Abstract
Hydroxyurea is the sole approved pharmacologic therapy for sickle cell disease (SCD). Higher fetal hemoglobin (HbF) levels diminish de-oxygenated sickle globin polymerization in vitro and clinically reduce the incidence of disease morbidities. Clinical and laboratory effects of hydroxyurea largely result from induction of HbF expression, though to a highly variable extent. Baseline and hydroxyurea-induced HbF expression are both inherited complex traits. In children with SCD, baseline HbF remains the best predictor of drug-induced levels, but accounts for only portion of the induction. A limited number of validated genetic loci are strongly associated with higher baseline HbF levels in SCD. For induced HbF levels, genetic approaches using candidate single nucleotide polymorphisms (SNP) have identified some of these same loci as also associated with induction. However, SNP associations to induced HbF are only partially independent of baseline levels. Additional approaches to understanding the impact of hydroxyurea on HbF and its other therapeutic effects on SCD include pharmaco-kinetic, gene expression and epigenetic analyses in patients and through existing murine models for SCD. Understanding the genetic and other factors underlying the variability in therapeutic effects of hydroxyurea for pediatric SCD is critical for prospectively predicting good responders and for designing other effective therapies.
INTRODUCTION
Healthy People 2020, the federal public health agenda, has set a goal of “Increase(ing) the proportion of persons with hemoglobinopathies who receive disease-modifying therapies”1. For the vast majority of people with sickle cell disease (SCD), the Healthy People goal will be reached through increased use of hydroxyurea (HU). Critical questions surrounding its use include how this drug works to ameliorate the clinical severity of SCD and what sub-population of children with SCD benefit most from its use. This review addresses these questions from a translational science perspective.
Sickle cell disease (SCD) affects an estimated 90,000 people in the U.S.2 with over 1900 newborns detected annually through universal newborn screening2. Infant screening, early preventive therapy and parental guidance have largely eliminated early child mortality from SCD3–5. Moreover, specialized care and on-going preventive services have prolonged average life expectancy6. Despite these successes, multi-organ damage and mortality accumulate by early adulthood, resulting in shortened lifespan6.
HU holds expanding promise for improved clinical outcomes. Over two decades ago, the seminal Multicenter Study of Hydroxyurea (MSH) phase III trial for adults demonstrated the striking clinical impact of HU: 40% reduction in the incidence of acute pain episodes, acute chest syndrome and hospitalization7. These results led to approval in 1998 of HU for use in symptomatic SCD by the United States Food Drug Administration (FDA). HU remains the only FDA-approved drug for SCD, but approval does not extend to pediatric use. The approval gap for children is partially attributed to the lack of a commercial pharmaceutical sponsor. Helping to span gap is the FDA’s recent commissioning of a pediatric study of the pharmacokinetics of HU and its relative bioavailability of the liquid formulation (http://clinicaltrials.gov/show/NCT01506544).
Clinical efficacy of HU treatment varies between individuals, although most patients with severe phenotypes benefit from its use7,8. This review describes newly identified mechanisms for the effects of HU, including genetic regulation of fetal hemoglobin (HbF) as a disease modifier and the biologic effects of HU on blood vessels and gene regulation. These recent advances improve the prospects for prospectively assessing efficacy of HU therapy, are inspiring clinical trials for additional salutatory effects of HU and may guide future drug development.
CLINICAL EFFECTS
The profound clinical effects of HU for children with SCD have been recently reviewed9–11 summarized here and in Table 1. Much of the work on HU in children with SCD has come from phase III trials led by Ware and colleagues, including pivotal studies such as HUGS, HUG-KIDS12–14, HUSOFT15, BABY-HUGS16–18 including an early pediatric trial published in 199912. French investigators have also contributed insights into the impact of HU19,20. Randomized pediatric trials with HU have demonstrated decreased pain episodes18, acute chest syndrome, hospitalization8,11,18, transfusion and splenic auto-infarction18 and improved quality of life21,22. Prolonged use sustains the laboratory effects of decreased anemia, markers of hemolysis, white blood cell and platelet counts and increased red cell mean corpuscular volume (MCV)23. Early HU use stabilizes renal hyperfiltration24 and hyposthenuria25, as well as age-dependent decreased HbF18. Induction of HbF is described below.
Table 1.
Clinical Effects of Hydroxurea on Children with SCDa
| Effects | Blood/Circulation | Organ/Whole body |
|---|---|---|
| Laboratory/Physiologic measurement | Elevate HbF levels8,14,23 and stabilize high infant levels18 Increase hemoglobin13–15 Increase MCV8,14,15,22 Decrease hemolysis13–15 Decrease WBC and platelet counts8,13–15 |
Brain: Improve TCD flow velocity 26,27 Spleen: Preserve blood flow15,18 b Lungs: Decrease acute chest syndrome15,18 Renal: Decrease hyperfiltration24 and hyposthenuria25 |
| Clinical/Well-being** |
b Fewer acute pain crises8,13,18 b Reduce dactylitis18 b Fewer transfusions18 b Fewer hospitalizations8,13,18 |
b,c Reduce mortality30–32 b Improve growth13,15 Improve quality of life21,22 |
| Not yet known | Stabilize HbF as adults Reduce allo-immunization (through reducing transfusion) Reduce transfusion-related iron toxicity (through reducing transfusion) |
b Improve overall lifespan for children b Improve cognitive development Protect from stroke/infarct Prevent long-term renal, lung, cardiac effects Reduce cholelithiasis Reduce retinopathy b Normalize timing of physical maturation b Maintain fertility |
MCV – Mean red cell volume TCD – Transcranial Doppler
Some of the effects have not been demonstrated across all pediatric age ranges nor by prospective randomized trials. Reports demonstrating effect by randomized trials are preferentially cited.
Patient-oriented outcomes
Established long-term outcome for adults
Of note, while the laboratory effects of HU apply across the pediatric ages tested, many of the various clinical improvements noted for one age range have not necessarily been assessed for other ranges. For example, reduced dactylitis, hyposthenuria and transfusions were noted in the BABY HUG trial of children enrolled at age 9–13 months17,18. Improved transcranial Doppler blood flow through large cerebral arteries has been demonstrated in school-aged children26,27. Despite positive findings, some of these trials had mixed results. For example, the primary endpoints of the BABY-HUG study were not met18. In the SWiTCH study for secondary stroke prevention, continued chronic transfusion was advantageous over HU with phlebotomy28. Moreover, HU reduces but does not eliminate the symptoms and morbidity of SCD. For example, the SWiTCH trial demonstrated that chronic transfusions more effectively prevented pain episodes than HU with phlebotomy29.
Two long-term studies demonstrated substantially improved lifespans from prolonged use of HU in adults, including a study based on the MSH trial30,31. Prospective lifespan data for children taking HU are not yet available due to later uptake into pediatric trials. Nonetheless, a recent retrospective study from Brazil reported improved childhood mortality for those taking HU for up to six years32. Collectively, these data are increasingly persuasive about the enduring impact of HU on SCD.
The pharmacokinetics of HU appears to follow a bi-phenotypic metabolism in children33. Multiple single base polymorphisms (SNP) are associated with two apparent pharmaco-kinetic profiles of HU uptake and excretion. However, these genotypes do not correlate with response by the biomarker HbF.
FETAL HEMOGLOBIN
The clinical severity of SCD is highly variable. Children experience multiple different clinical complications of differing severity and frequency. Fetal hemoglobin (HbF) is of critical importance in the major sickle sub-type of HbSS (and HbS-Beta zero thalassemia, here collectively referred to as HbSS). Lower HbF levels correlate with overall more severe disease manifestations34. Unlike HbA, HbF actively inhibits the polymerization of sickle hemoglobin, the underlying patho-physiology of SCD. In solution, fetal hemoglobin concentration higher than 15% prevents sickle globin polymerization35. The cut-off for defining lower risk of severe complications has been estimated at 20%36.
Sharp declines of HbF during infancy occur as HbF-producing gamma globin is replaced by beta globin. This switch leads to the predominant expression of either HbA or HbS. The F-to-S switch in children affected by HbSS37 occurs more gradually than the F-to-A switch in non-anemic children. HbF levels in toddlers with SCD stabilize by age 3 or 4 and are generally constant throughout childhood. Despite bearing the same beta globin sickle variant, affected populations with African ancestry exhibit wide variations in HbF levels37–40. In the U.S., pediatric levels vary from 3–20% of total hemoglobin, compared to only 0.5–2% for non-anemic individuals. The average HbF level in the U.S SCD pediatric population is approximately 10%36.
HU USE
Mechanism of action
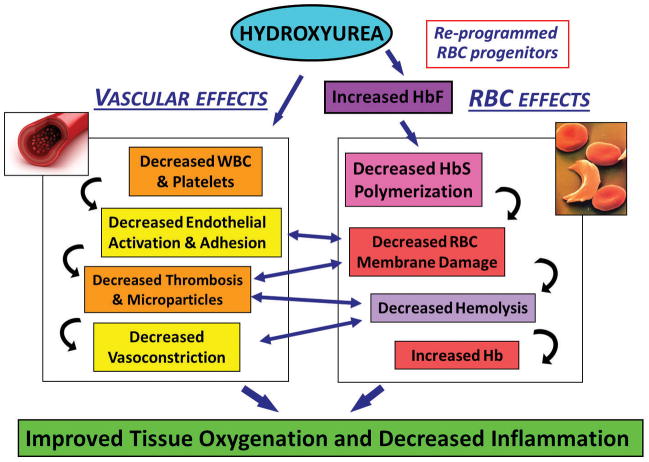
The physiology of the HU effect is complex, and can generally be generally categorized into two overlapping pathways: effects on HbF production and improved blood flow through reduced intercellular adhesion (Figure 1). HU is a short-acting cytotoxic drug that induces a state of “stress erythropoiesis.” Enhanced HbF production from intermittent mild marrow toxicity is believed to stem from steady shifting of marrow physiology to the stressed state. The marrow responds to the repetitive pharmacologic injury of daily use by enhanced erythropoiesis and increased HbF production34,41. Paradoxically, the net effect of marrow toxicity is induced HbF and stabilization of cellular hemoglobin solubility. These effects lead to decreased RBC membrane damage and hemolysis34,41.
Figure 1.
Physiologic Effects of Hydroxyurea on Sickle Cell Disease. Hydroxyurea has pleiotropic effects on ameliorating sickle cell disease, with complex and interacting vascular and red blood cell effects.
HbF induction usually occurs within the first few months after initiating HU, and is reversible upon cessation or diminution of dosing (Figure 2). Relevance of HU induction of HbF was demonstrated through a proof-of-principle murine model for SCD. Lack of expression of human HbF precluded HU induction in those mice. In that murine model, HU itself had no effect on improving anemia or protecting organs from SCD damage. In contrast, HbF gene therapy markedly improved the blood smear, microscopic and organ-level pathologic effects of SCD42.
Figure 2.
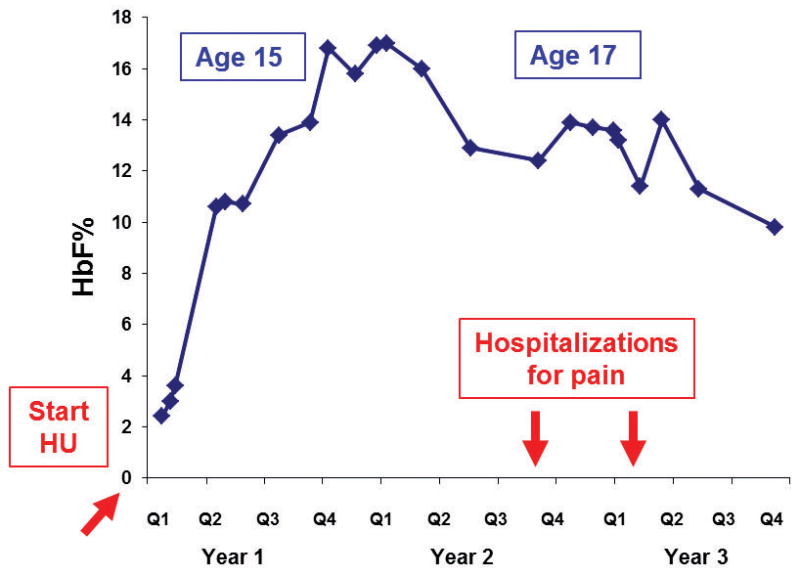
HbF Levels of a Teenager with HbSS on Hydroxyurea. Prior to hydroxyurea, this teenager had 2-3 hospitalizations for pain each year. She had no admissions for 1.7 years after beginning hydroxyurea. Her HbF baseline was 2.4% and HbF maximum recorded level was 16.9%. She acknowledged intermittent adherence in years 2–3, during which time she had 2 admissions for acute pain episodes. (Blue diamonds refer to HbF data points.)
HU appears to influence red blood cell-endothelial interactions. Decreased expression of RBC, WBC and endothelial integrins and other adhesion molecules probably improves micro-vascular blood flow and reduces pro-inflammatory cell-cell interactions43,44. Microvascular effects of SCD and HU appeared to be replicated using an interesting microfluidic model of blood flow and endothelialized microfluidic channels44. Whole blood samples from SCD lead to microvascular occlusion and thrombosis. Blood samples from patients with SCD had diminished velocity and greater tendency to obstruct in the microchannels. These effects nearly normalized using blood from patients on HU44. HU may be associated with reduced generation of microparticles, suggesting a reduction in markers of inflammation and thrombosis45.
HU may reduce cellular adhesion in general and/or adhesion provoked by infection or inflammation. Integrins and other cell surface glycoproteins regulate neutrophil migration and red blood cell flow through endothelial interactions. In a murine model for SCD and pneumococcal pneumonia and sepsis, HU provided some protection by decreasing the recruitment of neutrophils into infected lungs. Mice genetically engineered to lack E-selectin were not protected by HU46. This finding strengthens the view that HbF-independent effects of HU include decreasing leukocyte-endothelial adhesion.
HU may also stimulate nitric oxide production as an NO donor or through stimulating intermediates (discussed below). As a potent vasodilator, NO repletion contributes to improved vascular health in SCD (Figure 1)47. Along with decreased “sticky” interaction between of blood cells and the endothelium, enhanced nitric oxide-induced local vasodilation may also benefit blood flow (Figure 1)48,49. However, questions have arisen regarding these effects from NO50. In all, decreased pathology from damaged red blood cells and pathologic interactions between red blood cells and endothelial cells appear to synergistically reduce clinical signs symptoms and morbidities of the disease (Table 1). The ameliorative effects of HU appear to persist for as long as it is taken and the pharmaco-kinetics are maintained.
HbF response to HU
The individual extent of HU-induced HbF is highly variable. Standard pediatric dosing of HU adjusts for dose-dependent myelotoxicity14,33. Under these conditions, HU generally induces HbF an additional 8–18% over baseline levels14,33,51,52. In contrast to the bio-marker HbA1c for diabetes, no absolute HbF target exists. Nonetheless, peak attained HbF levels remain fairly constant in childhood 23. No absolute limit to the therapeutic amount of HbF induction has been described. For example, Southeast Asians or Saudis with SCD have baseline HbF levels averaging 16–20%. HU induction raises their levels 1.5 to 2-fold, associated with further diminution of their already tempered clinical symptoms53.
Children with SCD generally have higher baseline HbF levels than adults and more pronounced HbF response to HU14,54. Factors responsible for differences may include the need for highly regenerative marrow red cell precursors and, for HU, normal renal function for prompt excretion. Adults normally experience age-dependent decreased marrow cellularity. In SCD, disease-related marrow infarcts and other age-related physiologic effects could exacerbate normal marrow regression. Age-related diminution of response to HU increases the likelihood that genetic studies using pediatric populations may reveal more precise basic biologic insights.
Genetic analysis of HU-induced HbF
Analyses of HbF regulation are crucial to understanding the spectrum of SCD severity, variability of HU response and design of novel therapies. In addition to the established observations of ethnic variability of HbF levels, several key observations drive the rationale for identifying genetic components of HU induction of HbF in U.S. populations of SCD:
HbF induction from HU therapy is also a heritable trait55;
Genome-wide SNP studies in normal non-anemic adults identified on a few major loci associated with variation of low HbF levels. These regions are both cis and trans to the beta globin gene locus56,57;
These same loci are associated with baseline HbF in people with SCD in the U.S. 56,58–62. Additional loci have been identified but not yet replicated;
A modest correlation in children exists between levels of HbF at baseline and on HU14,33,51.
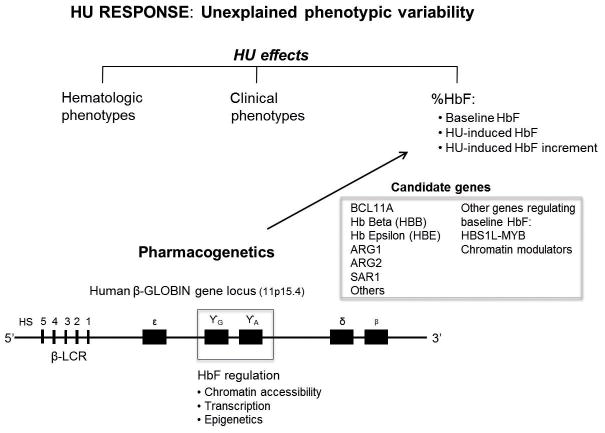
Taken together, these findings lead to the prediction that genetic regulation of HbF expression at baseline overlaps with the control of HU-induced HbF The three major loci related to HbF expression in normal and SCD populations are: a SNP upstream of the gamma globin gene within the globin locus on chromosome 11, previously identified by restriction enzyme analysis as the XmnI site37,60,61; BCL11A, a transcription factor now recognized as a major silencer of HbF expression51,58,59,61,63; the intergenic interval between the HBS1L and MYB56,58,61. Additional loci have been identified and await replication59,64,65, in addition to epigenetic effects, as well as probable epigenetic effects (Figure 3)66.
Figure 3.
Phenotypic Variability in Hydroxyurea Response. A diagram synthesizes the varying clinical and genetic effects of hydroxyurea in sickle cell disease. The beta globin locus is shown below.
Only a few published studies report on the genetics of HbF response to HU in SCD33,51,54,64. Compared to genomic studies of more common disorders, sample sizes of HU effects on SCD are inevitably modest. Using the retrospective cohort from the MSH adult trial and assessing over two dozen candidate genes, Ma et al. reported significant associations between SNPs and HbF response to HU in loci of genes involved in the metabolism of arginine to nitric oxide and in a transcription factor that induces DNA bending. This report pre-dated the identification of BCL11A as a central regulator of HbF expression. Most of the MSH patients exhibited a small HbF response to HU54, with less than a 5% change in HbF from baseline. This blunted response is not universal in U.S. adults with SCD, and may be influenced by patient characteristics as well as adherence to HU regimen.
Whether HbF induction by HU occurs directly through via the direct influence of BCL11A, is a concept awaiting direct testing. BCL11A effects on HbF are probably mediated through its protein partners, upstream or downstream effectors, chromatin structure and/or telomerase function (recently reviewed)67. Other reports include associations between HU response in SCD and polymorphisms in the guanosine triphosphate (GTP)-binding protein gene sar1a64, underscoring the complexity of the genetic pathways regulating the HbF response to HU (Figure 3).
Two pediatric pharmaco-genetics analyses using candidate single marker SNP polymorphisms suggested that just a few genes are associated with baseline HbF, including several SNPs within BCL11A (Table 2). SNP associations with induced HbF are generally not independent of baseline HbF levels33,51. In contrast to the induced HbF level, the treatment-associated increment appears to be a less relevant marker. Both of these observations probably reflect the association between baseline and induced levels.
Table 2.
SNP Polymorphisms Associated with HbF
| Gene | Phenotype | Clinic | Population | Ethnicity | N | SNP | β | p | Ref. # |
|---|---|---|---|---|---|---|---|---|---|
| ARG1 | HbF induced by HU | pediatric | USA | AA | 174 | rs17599586 | NA | 4 × 10−3 | 33 |
| ARG2 | HbF induced by HU | pediatric | USA | AA | 174 | rs2295644 | NA | 3 × 10−3 | 33 |
| ARG2 | HbF induced by HU | adult | USA | AA | 137 | rs10483801 | NA | 1 × 10−3 | 54 |
| BCL11A | HbF baseline | pediatric | USA | AA, H | 108 | rs4671393 | 2.88 | 5 × 10−5 | 51 |
| BCL11A | HbF baseline | pediatric | USA | AA | 174 | rs4671393 | NA | 3 × 10−4 | 33 |
| BCL11A | HbF baseline | adult | USA | AA | 255 | rs766432 | NA | 2 × 10−10 | 63 |
| BCL11A | HbF baseline | pediatric + adult | USA | AA | 1032 | rs4671393 | 0.60 | 4 × 10−37 | 60 |
| BCL11A | HbF baseline | pediatric + adult | USA | AA | 1275 | rs4671393 | 0.60 | 2 × 10−42 | 61 |
| BCL11A | HbF baseline | pediatric + adult | Brazil | NA | 350 | rs4671393 | 0.50 | 3 × 10−8 | 61 |
| BCL11A | HbF induced by HU | pediatric | USA | AA, H | 47 | rs1186868 | 3.37 | 0.019 | 51 |
| FTL1 | HbF induced by HU | adult | USA | AA | 137 | rs2182008 | NA | 0.003 | 54 |
| GLP2R | F cells baseline | pediatric + adult | USA | AA | 440 | rs12103880 | −1.36 | 3 × 10−8 | 59 |
| HAO2 | HbF induced by HU | adult | USA | AA | 137 | rs10494225 | NA | 2 × 10−3 | 54 |
| HBB | HbF baseline | pediatric | USA | AA, H | 108 | rs7482144 | 3.88 | 2 × 10−4 | 51 |
| HBB | HbF baseline | pediatric + adult | USA | AA | 1032 | rs10128556 | 0.42 | 2 × 10−9 | 60 |
| HBB | HbF baseline | pediatric + adult | USA | AA | 1275 | rs7482144 | 0.41 | 4 × 10−7 | 61 |
| HBE | HbF baseline | pediatric | USA | AA, H | 108 | rs7130110 | 2.86 | 6 × 10−5 | 51 |
| HBE | HbF baseline | pediatric | USA | AA | 174 | rs7130110 | NA | 3 × 10−5 | 33 |
| HBE | HbF induced by HU | pediatric | USA | AA, H | 38 | rs7130110 | 6.04 | 0.004 | 51 |
| HBS1L-MYB | HbF baseline | pediatric + adult | USA | AA | 1032 | rs9402686 | 0.65 | 2 × 10−13 | 60 |
| HBS1L-MYB | HbF baseline | pediatric + adult | USA | AA | 1275 | rs9399137 | 0.60 | 5 × 10−11 | 61 |
| NOS1 | HbF induced by HU | adult | USA | AA | 137 | rs7977109 | NA | 0.023 | 54 |
| OR51B5/B6 | HbF induced by HU | pediatric | USA | AA | 1153 | rs5006884 | 0.20 | 3 × 10−8 | 65 |
| OR51B6 | HbF baseline | pediatric | USA | AA, H | 108 | rs5024042 | 1.70 | 0.031 | 51 |
| SAR1A | HbF induced by HU | adult | USA | AA | 32 | rs4282891 | NA | < 0.05 | 64 |
AA: African American; H: Hispanics; AC: African Caribbean; WA: West African; N: sample size; All: phenotype associated allele
In our own, smaller multi-site analysis, baseline levels candidate were significantly associated with SNPs within the BCL11A and the beta and epsilon globin loci (HBB and HBE, respectively), with an additive attributable variance from these loci of 23% (Table 2)51. Consistent with studies by Ware and colleagues14,33, we reported that baseline HbF levels explained 33% of the variance in induced levels. The variant in HBE accounted for an additional 13% of the variance in induced levels, while variants in the HBB and BCL11A loci did not contribute beyond baseline levels. Thus our data suggest that the combined effects of baseline HbF and one SNP marker contributed an estimated 46% of the variance in HbF51.
By trend analysis, children with an allele associated with higher HbF (“favorable” allele) in one of the BCL11A and/or either globin marker had significantly higher average values of baseline HbF than those who lacked a favorable allele51. Effects on baseline HbF from a SNP in each these two genes were additive, and were associated with two-fold higher HbF for patients with favorable alleles in both loci. Similarly, having at least one favorable allele in either globin locus and BL11A was associated with a higher level of induced HbF. Statistical significance did not withstand adjustment for baseline fetal hemoglobin, likely reflecting the inter-relatedness of HbF regulation under both physiologic conditions. Genetic studies examining larger pediatric populations on HU, unusual responders and the influence of specific sequence variants are needed to the contribution of these and other genetic loci responsible for HbF response.
OTHER PHYSIOLOGIC EFFECTS OF HU
Cellular biology
The effects of HU largely depend upon its effects on nucleic acid synthesis in dividing red blood cell progenitors. HU affects the S-phase by inhibiting ribonucleotide reductase, an enzyme important for DNA synthesis. Depletion of DNA precursors by HU causes arrest of the replication fork, leading to cell death. A cell-based ex vivo assay for HbF induction, burst-forming unit erythroid (BFU-E) colonies grown in methylcellulose from blood of children with SCD, demonstrated that HU decreases the number of BFU-E colonies. HU and other ribonucleotide reductase inhibitors increase HbF production in that system. Interestingly, other cytotoxic agents that are not of that drug category, such as cytarabine and alkylating agents, decreased BFU-E counts but did not induce HbF41,68.
HU’s lethal effect of on ribonucleotide reductase and cell survival are also seen in laboratory bacteria such as E. coli69. Whether the bactericidal effects influence investigation using animal models or even patients has not previously been raised. Direct bacterial effects should be addressed on the HU-dampened expression of adhesion molecules in a murine model of bacterial infection.
HbF response to temporary marrow toxicity is probably attributable to transcriptional and epigenetic effects on the progenitor developmental program66,70. HU signaling appears to involve cyclic guanine monophosphate (cGMP), cAMP, p38MAPK, and others pathways. Activation of cGMP may induce HbF via enhancing production of nitric oxide (NO)47,68,71,72. NO may also support HbF production47. HU induces a small guanosine triphosphate (GTP)-binding protein, secretion-associated and RAS-related (SAR)73. SAR may be involved in the activation of transcription factors and signal transduction pathways in erythroleukemia K562 cells and in human bone marrow-derived progenitor cells. HU may also function through kinase and signal transduction pathways, such as GATA-1, to enhance gamma and beta globin synthesis in erythroid cells47.
Gene expression
Comparing whole mRNA pre- and post- initiation of therapy revealed that HU affects expression of a number of genes involved in transcription and translation, such as ribosome assembly and chromosomal organization66,70. Results may vary with age, dosing or other clinical conditions. Variation in cell source would be expected to affect detection of expressed genes, whether from bone marrow or purified early reticulocytes. HU may also affect expression of genes that link HU and HbF to BLCL11A66,70. Epigenetic analysis of the gamma globin promoter did not find reveal much impact from HU74. Interestingly, HU appears to up-regulate specific miRNAs74. These results require further investigation, but underscore the view that HU is involved with complex pathways of gene regulation.
Testing for oncogenicity
The primary effect is damaging DNA replication by inhibiting ribonucleotide reductase. This effect raises concerns about an oncogenic potential, especially after prolonged use. These fears have been amplified by its original use as chemotherapy for chronic myeloid leukemia (CML), the latent phase of acute leukemia. While links acute leukemia outside of CML have been disproven75, concerns for the safety of long-term use in children persist. Several studies have tested DNA and cellular toxicity from pediatric HU users. No genotoxicity was detected using several different assays in vitro, including karyotype, illegitimate VDJ recombination, chromatid breaks9. Increased reticulocyte micronuclei were observed, but this effect was highly variable between patients and did not increase with time76. In all, oncogenicity of HU is probably quite low or non-existent. A few cases of acute leukemia were reported in patients after many years of HU treatment, but do not appear to be more frequent than in the untreated population75.
POTENTIAL PHARMACOLOGIC ALTERNATIVES TO HU
Other HbF inducers have been assessed over the past few decades, including nucleoside analogs such as 5-azacytidine and decitabine. However, they are often poorly tolerated potentially oncogenic and lack proof of effectiveness comparable to HU (recently reviewed)41. Additional HbF-inducing drugs are histone deacetylase inhibitors, erythropoietin (already high in SCD and shown not to induce HbF in SCD), valproate, thalidomide derivatives (e.g. pomalidimide), kit ligand. In all, a variety of cellular stresses and stimuli can promote coordinated stress responses, including activation of the gamma globin gene66,70. Based on results from use of SCD mouse models, inhibitor of phosphodiesterase 971 or inducible factor-1α (HIF-1α)77, alone or in combination with HU, may be clinically useful to stimulate cyclic GMP (cGMP) and nitric oxide for HbF production and/or to enhance its anti-sickling impact71.
BARRIERS TO HU UTILIZATION
Outside of clinical trials with HU, ample documentation exists of incomplete clinical effectiveness of HU. Uneven drug adherence has been well documented78,79. Provider non- and under-utilization is well documented75,80. Our recent multi-site survey of parents of children with SCD revealed several family barriers to use of HU such as lack of FDA approval, near-universal safety concerns and highly varied knowledge about its benefits, including many for whom its basic property of decreasing episodes of pain was unknown81. Use of HU was positively correlated with fundamental knowledge of parents in the basic positive effects of HU on disease, independent of parental demographics such as education level, language spoken or ethnicity. Barriers in effective communication between providers and families may be exacerbated by issues arising from medical delivery systems.
The mixed uptake of HU by families may also reflect family perspectives on the long-term effects of SCD. A single site survey of parents revealed that the majority believed that the disease effects were going to diminish over time, and would not impact life goals or lifespan82. These poignant perceptions will need to be addressed if families are to embrace the long-term benefits of HU against its inconveniences and largely theoretical risks.
CONCLUSION
HU is a remarkably effective drug for a large proportion of children with SCD. Expanded understanding of the scientific underpinnings of its effects on SCD, the ability to predict individual response and the clinical applications for modifying disease effects are ongoing. Clinical trials will continue to test the uncertain benefits of HU (Table 1), such as primary prevention of brain infarcts (clinicaltrials.gov/show/NCT01389024). Murine models will facilitate insight into the benefits provided by induced HbF, altered expression of adhesion molecules, reduced BCL11A and other mechanisms. Genetic epidemiology will be used to identify specific variants in regulatory genes and gene pathways.
The accumulating science of HU is anticipated to lead to three direct effects for children with HbSS: 1) Use at earlier ages; 2) Wider clinical indications; and 3) Delineation of children who are less likely to enjoy substantive benefit from HU. For this last group, more aggressive consideration of chronic transfusion, hematopoietic stem cell transplantation or trial of emerging alternative agents may be warranted. To date, early clinical trials of other experimental HbF-inducing drugs have demonstrated considerable short- and long-term toxicity compared to HU. Therefore, HU is predicted to remain the mainstay of pharmacologic therapy for SCD in the foreseeable future.
Progress towards the Healthy People 2020 goals will occur through increased use of HU. Nonetheless, the entire SCD population may not benefit from HU alone. Dampened impact on clinical complications and HbF induction occurs for those with certain genotypes, many patients with HbSC, some adult patients and those with renal compromise. New, effective and safe therapies, alone or in combination with HU, are still needed to maximize pharmacologic benefit for everyone living with SCD. Constructive engagement must be made to assist families in undertaking long-term HU use to help them to balance the optimism of HU treatment and its potential toxicities with the risk of accumulating disease consequences. While several important crisis-modulators are currently under investigation, disease modifiers that prevent crises and other morbidities is arguably the primary therapeutic target.
Acknowledgments
STATEMENT OF FINANCIAL SUPPORT: The authors acknowledge support from the Clinical Translational Science Award (CTSA) at Columbia University, 5UL1RR024156 (H.N. Ginsberg, PI)
Footnotes
DISCLOSURES: No commercial or conflict of interest is reported by the authors.
References
- 1. [Accessed 6 September 2012];Healthy People 2020. http://healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=4.
- 2.Hassell KL. Population estimates of sickle cell disease in the U. S Am J Prev Med. 2010;38:S512–21. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999–2009) Pediatr Blood Cancer. 2013;60:1482–6. doi: 10.1002/pbc.24557. [DOI] [PubMed] [Google Scholar]
- 4.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115:3447–52. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanni E, Grosse SD, Yang Q, Olney RS. Trends in pediatric sickle cell disease-related mortality in the United States, 1983–2002. J Pediatr. 2009;154:541–5. doi: 10.1016/j.jpeds.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 6.Lanzkron S, Carroll CP, Haywood C., Jr Mortality rates and age at death from sickle cell disease: U.S. 1979–2005. Public Health Rep. 2013;128:110–6. doi: 10.1177/003335491312800206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332:1317–22. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 8.Ferster A, Vermylen C, Cornu G, et al. Hydroxyurea for treatment of severe sickle cell anemia: a pediatric clinical trial. Blood. 1996;88:1960–4. [PubMed] [Google Scholar]
- 9.McGann PT, Ware RE. Hydroxyurea for sickle cell anemia: what have we learned and what questions still remain? Curr Opin Hematol. 2011;18:158–65. doi: 10.1097/MOH.0b013e32834521dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strouse JJ, Heeney MM. Hydroxyurea for the treatment of sickle cell disease: efficacy, barriers, toxicity, and management in children. Pediatr Blood Cancer. 2012;59:365–71. doi: 10.1002/pbc.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strouse JJ, Lanzkron S, Beach MC, et al. Hydroxyurea for sickle cell disease: a systematic review for efficacy and toxicity in children. Pediatrics. 2008;122:1332–42. doi: 10.1542/peds.2008-0441. [DOI] [PubMed] [Google Scholar]
- 12.Kinney TR, Helms RW, O’Branski EE, et al. Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Pediatric Hydroxyurea Group. Blood. 1999;94:1550–4. [PubMed] [Google Scholar]
- 13.Wang WC, Helms RW, Lynn HS, et al. Effect of hydroxyurea on growth in children with sickle cell anemia: results of the HUG-KIDS Study. J Pediatr. 2002;140:225–9. doi: 10.1067/mpd.2002.121383. [DOI] [PubMed] [Google Scholar]
- 14.Ware RE, Eggleston B, Redding-Lallinger R, et al. Predictors of fetal hemoglobin response in children with sickle cell anemia receiving hydroxyurea therapy. Blood. 2002;99:10–4. doi: 10.1182/blood.v99.1.10. [DOI] [PubMed] [Google Scholar]
- 15.Hankins JS, Ware RE, Rogers ZR, et al. Long-term hydroxyurea therapy for infants with sickle cell anemia: the HUSOFT extension study. Blood. 2005;106:2269–75. doi: 10.1182/blood-2004-12-4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thornburg CD, Dixon N, Burgett S, et al. A pilot study of hydroxyurea to prevent chronic organ damage in young children with sickle cell anemia. Pediatr Blood Cancer. 2009;52:609–15. doi: 10.1002/pbc.21738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornburg CD, Files BA, Luo Z, et al. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood. 2012;120:4304–10. doi: 10.1182/blood-2012-03-419879. quiz 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377:1663–72. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernaudin F, Verlhac S, Arnaud C, et al. Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood. 2011;117:1130–40. doi: 10.1182/blood-2010-06-293514. quiz 436. [DOI] [PubMed] [Google Scholar]
- 20.de Montalembert M, Brousse V, Elie C, et al. Long-term hydroxyurea treatment in children with sickle cell disease: tolerance and clinical outcomes. Haematologica. 2006;91:125–8. [PubMed] [Google Scholar]
- 21.Thornburg CD, Calatroni A, Panepinto JA. Differences in health-related quality of life in children with sickle cell disease receiving hydroxyurea. J Pediatr Hematol Oncol. 2011;33:251–4. doi: 10.1097/MPH.0b013e3182114c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dampier C, Lieff S, LeBeau P, et al. Health-related quality of life in children with sickle cell disease: a report from the Comprehensive Sickle Cell Centers Clinical Trial Consortium. Pediatr Blood Cancer. 2010;55:485–94. doi: 10.1002/pbc.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmerman SA, Schultz WH, Davis JS, et al. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103:2039–45. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 24.Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. American journal of hematology. 2013;88:116–9. doi: 10.1002/ajh.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez O, Miller ST, Wang WC, et al. Effect of hydroxyurea treatment on renal function parameters: results from the multi-center placebo-controlled BABY HUG clinical trial for infants with sickle cell anemia. Pediatr Blood Cancer. 2012;59:668–74. doi: 10.1002/pbc.24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kratovil T, Bulas D, Driscoll MC, Speller-Brown B, McCarter R, Minniti CP. Hydroxyurea therapy lowers TCD velocities in children with sickle cell disease. Pediatr Blood Cancer. 2006;47:894–900. doi: 10.1002/pbc.20819. [DOI] [PubMed] [Google Scholar]
- 27.Zimmerman SA, Schultz WH, Burgett S, Mortier NA, Ware RE. Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood. 2007;110:1043–7. doi: 10.1182/blood-2006-11-057893. [DOI] [PubMed] [Google Scholar]
- 28.Ware RE, Helms RW, Investigators SW. Stroke With Transfusions Changing to Hydroxyurea (SWiTCH) Blood. 2012;119:3925–32. doi: 10.1182/blood-2011-11-392340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez O, Yovetich NA, Scott JP, et al. Pain and other non-neurological adverse events in children with sickle cell anemia and previous stroke who received hydroxyurea and phlebotomy or chronic transfusions and chelation: Results from the SWiTCH clinical trial. American journal of hematology. 2013 doi: 10.1002/ajh.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17. 5 year follow-up. American journal of hematology. 2010;85:403–8. doi: 10.1002/ajh.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS) Blood. 2010;115:2354–63. doi: 10.1182/blood-2009-05-221333. [DOI] [PubMed] [Google Scholar]
- 32.Lobo CL, Pinto JF, Nascimento EM, Moura PG, Cardoso GP, Hankins JS. The effect of hydroxcarbamide therapy on survival of children with sickle cell disease. Br J Haematol. 2013;161:852–60. doi: 10.1111/bjh.12323. [DOI] [PubMed] [Google Scholar]
- 33.Ware RE, Despotovic JM, Mortier NA, et al. Pharmacokinetics, pharmacodynamics, and pharmacogenetics of hydroxyurea treatment for children with sickle cell anemia. Blood. 2011;118:4985–91. doi: 10.1182/blood-2011-07-364190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Platt OS. Hydroxyurea for the treatment of sickle cell anemia. N Engl J Med. 2008;358:1362–9. doi: 10.1056/NEJMct0708272. [DOI] [PubMed] [Google Scholar]
- 35.Bunn HF. Subunit assembly of hemoglobin: an important determinant of hematologic phenotype. Blood. 1987;69:1–6. [PubMed] [Google Scholar]
- 36.Powars DR, Weiss JN, Chan LS, Schroeder WA. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood. 1984;63:921–6. [PubMed] [Google Scholar]
- 37.Green NS, Fabry ME, Kaptue-Noche L, Nagel RL. Senegal haplotype is associated with higher HbF than Benin and Cameroon haplotypes in African children with sickle cell anemia. American journal of hematology. 1993;44:145–6. doi: 10.1002/ajh.2830440214. [DOI] [PubMed] [Google Scholar]
- 38.Makani J, Menzel S, Nkya S, et al. Genetics of fetal hemoglobin in Tanzanian and British patients with sickle cell anemia. Blood. 2011;117:1390–2. doi: 10.1182/blood-2010-08-302703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagel RL, Erlingsson S, Fabry ME, et al. The Senegal DNA haplotype is associated with the amelioration of anemia in African-American sickle cell anemia patients. Blood. 1991;77:1371–5. [PubMed] [Google Scholar]
- 40.Solovieff N, Hartley SW, Baldwin CT, et al. Ancestry of African Americans with sickle cell disease. Blood Cells Mol Dis. 2011;47:41–5. doi: 10.1016/j.bcmd.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fathallah H, Atweh GF. Induction of fetal hemoglobin in the treatment of sickle cell disease. Hematology Am Soc Hematol Educ Program. 2006:58–62. doi: 10.1182/asheducation-2006.1.58. [DOI] [PubMed] [Google Scholar]
- 42.Lebensburger JD, Pestina TI, Ware RE, Boyd KL, Persons DA. Hydroxyurea therapy requires HbF induction for clinical benefit in a sickle cell mouse model. Haematologica. 2010;95:1599–603. doi: 10.3324/haematol.2010.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gambero S, Canalli AA, Traina F, et al. Therapy with hydroxyurea is associated with reduced adhesion molecule gene and protein expression in sickle red cells with a concomitant reduction in adhesive properties. Eur J Haematol. 2007;78:144–51. doi: 10.1111/j.1600-0609.2006.00788.x. [DOI] [PubMed] [Google Scholar]
- 44.Tsai M, Kita A, Leach J, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122:408–18. doi: 10.1172/JCI58753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nebor D, Romana M, Santiago R, et al. Fetal hemoglobin and hydroxycarbamide moduate both plasma concentration and cellular origin of circulating microparticles in sickle cell anemia children. Haematologica. 2013;98:862–7. doi: 10.3324/haematol.2012.073619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lebensburger JD, Howard T, Hu Y, et al. Hydroxyurea therapy of a murine model of sickle cell anemia inhibits the progression of pneumococcal disease by down-modulating E-selectin. Blood. 2012;119:1915–21. doi: 10.1182/blood-2011-08-374447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lou TF, Singh M, Mackie A, Li W, Pace BS. Hydroxyurea generates nitric oxide in human erythroid cells: mechanisms for gamma-globin gene activation. Exp Biol Med (Maywood) 2009;234:1374–82. doi: 10.3181/0811-RM-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions. American journal of hematology. 2009;84:618–25. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King SB. Nitric oxide production from hydroxyurea. Free Radic Biol Med. 2004;37:737–44. doi: 10.1016/j.freeradbiomed.2004.02.073. [DOI] [PubMed] [Google Scholar]
- 50.Bunn HF, Nathan DG, Dover GJ, et al. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010;116:687–92. doi: 10.1182/blood-2010-02-268193. [DOI] [PubMed] [Google Scholar]
- 51.Green NS, Ender KL, Pashankar F, et al. Candidate Sequence Variants and Fetal Hemoglobin in Children with Sickle Cell Disease Treated with Hydroxyurea. PloS One. 2013 doi: 10.1371/journal.pone.0055709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meier ER, Byrnes C, Weissman M, Noel P, Luban NL, Miller JL. Expression patterns of fetal hemoglobin in sickle cell erythrocytes are both patient- and treatment-specific during childhood. Pediatr Blood Cancer. 2011;56:103–9. doi: 10.1002/pbc.22643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Italia K, Jain D, Gattani S, et al. Hydroxyurea in sickle cell disease--a study of clinico-pharmacological efficacy in the Indian haplotype. Blood Cells Mol Dis. 2009;42:25–31. doi: 10.1016/j.bcmd.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Ma Q, Wyszynski DF, Farrell JJ, et al. Fetal hemoglobin in sickle cell anemia: genetic determinants of response to hydroxyurea. Pharmacogenomics J. 2007;7:386–94. doi: 10.1038/sj.tpj.6500433. [DOI] [PubMed] [Google Scholar]
- 55.Steinberg MH, Voskaridou E, Kutlar A, et al. Concordant fetal hemoglobin response to hydroxyurea in siblings with sickle cell disease. American journal of hematology. 2003;72:121–6. doi: 10.1002/ajh.10264. [DOI] [PubMed] [Google Scholar]
- 56.Thein SL, Menzel S. Discovering the genetics underlying foetal haemoglobin production in adults. Br J Haematol. 2009;145:455–67. doi: 10.1111/j.1365-2141.2009.07650.x. [DOI] [PubMed] [Google Scholar]
- 57.Garner C, Tatu T, Reittie JE, et al. Genetic influences on F cells and other hematologic variables: a twin heritability study. Blood. 2000;95:342–6. [PubMed] [Google Scholar]
- 58.Bae HT, Baldwin CT, Sebastiani P, et al. Meta-analysis of 2040 sickle cell anemia patients: BCL11A and HBS1L-MYB are the major modifiers of HbF in African Americans. Blood. 2012;120:1961–2. doi: 10.1182/blood-2012-06-432849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhatnagar P, Purvis S, Barron-Casella E, et al. Genome-wide association study identifies genetic variants influencing F-cell levels in sickle-cell patients. J Hum Genet. 2011;56:316–23. doi: 10.1038/jhg.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galarneau G, Palmer CD, Sankaran VG, Orkin SH, Hirschhorn JN, Lettre G. Fine-mapping at three loci known to affect fetal hemoglobin levels explains additional genetic variation. Nat Genet. 2010;42:1049–51. doi: 10.1038/ng.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lettre G, Sankaran VG, Bezerra MA, et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci U S A. 2008;105:11869–74. doi: 10.1073/pnas.0804799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sebastiani P, Solovieff N, Hartley SW, et al. Genetic modifiers of the severity of sickle cell anemia identified through a genome-wide association study. American journal of hematology. 2010;85:29–35. doi: 10.1002/ajh.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sedgewick AE, Timofeev N, Sebastiani P, et al. BCL11A is a major HbF quantitative trait locus in three different populations with beta-hemoglobinopathies. Blood Cells Mol Dis. 2008;41:255–8. doi: 10.1016/j.bcmd.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumkhaek C, Taylor JGt, Zhu J, Hoppe C, Kato GJ, Rodgers GP. Fetal haemoglobin response to hydroxycarbamide treatment and sar1a promoter polymorphisms in sickle cell anaemia. Br J Haematol. 2008;141:254–9. doi: 10.1111/j.1365-2141.2008.07045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solovieff N, Milton JN, Hartley SW, et al. Fetal hemoglobin in sickle cell anemia: genome-wide association studies suggest a regulatory region in the 5′ olfactory receptor gene cluster. Blood. 2010;115:1815–22. doi: 10.1182/blood-2009-08-239517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flanagan JM, Steward S, Howard TA, et al. Hydroxycarbamide alters erythroid gene expression in children with sickle cell anaemia. Br J Haematol. 2012;157:240–8. doi: 10.1111/j.1365-2141.2012.09061.x. [DOI] [PubMed] [Google Scholar]
- 67.Bauer DE, Orkin SH. Update on fetal hemoglobin gene regulation in hemoglobinopathies. Curr Opin Pediatr. 2011;23:1–8. doi: 10.1097/MOP.0b013e3283420fd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang YM, Pace B. Pharmacologic induction of fetal hemoglobin synthesis: cellular and molecular mechanisms. Pediatr Pathol Mol Med. 2001;20:87–106. [PubMed] [Google Scholar]
- 69.Bollenbach T, Quan S, Chait R, Kishony R. Nonoptimal microbial response to antibiotics underlies suppressive drug interactions. Cell. 2009;139:707–18. doi: 10.1016/j.cell.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costa FC, da Cunha AF, Fattori A, et al. Gene expression profiles of erythroid precursors characterise several mechanisms of the action of hydroxycarbamide in sickle cell anaemia. Br J Haematol. 2007;136:333–42. doi: 10.1111/j.1365-2141.2006.06424.x. [DOI] [PubMed] [Google Scholar]
- 71.Almeida CB, Scheiermann C, Jang JE, et al. Hydroxyurea and a cGMP-amplifying agent have immediate benefits on acute vaso-occlusive events in sickle cell disease mice. Blood. 2012;120:2879–88. doi: 10.1182/blood-2012-02-409524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cokic VP, Andric SA, Stojilkovic SS, Noguchi CT, Schechter AN. Hydroxyurea nitrosylates and activates soluble guanylyl cyclase in human erythroid cells. Blood. 2008;111:1117–23. doi: 10.1182/blood-2007-05-088732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang DC, Zhu J, Liu W, et al. The hydroxyurea-induced small GTP-binding protein SAR modulates gamma-globin gene expression in human erythroid cells. Blood. 2005;106:3256–63. doi: 10.1182/blood-2003-10-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walker AL, Steward S, Howard TA, et al. Epigenetic and molecular profiles of erythroid cells after hydroxyurea treatment in sickle cell anemia. Blood. 2011;118:5664–70. doi: 10.1182/blood-2011-07-368746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lanzkron S, Strouse JJ, Wilson R, et al. Systematic review: Hydroxyurea for the treatment of adults with sickle cell disease. Ann Intern Med. 2008;148:939–55. doi: 10.7326/0003-4819-148-12-200806170-00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flanagan JM, Howard TA, Mortier N, et al. Assessment of genotoxicity associated with hydroxyurea therapy in children with sickle cell anemia. Mutat Res. 2010;698:38–42. doi: 10.1016/j.mrgentox.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaul DK, Fabry ME, Suzuka SM, Zhang X. Antisickling fetal hemoglobin reduces hypoxia-inducible factor-1alpha expression in normoxic sickle mice: microvascular implications. Am J Physiol Heart Circ Physiol. 2013;304:H42–50. doi: 10.1152/ajpheart.00296.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Candrilli SD, O’Brien SH, Ware RE, Nahata MC, Seiber EE, Balkrishnan R. Hydroxyurea adherence and associated outcomes among Medicaid enrollees with sickle cell disease. American journal of hematology. 2011;86:273–7. doi: 10.1002/ajh.21968. [DOI] [PubMed] [Google Scholar]
- 79.Thornburg CD, Rogers ZR, Jeng MR, et al. Adherence to study medication and visits: data from the BABY HUG trial. Pediatr Blood Cancer. 2010;54:260–4. doi: 10.1002/pbc.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haywood C, Jr, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc. 2009;101:1022–33. doi: 10.1016/s0027-9684(15)31069-5. [DOI] [PubMed] [Google Scholar]
- 81.Oyeku SO, Driscoll MC, Cohen HW, et al. Parental and other factors associated with hydroxyurea use for pediatric sickle cell disease. Pediatr Blood Cancer. 2013;60:653–8. doi: 10.1002/pbc.24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roth M, Krystal J, Manwani D, Driscoll C, Ricafort R. Stem cell transplant for children with sickle cell anemia: parent and patient interest. Biol Blood Marrow Transplant. 2012;18:1709–15. doi: 10.1016/j.bbmt.2012.05.013. [DOI] [PubMed] [Google Scholar]