Introduction
Posttraumatic stress disorder (PTSD) is a disabling yet prevalent disorder (1). PTSD is hypothesized to represent the endpoint of negative affects associated with reminders of a traumatic exposure, fueled by memories of the exposure because the exposure itself occurred in the past and is no longer occurring. In a recent report, the National Academies of Science Institute of Medicine (IOM) concluded that the treatment of PTSD has not received adequate research attention and found little evidence to support the efficacy of most available treatments (2). The exception, however, was exposure therapy; the IOM referenced a number of studies demonstrating significant therapeutic effects of exposure therapy on PTSD symptoms (3,4, 5).
Exposure therapy promotes memory extinction. Memory extinction involves learning new emotional associations to reminders of a traumatic stimulus, rather than forgetting or erasure of the original memory (6). Learned conditioned responses can be attenuated through presentation of a conditioned stimulus (a reminder of a trauma exposure) without reinforcement by the unconditioned stimulus (actual trauma exposure) (6). The trauma memory is thus replaced with a new memory involving neutral or less upsetting emotions, disarticulating previously conditioned negative emotions from subsequent trauma reminders. This extinction process is amenable to pharmaceutical enhancement (7,8), stimulating much interest in potential pharmacologic agents for the treatment of PTSD.
Studies designed to examine pharmacologic enhancement of the extinction process have been informed by animal models, and glucocorticoids have been shown to enhance this process in rodents (9,10,). Consolidation of fear memories can also be enhanced with corticosterone in animal models (11). Regulation of glucocorticoids (primarily cortisol in humans) is significantly altered in PTSD subjects (12,13), suggesting that disruption in cortisol secretion may underlie the difficulties PTSD patients have in extinguishing trauma memories. Non-experimental research on administration of exogenous glucocorticoids indicates promise for cortisol as an intervention for PTSD. For example, several studies of Intensive Care Unit (ICU) patients by Schelling and colleagues found that the administration of higher doses of hydrocortisone was coupled with a decreased likelihood of developing PTSD (14). Indeed one recent, small study suggests that the chronic daily administration of a glucocorticoid can reduce PTSD symptoms during treatment and may persist even after the medication is stopped (15). Effects of continuing steroid use, however, are fraught with adverse side effects. If glucocorticoids exert lasting beneficial effects on extinction, then perhaps a focused approach pairing reactivation of traumatic memories to brief glucocorticoid administration could suffice.
When fear memories were previously established in rodents through the pairing of foot shock and specific contextual cues, our team observed that the injection of a single dose of corticosterone following memory reactivation reduced behaviors indicating fear memories previously established through pairing foot shock with specific contextual cues (9). The delivery of small “reminder” shocks insufficient to stimulate new fear memories but sufficient to reawaken the established fear responses reversed the dampening effects of corticosterone on the fear responses. Similarly, the passage of time also reduced the dampening effects of a single dose of corticosterone (9). The reversal of the corticosterone effects by these maneuvers indicates that the mechanism by which the corticosterone dampens fear response behaviors involves actual augmentation of extinction rather than interference with fear memory reconsolidation (16) . Based on our previous rodent research, this pilot study aimed to assess the effectiveness of glucocorticoids in augmenting extinction memories and reducing clinical symptoms in veterans with combat-related PTSD. In this double-blind, placebo-controlled study, subjects were exposed to a memory reactivation task followed by either hydrocortisone or placebo.
Methods and Materials
VA Institutional Review Board (IRB) approval for the study was obtained before the commencement of the research. Men diagnosed with PTSD related to combat or combat support were eligible for participation. Women were not included to preclude interference of menstrual phase with measurement of HPA axis response. Other exclusion criteria were substance (except nicotine) abuse or dependence within the past six months; current steroid, benzodiazepine, or barbiturate use; psychosis; brain damage; and medical contraindications to steroid administration. Psychiatric medications were not exclusionary and any changes or new medications during the study period were monitored. A volunteer sample of 22 male combat veterans was recruited from various mental health programs at a large southwestern Veterans Affairs Hospital. All participants provided written informed consent, after which they were randomized to receive either an intravenous bolus of glucocorticoid (hydrocortisone sodium succinate, 4mg/kg) or saline in a double-blind fashion. The dose was selected based on effective doses in our animal studies and discussions with study physicians and a PharmD, who is a drug information specialist, regarding typical and safe human doses that would be equivalent to the most efficacious animal dose. All four study physician co-authors and the PharmD agreed on the final dose.
Baseline assessment included the Clinician Administered PTSD Scale for DSM-IV (CAPS) to confirm the PTSD diagnosis (17), a Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID) to diagnose lifetime and current psychiatric comorbidity (18), the Impact of Event Scale-Revised (IES-R) for the presence and severity of related PTSD symptoms by DSM-IV-TR groupings (re-experience, avoidance/numbing, and arousal) for the past week (19), and the Quick Inventory of Depressive Symptomatology (Self-Report) (QIDS-SR) for depression symptoms for the past week (20). After baseline, participants had three return study visits (treatment visit, 1-week post-treatment memory reactivation visit, and 1-month post-treatment assessment visit). Two participants dropped out of the study after the initial assessment, yielding a total of 20 study completers.
At the treatment visit, an IV was inserted in the non-dominant arm and participants rested for 45 minutes prior to being asked to write a one-page description of their two “worst” combat-related trauma memories and to identify from a list any bodily sensations (e.g., sweaty palms, breathing fast, etc.) that they experienced at the time of the trauma. (From the material provided by participants, a 30-second script was audiotaped by a male research staff member incorporating material from participants’ own trauma descriptions to be played during the 1-week post-treatment assessment visit to activate their combat memories.) Next, participants filled out the IES-R once for each of these two traumatic events. Immediately after addressing both traumas, the participant was administered the intravenous bolus of either hydrocortisone or saline and then completed the QIDS. They were then instructed to rest quietly for a minimum of one hour while the study nurse observed to make sure there were no side effects from the medication.
At the 1-week post-treatment memory reactivation visit, two standardized neutral scripts (21) were presented to participants along with their two personal trauma narratives. These scripts were presented in randomly alternating order. Four physiological measures (heart rate, skin conductance, and corrugator and frontalis muscle electromyogram) were recorded during presentation of the scripts. After the imagery procedure was completed, an IES-R for each traumatic event and the QIDS were completed. During the one month post-treatment visit, only the IES-R, QIDS, and CAPS were administered.
Average baseline psychophysiologic measure variables were out of established normal range for two participants, leaving 19 participants (9 administered hydrocortisone and 10 administered placebo) with complete information for this study’s data analysis. Findings are summarized and presented as raw numbers, percentages, means, and standard deviations. Comparisons of two dichotomous variables were performed using χ2 analysis (substituting Fisher’s exact tests for expected cell sizes <5). Student’s t-tests were used to compare dichotomous with numerical variables.
Results
Baseline characteristics
The hydrocortisone and saline groups were indistinguishable on demographic variables (age, ethnicity, education, marital status, work status, or service connection status), psychiatric comorbidity, or baseline symptoms on the CAPS (total and groups B, C, and D scores respectively representing PTSD intrusion, avoidance/numbing, and arousal symptom clusters), IES-R (total and intrusion, avoidance/numbing, and arousal scores), and QIDS. Comorbid lifetime psychiatric disorders were diagnosed in 84% of the participants (major depression in 74%, generalized anxiety disorder in 16%, social phobia in 11%, panic disorder in 11%, and anxiety disorder NOS in 11%). Regarding past diagnoses of substance abuse/dependence, 10% had a history of alcohol abuse, 40% alcohol dependence, 10% cannabis dependence, 5% amphetamine dependence, and 20% cocaine dependence. Nicotine dependence was not assessed. Five of the study participants were receiving disability payments (service connected) for PTSD by the VA.
Treatment Visit (Pairing of Hydrocortisone/Saline with Traumatic Memory Activation, 1 week after baseline)
At this visit, no change from baseline was found in IES-R scores, as expected, reflecting stability of the measurement. The hydrocortisone group did not differ from the saline group in IES-R total and symptom subgroup scores. Curiously, however, compared to the saline group, the hydrocortisone group had a significantly higher QIDS score (mean=15.7, SD=4.4 vs. mean=11.3, SD=3.5; t=2.38, df=17, p=.029). No side effects were noted for either the medication or control group.
1-Week Post-Treatment Memory Reactivation Visit
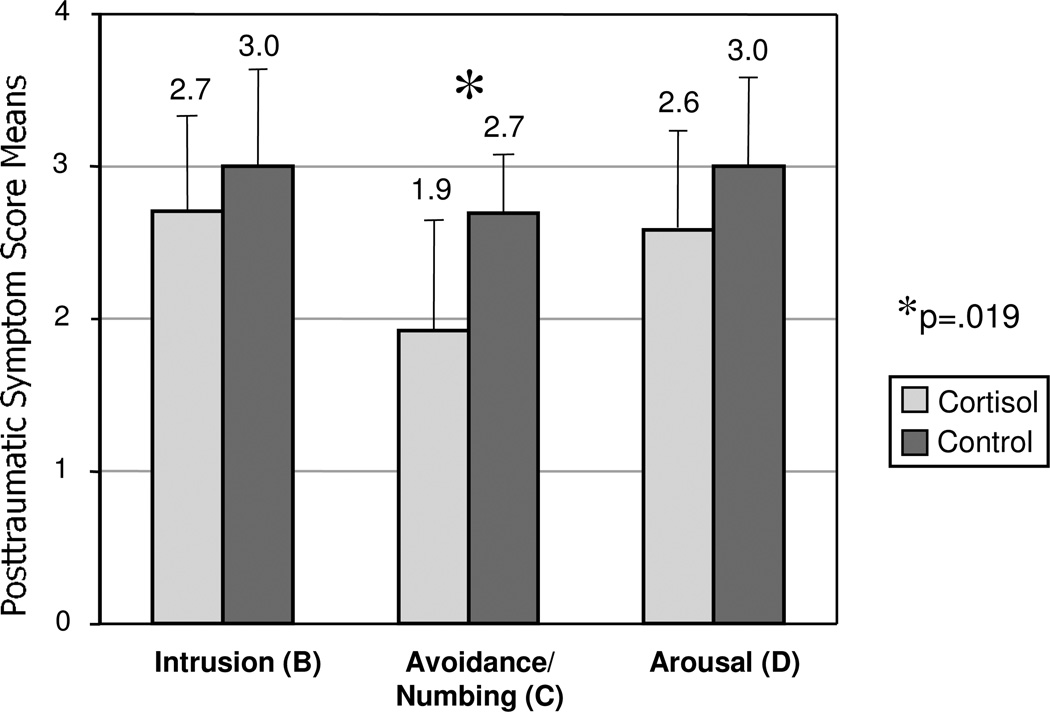
Following memory reactivation one-week post-treatment, IES-R avoidance/numbing scores were significantly lower for the hydrocortisone group compared to the saline group (worst trauma: mean=1.9, SD=0.8 vs. mean=2.7, SD=0.4, t=2.58, df=17, p=.019; next worst trauma: mean=1.9, SD=1.0 vs. mean=2.6, SD=0.6, t=1.84, df=17, p=.084; average of the two traumas: mean=1.9, SD=0.9 vs. mean=2.6, SD=0.5, t=2.21, df=17, p=.041.) See Figure 1 for a graphic presentation of the findings for the worst trauma. IES-R intrusion and arousal scores did not differ between the treatment groups. Between-group differences in total IES-R scores for the worst (cortisol=52.6, SD=14.4 vs. saline=63.3, SD=12.0; t=1.77, df=17, p=.094) and second worst (cortisol=51.9, SD=15.7 vs. saline=63.6, SD=12.5; t=1.81, df=17, p=.088) traumas fell short of statistical significance. QIDS scores on this visit were not significantly different between treatment groups, as at baseline.
Figure 1.
Mean Posttraumatic Symptom Scores by Symptom Group, for Worst Trauma
There were no differences on any of the four physiological variables between the groups for any traumatic or neutral scripts or for their averages.
1-Month Post-Treatment Assessment
One month after the last treatment visit, no significant between-group differences were detectable on any of the total or subscale scores of the IES-R, CAPS, and QIDS.
Discussion
Previous research has suggested the potential for benefit of brief glucocorticoid administration for PTSD. First, non-experimental studies have shown that chronic glucocorticoid administration may reduce the likelihood of PTD in traumatized individuals (14). Second, our prior experimental research demonstrated that brief glucocorticoid administration reduced freezing behavior in rodents after contextual fear conditioning (9). Together, the findings from these studies suggested the potential for brief glucocorticoid administration in humans to provide therapeutic effects. Successfully applying experimental methods from our rodent experiment to humans with PTSD, this study probed the effects of pairing a single administration of a glucocorticoid with a traumatic memory reactivation task. Consistent with our animal studies, the administration of an exogenous glucocorticoid following fear memory reactivation reduced the response to trauma reminders in our patients with PTSD. This small pilot study represents the first randomized, double-blind, placebo-controlled clinical trial testing the effects of exogenous glucocorticoids on patients with PTSD.
Specifically, this study demonstrated that pairing of traumatic memory activation with a single dose of glucocorticoid resulted in lower Group C (avoidance/numbing) symptom scores in the hydrocortisone group compared to the saline group one week after treatment. Treatment effects were not observed in Group B (intrusion) or Group D (arousal) symptoms, but other studies have demonstrated the avoidance/numbing symptom cluster to be a marker of psychopathology in PTSD (22,23,24), and reduction of problematic avoidance behaviors a major therapeutic endpoint of exposure therapies (25). The finding that such effects were not observed one month later suggests that the observed effects of a single glucocorticoid dose may be relatively transient.
The strengths of this study are the elements of the experimental design: blinding of assessments using a placebo comparison group; systematized, validated measurement tools, and implementation of a standardized procedure for trauma memory reactivation; and simultaneous measurement of psychophysiologic recording methods.
This study had several limitations, some of which may have constrained the study’s observable findings. Perhaps the most serious limitation is the small sample size that constrained statistical power. While one-week worst-trauma avoidance/numbing symptom scores were significantly lower in the cortisol group one week post-treatment than in the control group, the one-week treatment group difference in avoidance/numbing symptom scores specifically for the second worst trauma fell short of statistical significance. The finding that the average of between-group difference on both trauma scores combined was statistically significant suggests that limited statistical power may have obscured potential detection of a real difference. Additionally, the significant difference in avoidance/numbing symptom scores between treatment groups at one week post-treatment represents merely a between-group difference and not a measure of change over time from pre-treatment to post-treatment. No change scores over time were significantly different between treatment groups even though differences between groups were apparent post-treatment. Lack of findings in the change scores may also reflect limited statistical power due to small sample size. The change values were much smaller in magnitude than the actual scores, reducing the ability to detect differences.
Additionally, this study was limited by its testing of only a single administration of cortisol of limited dosage, a procedure that may not have been powerful enough to produce observable changes in symptom scores over time. Using a higher dose might have produced more measurable and durable effects. Because our animal studies showed that multiple pairings of reactivation and glucocorticoid resulted in a much larger and longer lasting effects than a single pairing on fear memory (9), it is reasonable to predict that multiple pairings of memory activation with cortisol might further enhance the effects and extend them across all PTSD symptom domains.
Finally, the volunteer sample used for this study may not be representative of veterans in general. The medical and pharmacologic agent exclusion criteria hindered the study’s ability to gather a sample because so many potential recruits were not eligible for participation. A substantial number of veterans screened were ineligible because of medical (e.g., diabetes and heart) problems and/or use of psychotropic medication. Additional study exclusions of women limit the ability of this study to generalize to populations other than men. Lastly, combat veterans in treatment for PTSD may not be representative of other populations with PTSD.
Conclusions
In conclusion, even the short-lived effect on IES-R avoidance/numbing scores after only a single pairing of reactivation and cortisol in the present study are promising indications that repeated sessions of memory reactivation paired with glucocorticoids might lead to significantly enhanced and prolonged clinical benefits. We conclude that the use of traumatic memory reactivation temporally paired with glucocorticoid administration holds potential for developing a viable therapeutic option worthy of future study.
Acknowledgements
The authors wish to thank Roger Pittman, M.D. for training on the imagery procedure and Scott Orr, Ph.D., for consultation on psychophysiology data interpretation. We also recognize Lisa Thoman, Broc Sanchez, and Sharon Marcus for their dedication and contributions to data gathering.
Declaration of Interest
This manuscript was supported by a grant to the first author from NARSAD. Dr. Suris also reports grant funding from the VA, Dallas VA Research Corporation, and the NIH National Center for Research Resources. Dr. North reports grant funding from NIMH, NIAAA, and the VA and paid consulting relationships with Applied Research Associates and Cubic Corporation. Dr. Powell reports funding from NIMH, NAAR-Autism Speaks and the NIH National Center for Research Resources. Dr. Adinoff reports funding from VA, NIAAA, and NIH/NIDA. Dr. Greene reports funding from VA, NIMH, Sepracor, and Astra Zenaca.
References
- 1.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.National Academy of Sciences Institute of Medicine: Treatment of Posttraumatic Stress Disorder: An Assessment of the Evidence. Washington, DC: National Academy Press; 2008. [Google Scholar]
- 3.Schnurr PP, Friedman MJ, Engel CC, Foa EB, Shea MT, Chow BK, Resick PA, Thurston V, Orsillo SM, Haug R, Turner C, Bernardy N. Cognitive behavioral therapy for posttraumatic stress disorder in women: A randomized controlled trail. JAMA. 2007;297(8):820–830. doi: 10.1001/jama.297.8.820. [DOI] [PubMed] [Google Scholar]
- 4.Foa EB, Hembree EA, Cahill SP, Rauch SA, Riggs DS, Feeny NC, Yadin E. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: Outcome at academic community clinics. J Clin Psychol. 2005;79(2):953–964. doi: 10.1037/0022-006X.73.5.953. [DOI] [PubMed] [Google Scholar]
- 5.Bryant A, Moulds MI, Guthrie RM, Dang ST, Nixon RD. Imaginal exposure alone and imaginal exposure with cognitive restructuring in treatment of posttraumatic stress disorder. J Clin Psychol. 1999;71(4):706–712. doi: 10.1037/0022-006x.71.4.706. [DOI] [PubMed] [Google Scholar]
- 6.Rescorla RA. Experimental extinction. In: Mowrer RR, Klein S, editors. Handbook of Contemporary Learning Theories. Mahwah, NJ: Erlbaum; 2001. pp. 119–154. [Google Scholar]
- 7.Bouton ME. Context, time, and memory retrieval in the interference paradigms of pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- 8.Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 9.Cai W, Blundell J, Han J, Greene RW, Powell CM. Post-reactivation Glucocorticoids Impair Recall of Established Fear Memory. J of Neurosci. 2006;26:9560–9566. doi: 10.1523/JNEUROSCI.2397-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- 11.Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y. Administration of corticosterone after memory reactivation disrupts subsequent retrieval of a contextual conditioned fear memory: Dependence upon training intensity. Neurobiol Learn Mem. 2008;89:178–184. doi: 10.1016/j.nlm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(Suppl 17):41–46. [PubMed] [Google Scholar]
- 13.Newport DJ, Nemeroff CB. Neurobiology of posttraumatic stress disorder. Cog Neuro. 2000;10:211–218. doi: 10.1016/s0959-4388(00)00080-5. [DOI] [PubMed] [Google Scholar]
- 14.Schelling G, Stoll H, Kapfhammer HP, Rothenhausler HB, Krauseneck T, Durst K, Haller M, Briegel J. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder and health-related quality of life in survivors. Cril Care Med. 1999;27:2678–2683. doi: 10.1097/00003246-199912000-00012. [DOI] [PubMed] [Google Scholar]
- 15.de Quervain DJ. Glucocorticoid-induced reduction of traumatic memories: Implications for the treatment of PTSD. Prog Brain Res. 2008;167:239–247. doi: 10.1016/S0079-6123(07)67017-4. [DOI] [PubMed] [Google Scholar]
- 16.Diergaarde L, Schoffelmeer AN, DeVries TJ. Pharmacological manipulation of memory reconsolidation: Towards a novel treatment of pathogenic memories. Eur J Pharmacology. 2008;585:453. doi: 10.1016/j.ejphar.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Klauminzer G, Charney DS, Keane TM. A clinician rating scale for assessing current and lifetime PTSD: The CAPS-1. Behav Ther. 1990;13:187–188. [Google Scholar]
- 18.First MB, Gibbon M, Spitzer RL, Williams JBW. User’s Guide for the Structured Clinical Interview for DSM-IV-TR Axis I Disorders- Research Version (SCID-I, for DSM-IV-TR, February 2001 Revision) 2001. [Google Scholar]
- 19.Weiss DS, Marmar CR. In: The Impact of Event Scale–Revised, in Assessing Psychological Trauma and PTSD. Wilson JP, Keane TM, editors. New York: Guilford; 1997. pp. 399–411. [Google Scholar]
- 20.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-item Quick Inventory of Depressive Symptomatology (QIDS) Clinician Rating (QIDS-C) and Self-Report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 21.Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of post-traumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry. 1987;44:970–975. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- 22.North CS, Nixon SJ, Shariat S, Mallonee S, McMillen JC, Spitznagel EL, Smith EM. Psychiatric disorders among survivors of the Oklahoma City bombing. JAMA. 1999;282:755–762. doi: 10.1001/jama.282.8.755. [DOI] [PubMed] [Google Scholar]
- 23.Ehlers A, Mayou RA, Bryant B. Psychological predictors of chronic posttraumatic stress disorder after motor vehicle accidents. J Abnorm Psycho.l. 1998;107:508–519. doi: 10.1037//0021-843x.107.3.508. [DOI] [PubMed] [Google Scholar]
- 24.Maes M, Delmeire L, Schotte C, Aleksander J, Creten T, Mylle J, Struyf A, Pison G, Rousseeuw PJ. Epidemiologic and phenomenological aspects of post-traumatic stress disorder: DSM-III-R diagnosis and diagnostic criteria not validated. Psychiat Res. 1998;81:179–193. doi: 10.1016/s0165-1781(98)00095-x. [DOI] [PubMed] [Google Scholar]
- 25.Foa E. Psychosocial therapy for posttraumatic stress disorder. J Clin Psychiatry. 2006;67(Suppl 2):40–45. [PubMed] [Google Scholar]