Summary
Improvements in comprehensive burn care, as practiced in dedicated burns units, have reduced mortality and morbidity rates significantly. Strategies deemed most important include the application of fluid resuscitation and nutrition protocols, intensive care and antimicrobial dressings, as well as early excision and grafting. Autografting is limited, however, by availability in very extensive burns, despite the use of expanded (meshed) skin. Alternatives have therefore been required, and deceased donor allograft is considered the gold standard. Fresh allograft use is limited by supply, and legislative and cultural restrictions have significantly influenced availability, despite evidence of its efficacy. This necessitates the establishment of a deceased donor skin bank in South Africa, with a mandate to procure and store allograft for distribution to burns units when required.
Keywords: major burn injuries, burn care in developing countries, skin banking, deceased donor/cadaver skin, allograft
Abstract
L’amélioration des soins aux brûlés, tels qu’ils sont pratiqués dans les unités réservées aux brûlés, a réduit significativement la mortalité et la morbidité. Stratégies jugées les plus importantes comprennent l’application des protocoles sur les fluides de réanimation et de la nutrition, les soins intensifs et les pansements antimicrobiens, ainsi que l’excision précoce et le greffage. L’autogreffe est cependant limitée par la disponibilité chez les grandes brûlures, malgré l’utilisation de la peau étendue (maillée). Des alternatives ont donc été nécessaires, et allogreffe d’un donneur décédé est considérée comme l’étalon-or. Utilisation d’allogreffe fraîche est limitée par l’offre, et les restrictions législatives et culturelles ont influencé de façon significative la disponibilité, en dépit des preuves de son efficacité. Cela nécessite la mise en place d’une banque de peau d’un donneur décédé en Afrique du Sud, qui a pour mandat de se procurer et stocker allogreffe pour la distribution aux unités des brûlures en cas de besoin.
Introduction
Besides depth, the extent of burn injury predicts outcomes and dictates therapy. Patients with large surface area burns suffer a number of sequelae as a result of the disruption to the skin, which plays a critical role in the homeostasis of temperature and fluid, and protects the body from physical and biological trauma. Major burns impose a significant catabolic stress on the body, and patients are profoundly immunocompromised, frequently suffering from derangements of electrolytes, nutritional state and fluid balance. A number of advances have been made in recent decades with regard to fluid resuscitation protocols, dressings, infection control strategies, antimicrobials, intensive care and nutrition for the management of severe burn victims. These measures have reduced mortality and morbidity rates significantly over the last few decades, but the single most important intervention has undoubtedly been the implementation of early excision and autografting.1-3 This is limited, however, by the availability of autograft in very extensive burns, despite the use of expanded (meshed) skin. Alternatives to autograft have therefore been required, and options include various manufactured skin substitutes and deceased donor allograft. These are not definitive therapies in deep burns, and autografting will still be required when available.4,5
Burn care in South Africa
Burn injuries remain a prominent external cause of morbidity and mortality in South Africa. These injuries are most prevalent in the very young (7-15/10 000 children annually), with the majority being scald burns occurring in domestic settings6 and requiring referral to secondary or tertiary hospitals. In 2011, 1,223 burn victims were admitted to the Red Cross War Memorial Children’s Hospital specialist burns unit, comprising 14.7% of all trauma presentations to this hospital. In adults, proportionally more burn injuries are caused by flame, often as a result of fires in informal settlements, inter-personal violence or following epileptic seizures. Burns comprise 12% of fatal accidents in South Africa, and are the commonest external cause of death under the age of 4 years, and the 3rd most common under the age of 18. The burn mortality rate in South Africa is 7.9 per 100,000 person years, at least double the rate of most developed countries.6,7
The term ‘burns unit’ is a tertiary (specialist/multidisciplinary) care concept, but more often than not burns victims in South Africa are managed in regional hospital wards without the necessary support required to optimize outcomes. Burns surgery is often undertaken by those ‘obliged’, rather than dedicated to do so. As such, many burns patients are unlikely to benefit from modern burn care principles, dressings, intensive care, nutrition, physiotherapy or rehabilitation. There are no formal accreditation processes, no burns disaster plans or national burns unit bed management systems, and few career burn surgeons. 6,8 It is the priority of the South African Burn Society to address these deficiencies. In line with these is the formulation of a national burns disaster plan using international experience to prepare for such an event.3 Cadaver skin was identified as the single major resource limitation during the Bali bombing disaster in 2002, and the Singapore burn unit had to request donations from other national skin banks.8,9
Cadaver skin is recognized as the gold standard for temporary cover following early excision when insufficient autograft is available, but fresh cadaver skin must be harvested and used for specific patients within days. The unpredictable supply of fresh cadaver skin for use in the management of major burns has necessitated the establishment of skin banks in most developed countries. In fact, there are in excess of fifty operational skin banks in North America alone. Jerusalem’s Hudassah Hospital houses the largest skin bank, which aims to store 200 m2 of skin, should there be a major disaster or another conflict in the region.
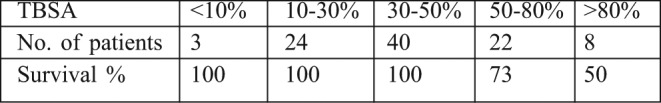
In comparison, few units utilise cadaver skin even on an occasional basis in South Africa, and as far as we know, there are no functional skin banks on the whole continent of Africa. Table I demonstrates the excellent outcomes achieved with the use of fresh cadaver skin at the Red Cross Children’s War Memorial Hospital in Cape Town. Cultural, ethnic and legislative issues have greatly reduced fresh cadaver donations and harvest in recent years in South Africa, with devastating consequences for major burns. The LD50 during this period was in excess of 70% TBSA, but declined to below 50% TBSA in the years following the legislative changes (when ready access to fresh cadaver skin was severely limited).
Table I. Fresh allograft use at Red Cross War Memorial Children’s Hospital (2000-2007). (TBSA = Total Body Surface Area Burnt).
Indications for deceased donor allograft skin
Superficial partial thickness burns involve the epithelial and papillary dermis with blistering as a characteristic feature. Clinically these are sensate, blanch when examined, and their potential for healing depends on spared dermal elements. Should these burns reach intermediate depth, careful monitoring with a conservative approach for as long as three weeks may be necessary until the wound declares its true healing potential. This is particularly applicable for the management of scald injuries. Allograft may be useful as a biological dressing in a freshly debrided partial thickness burn, as it has been shown to promote epithelialization.10,11 Exfoliative disorders such as Stevens-Johnson Syndrome (SJS), toxic epidermal necrolysis (TEN) and Staphylococcal Scalded Skin Syndrome (SSSS), where there is separation at the dermo-epidermal or intraepidermal level, leaving intact dermis and the potential for full re-epithelialization, may be considered similarly to superficial partial thickness burns. Allograft usage may achieve temporary tissue cover until spontaneous re-epithelialization is completed.12
Involvement of the reticular dermis or the full thickness of the skin removes the potential for regeneration. These are non-blanching, fixed-staining or leathery wounds that will only heal by very protracted and disorganized scarring; complete excision of the eschar to bleeding or to the next fascial layer (tangential or fascial excision) is imperative. If autograft is not an option due to lack of donor skin, prompt coverage of the excised burn wound with allograft will protect the debrided area and promote vascular ingrowth that will optimize future autograft take.13,14 This main indication for allograft for large, deeper burns has improved survival rates by reducing the heat and fluid loss and the susceptibility to infection during the acute period.15
If autograft is available to achieve wound cover but only by means of meshing and over-expansion of the autograft, then a sandwich technique as originally described by Alexander may be used. In this technique, widely meshed autograft is covered by unmeshed or non-expanded allograft; this provides a protective layer that prevents underlying tissue desiccation and contamination, and limits healing by fibrosis.16 Both the autograft and allograft adhere to the wound bed and as autologous epithelium grows across the interstices of the mesh graft, the allograft separates off the wound bed, a process known as creeping substitution.17 In areas of particular functional or aesthetic significance, such as the face, where a conservative approach is preferred to avoid overzealous debridement, allografts have proved to promote superior healing than topical wound management.18
Meningococcal septicaemia and purpura fulminans can cause similar full thickness cutaneous loss. The skin defects are usually conservatively treated until clearly demarcated. Once this occurs and the patient is clinically stable, it is possible to proceed to excision of the necrotic tissue and allografting of the defects with a view to subsequent autografting.19
Another indication for allograft use in burns surgery may be in the context of infected burns, when the risk of autograft loss is deemed significant. Following debridement, cadaver skin ‘take’ in this setting will suggest that the wound is better prepared for autografting. This same principle may also apply to large wounds from other causes, particularly if chronic or complex, as in the setting of malnutrition or peripheral arterial or venous diseases. In any setting where the allograft ‘takes’, it undergoes inosculation and angiogenesis, and when removed, leaves a wound bed ideally primed for autograft.
Deceased donor skin may also be de-epithelialized and rendered acellular, leaving a dermal scaffold that can be used as a dermal substitute in both acute burns and reconstructive surgery, for soft tissue augmentation, dural repair, hernia repair, and eyelid reconstruction. One such product is Alloderm (LifeCell, Woodlands, Tx, USA.).
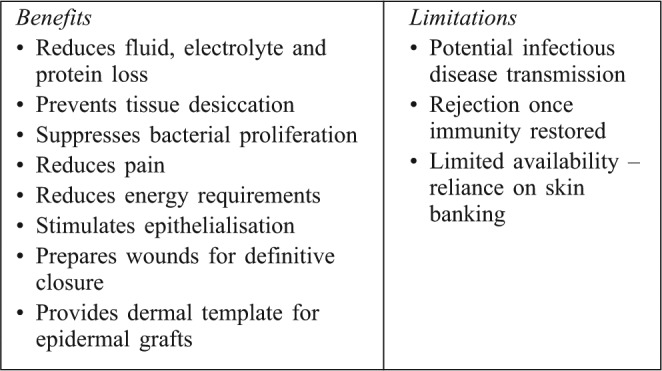
Some of the benefits and limitations of allografting are summarized in Table II.
Table II. Benefits and limitations of cadaver skin use in major burns.
Allograft preservation methods
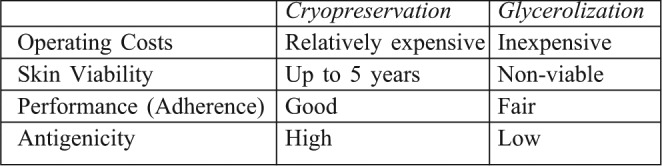
Allograft may be classified as either viable or non-viable. Viable allograft contains viable keratinocytes, fibroblasts, endothelial cells and Langerhans cells (dermal macrophages) and may be fresh or cryopreserved. Non-viable allograft may be glycerolized or gamma irradiated, freeze dried or ethylene oxide treated. Two schools of thought regarding allograft preservation have developed. The first, as practiced in the US, Singapore and Israel, makes use of cryopreservation. The European skin banks, on the other hand, have used glycerolization. The method of allograft preservation determines its performance. Fresh and cryopreserved grafts exhibit significantly greater adherence to the wound bed when compared to glycerol preserved allografts.4,10,20-22 Table III compares some of the key features of these two methods.
Table III. Features of the two major types of skin banking.
For cryopreservation, following harvest and transfer to the bank, grafts are stored overnight in saline and antibiotics in a refrigerator (4-8°C). Thereafter, following a protocol, they are washed extensively, placed in 50 ml centrifuge tubes and filled with skin graft preservation medium RPMI 1640 with an antibiotic solution and 15% glycerol. Skin samples are cryopreserved by programmed freezing (1°C/min) to -130°C, and then stored in liquid nitrogen tanks (-145°C to-180°C). Cryopreserved skin may be stored for up to five years without significant effect on performance.20 During the glycerolization process, on the other hand, grafts are placed in 50% glycerol in saline overnight, and then transferred to the processing room, where they are incubated in 85% glycerol for 4 hours at 37°C, before transfer to 98% glycerol and preserved at room temperature.
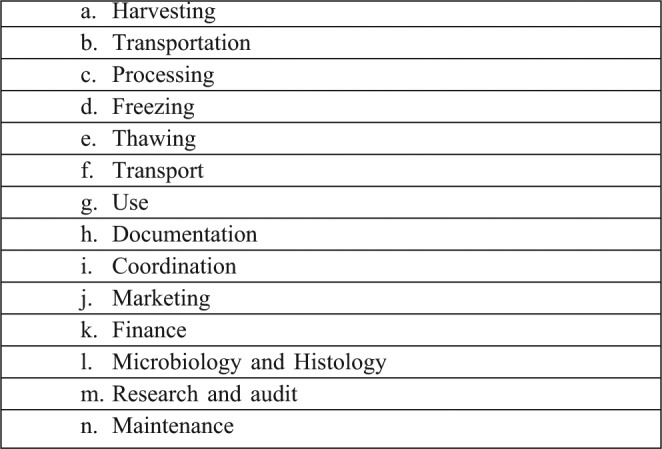
Stringent protocols for skin banking procedures (Table IC) are necessary to maintain the highest possible standards.
Table IV. Skin banking protocols and procedures.
Discussion
Despite access to other modern burn care technologies, outcomes in the management of major burn injuries are unlikely to improve without addressing the ‘Achilles heel’ of the burn surgeon: reliable access to cadaver skin. The South African Burn Society aims to establish a deceased donor skin bank as soon as possible, but a number of challenges will need to be addressed.
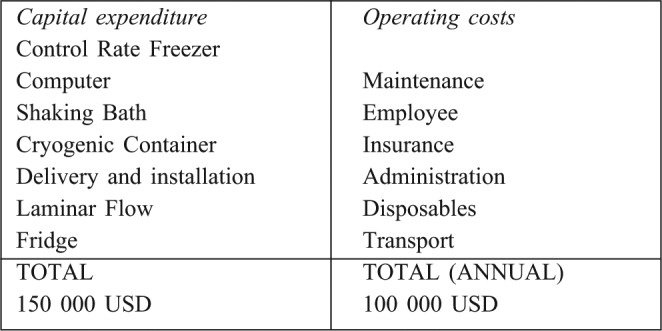
Not least of these is funding. Efforts have begun with the aim of raising the initial establishment and operational costs of the skin bank, with a view to relinquishing this ongoing responsibility to the national department of health. An outline of the budget is illustrated in Table V. It is likely that we will opt to implement the cryopreservation method of skin banking, in light of the improved performance demonstrated and the prevalence of major burn injury in this country.
Table V. Estimated initial capital expenditure and annual operating costs.
A fairly modest facility is required for processing, storage and administration. Obvious reciprocal benefits are envisaged with it being located adjacent to one of South Africa’s academic burns units. Accreditation as a provincial and national tissue bank is still required. Support from the local tertiary hospitals and university authorities is necessary to contribute to protocols, oversee functioning, authorize appropriate distribution of skin, conduct research and implement regular audit.
The bank will require the services of employees to maintain the skin bank, process, store and distribute skin, liaise with national tissue procurement organizations, and be involved with marketing and educational programs. A transport system for distribution will be required, making use of a national airline. A second skin bank may well be required in one of the other major centres, as demand will almost certainly exceed supply.
A further obstacle to the successful functioning of the skin bank will be obtaining enough skin to meet the obvious need for it. Those burns units and centres making use of the service will be required to participate in the procurement of skin regionally, with channels of communication and feedback established via the burn society. The national organ and tissue donation foundations have noted that a significant proportion of donations originate from private hospitals in South Africa. A minority of the population is managed in this setting, resulting in a small pool from which to draw donors. The blood donor population in South Africa is similarly stratified. Misconceptions regarding the use of the tissues and/or organs, as well as beliefs relating to disfigurement of the body, have certainly contributed; this has also resulted in many families agreeing to the donation of major organs, but not to the harvesting of skin. Recent legislation has restricted the harvesting of deceased donor allograft skin in the mortuaries in the absence of a state forensic officer. A concerted effort is required to address misconceptions and unnecessary bureaucracy regarding organ and tissue donation in South Africa. One of the employee’s functions will be to collaborate with the burn society and the organ and tissue donation foundations in the country to increase donation. Fortunately, the exclusion criteria for skin are less stringent than those imposed on the organ donation process (Table VI).
Table VI. Donor exclusion criteria for harvesting cadaver skin for banking.

Despite significant obstacles to establish a successful deceased donor skin bank for South Africa, we are confident these are not insurmountable, and that the benefits will translate to improved survival and functional outcomes for victims of major burns.
References
- 1.Janzekovic Z. A new concept in the early excision and immediate grafting of wounds. J Trauma. 1970;10:1103–8. [PubMed] [Google Scholar]
- 2.Tompkins RG, Remensnyder JP, Burkr JF, et al. Significant reduction in mortality for children with burn injuries through the concept of prompt eschar excision. Ann Surg. 1988;208:577–85. doi: 10.1097/00000658-198811000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers AD, Allorto NL, Wallis L, Rode H. The emergency management of severe burns course. SAMJ. 2013;51:38–9. doi: 10.7196/sajs.1309. [DOI] [PubMed] [Google Scholar]
- 4.Leon-Villapalos J, Eldariri M, Dziewulski P. The use of human deceased donor skin allograft in burn care. Cell and Tissue Bankong. 2010;11:99–104. doi: 10.1007/s10561-009-9152-1. [DOI] [PubMed] [Google Scholar]
- 5. Herndon, DN , editor. Total Burn Care. 3rd edn. Saunders Elsevier; 2007. The Skin Bank. pp. 229–38. [Google Scholar]
- 6.Rode H, Berg A, Rogers A. Burn care in South Africa. Ann. Burns and Fire Disasters. 2011;14:7–8. [PMC free article] [PubMed] [Google Scholar]
- 7.National Injury Mortality Surveillance System. MRC/UNISA.; 2005. [Google Scholar]
- 8.Rogers AD, Price CE, Wallis L, Rode H. Towards a national burns disaster plan. SAJS. 2012;49:174–7. [PubMed] [Google Scholar]
- 9.Chim H, Yew Ws, Song C. Managing burn victims of suicide bombing attacks: Outcomes, lessons learnt, and changes made from three attacks in Indonesia. Critical Care. 2007;11:15. doi: 10.1186/cc5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vloemans AFPM, Middelkop E, Kreis RW. A historical appraisal of the use of cryopreserved and glycerol-preserved allograft skin in the treatment of partial thickness burns. Burns. 2002;28:16–20. doi: 10.1016/s0305-4179(02)00087-6. [DOI] [PubMed] [Google Scholar]
- 11.Atiyeh BS, Hayek SN, Gunn SW, et al. New technologies for burn wound closure and healing: Review of the literature. Burns. 2005;31:944–56. doi: 10.1016/j.burns.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Spies M, Sanford AP, Aili Low JF, et al. Treatment of extensive toxic epidermal necrolysis in children. Pediatrics. 2001;108:1162–8. doi: 10.1542/peds.108.5.1162. [DOI] [PubMed] [Google Scholar]
- 13.Burd A, Lam PK, Lau H. Allogenic skin: Transplant or dressing? Burns. 2002;28:358–66. doi: 10.1016/s0305-4179(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 14.Tenenhaus M, Rennenkampff HO. Burn surgery. Clin Plast Surg. 2007;34:669–715. doi: 10.1016/j.cps.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe RA, Roi LD, Flora JD, et al. Mortality differences and speed of wound closure among specialised burn care facilities. J Am Med Assoc. 1983;250:37–41. [PubMed] [Google Scholar]
- 16.Alexander JW, MacMilan BG, Law E, et al. Treatment of severe burns with widely meshed skin autograft and meshed skin autograft overlay. J Trauma. 1981;21:433–8. [PubMed] [Google Scholar]
- 17.Hierner R, Degreef H, Vranckx J, et al. Skin grafting and wound healing: The ‘dermato-plastic team approach’. Clin Dermatol. 2005;23:343–52. doi: 10.1016/j.clindermatol.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 18.Horch RE, Jeschke MG, Spiler G, et al. Treatment of second degree facial burns with allografts: Preliminary results. Burns. 2005;31:597–602. doi: 10.1016/j.burns.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Lowery K, Shirley R, Shelley Op, et al. Purpura skin loss: Surgical management protocols at a regional burns centre. J Plast Reconstr Aesthet Surg. 2008;61:1520–3. doi: 10.1016/j.bjps.2007.01.085. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Bassat H, Chaouat M, Segal N, et al. How long can cryopreserved skin be stored to maintain adequate graft performance? Burns. 2001;27:425–431. doi: 10.1016/s0305-4179(00)00162-5. [DOI] [PubMed] [Google Scholar]
- 21.Chua A, Song C, Chai A, et al. The impact of skin banking and the use of cadaveric skin allografts for severe burn victims in Singapore. Burns. 2004;30:694–700. doi: 10.1016/j.burns.2004.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben-Bassat H, Chaouat M, Zumai E, et al. The Israel National Skin Bank: Quality assurance and graft performance of stored tissues. Cell and Tissue Banking. 2000;1:303–12. doi: 10.1023/A:1010183719662. [DOI] [PubMed] [Google Scholar]


