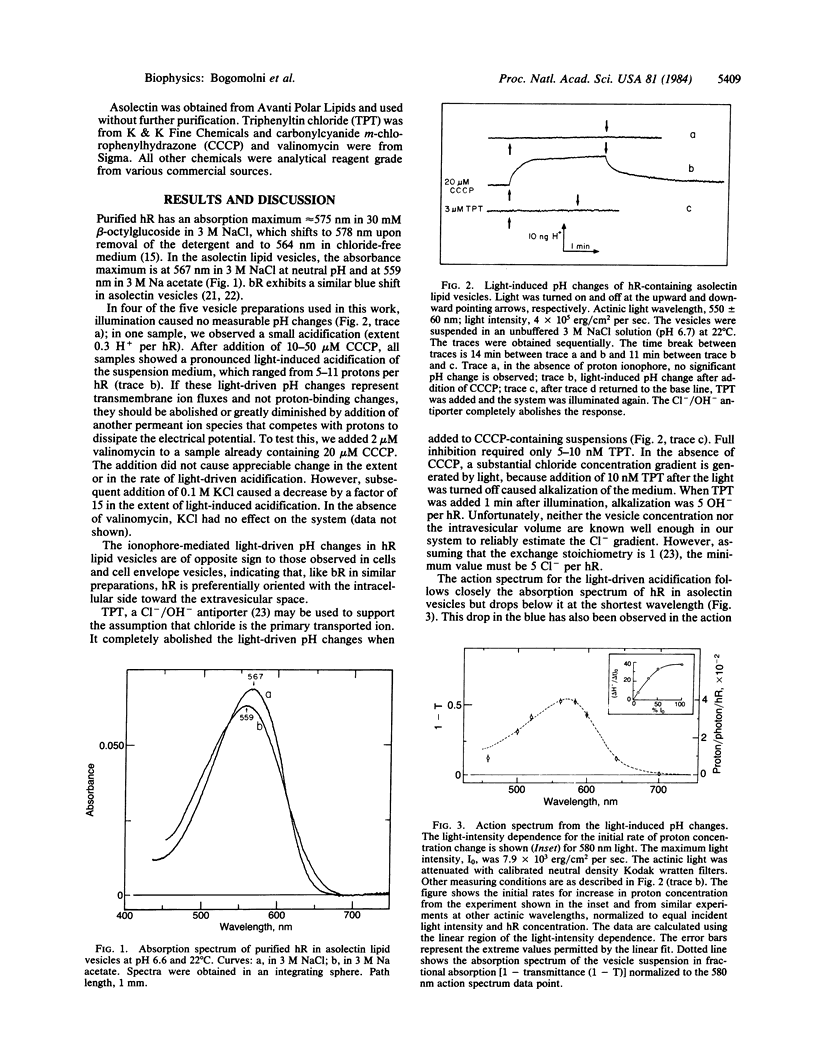
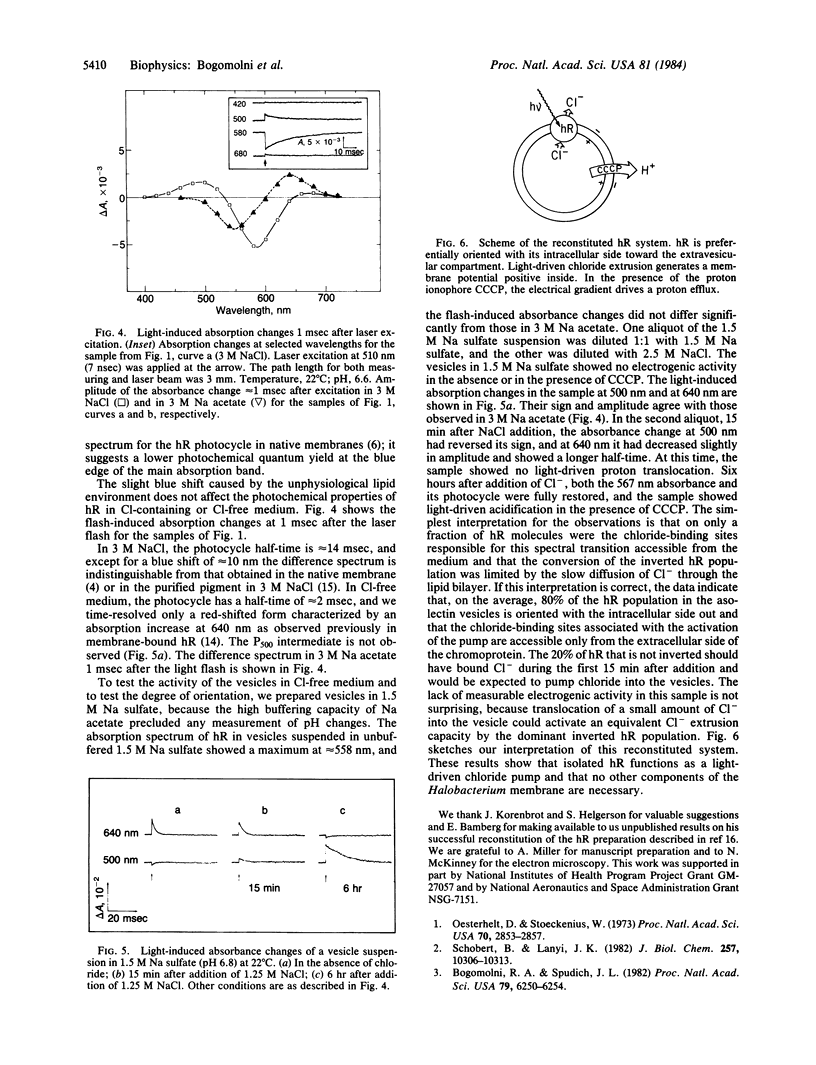
Asolectin lipid vesicles containing halorhodopsin show light-induced acidification in the presence of proton ionophores. This effect is abolished by triphenyltin chloride, a chloride/hydroxyl antiporter, and is greatly diminished by valinomycin in the presence of potassium ions, which collapse the membrane potential. This indicates that halorhodopsin orients in the lipid vesicles preferentially inside out, pumping chloride into the extravesicular compartment. The absorption maximum of halorhodopsin in asolectin vesicles in 3 M NaCl is at 567 nm, and the action spectrum for the light-induced pH changes followed closely the absorption spectrum. Replacement of chloride by acetate or sulfate causes a shift in the absorption maximum to approximately equal to 559 nm and renders the pump inactive. The different photocycles of the two forms were used to show that 80% of the molecules have the extracellular side exposed to the vesicle interior and that the halide-binding site(s) associated with the spectral transition is accessible from the extracellular side of the molecule. The data presented demonstrate that the purified chromoprotein is the light-driven chloride pump in Halobacterium halobium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogomolni R. A., Baker R. A., Lozier R. H., Stoeckenius W. Action spectrum and quantum efficiency for proton pumping in Halobacterium halobium. Biochemistry. 1980 May 13;19(10):2152–2159. doi: 10.1021/bi00551a024. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Spudich J. L. Identification of a third rhodopsin-like pigment in phototactic Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6250–6254. doi: 10.1073/pnas.79.20.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K., Weber H. J. Spectrophotometric identification of the pigment associated with light-driven primary sodium translocation in Halobacterium halobium. J Biol Chem. 1980 Jan 10;255(1):243–250. [PubMed] [Google Scholar]
- Lindley E. V., MacDonald R. E. A second mechanism for sodium extrusion in Halobacterium halobium: a light-driven sodium pump. Biochem Biophys Res Commun. 1979 May 28;88(2):491–499. doi: 10.1016/0006-291x(79)92075-8. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W., Bogomolni R. A., Hwang S., Stoeckenius W. Kinetics and stoichiometry of light-induced proton release and uptake from purple membrane fragments, Halobacterium halobium cell envelopes, and phospholipid vesicles containing oriented purple membrane. Biochim Biophys Acta. 1976 Sep 13;440(3):545–556. doi: 10.1016/0005-2728(76)90041-4. [DOI] [PubMed] [Google Scholar]
- Mukohata Y., Kaji Y. Light-induced membrane-potential increase, ATP synthesis, and proton uptake in Halobacterium halobium, R1mR catalyzed by halorhodopsin: Effects of N,N'-dicyclohexylcarbodiimide, triphenyltin chloride, and 3,5-di-tert-butyl-4-hydroxybenzylidenemalononitrile (SF6847). Arch Biochem Biophys. 1981 Jan;206(1):72–76. doi: 10.1016/0003-9861(81)90067-9. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogurusu T., Maeda A., Sasaki N., Yoshizawa T. Light-induced reaction of halorhodopsin prepared under low salt conditions. J Biochem. 1981 Nov;90(5):1267–1273. doi: 10.1093/oxfordjournals.jbchem.a133591. [DOI] [PubMed] [Google Scholar]
- Ogurusu T., Maeda A., Yoshizawa T. Absorption spectral properties of purified halorhodopsin. J Biochem. 1984 Apr;95(4):1073–1082. doi: 10.1093/oxfordjournals.jbchem.a134695. [DOI] [PubMed] [Google Scholar]
- Schobert B., Lanyi J. K. Halorhodopsin is a light-driven chloride pump. J Biol Chem. 1982 Sep 10;257(17):10306–10313. [PubMed] [Google Scholar]
- Selwyn M. J., Dawson A. P., Stockdale M., Gains N. Chloride-hydroxide exchange across mitochondrial, erythrocyte and artificial lipid membranes mediated by trialkyl- and triphenyltin compounds. Eur J Biochem. 1970 May 1;14(1):120–126. doi: 10.1111/j.1432-1033.1970.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Spectroscopic discrimination of the three rhodopsinlike pigments in Halobacterium halobium membranes. Biophys J. 1983 Aug;43(2):243–246. doi: 10.1016/S0006-3495(83)84345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M., Oesterhelt D. Isolation and properties of the native chromoprotein halorhodopsin. EMBO J. 1983;2(8):1379–1385. doi: 10.1002/j.1460-2075.1983.tb01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Taylor M. E., Bogomolni R. A., Weber H. J. Purification of photochemically active halorhodopsin. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6172–6176. doi: 10.1073/pnas.80.20.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]


