Abstract
Cardiovascular diseases are still the most important cause of morbidity and mortality worldwide and anti-thrombotic treatment is widely used as a result. The currently used drugs include heparin and its derivatives, vitamin K antagonists, though efficacious, have their own set of limitations like unpredictable pharmacokinetic profile, parenteral route (with heparin and its derivatives only), narrow therapeutic window, and constant laboratory monitoring for their efficacy and safety. This lead to the development of novel factor Xa inhibitors which could be given orally, have predictable dose response relationship and are associated with lesser hemorrhagic complications. They include rivaroxaban, apixaban, and edoxaban among others. Apixaban has currently been approved for use in patients undergoing total knee or hip replacement surgery and to prevent stroke in patients with atrial fibrillation. Many trials are ongoing for apixaban to firmly establish its place in future, among the anti-thrombotic drugs.
Keywords: Apixaban, atrial fibrillation, factor Xa inhibitors, thrombosis
INTRODUCTION
Thrombosis continues to be a menace, inspite of available drugs like heparin and its derivatives, vitamin K antagonists, and direct thrombin inhibitors (DTIs), which though efficacious, are fraught with their own set of limitations. The problems encountered with Unfractionated Heparin (UFH) are its intra-venous route, animal source, and heparin-induced thrombocytopenia (HIT). Warfarin has delayed onset and offset of action, a narrow therapeutic window, an unpredictable pharmacokinetics and pharmacodynamics, along with dietary and drug interactions. Also, both the drug groups need monitoring by APTT and INR, respectively. Low molecular weight heparins (LMWH), also have issues like parenteral route, HIT and inability to use in renal insufficiency.[1]
Direct factor Xa inhibitors are the new oral drugs on the block, theoretically supposed to be better than DTIs, because former have no effect on activity of thrombin, per se. Rivaroxaban (July, 2011) and apixaban (December 2012) have been approved by United States Food and Drug Administration (USFDA) for the use in clinical setting of thrombotic disorders. “Xabans” literally mean the drugs which “ban” factor “Xa”. They do not require monitoring and have a fast and reliable onset of action, with no risk of HIT.[2]
Efficacy profile of apixaban
Apixaban is an oral selective direct factor Xa inhibitor, approved by European commission, in May 2011 for the prevention of thromboembolic complications in patients undergoing elective knee or hip replacement. It has also been approved by USFDA in December 2012, for use in patients with atrial fibrillation (AF) to prevent stroke and embolism [Table 1].[3,4,5,6,7,8]
Table 1.
Various clinical trials of apixaban
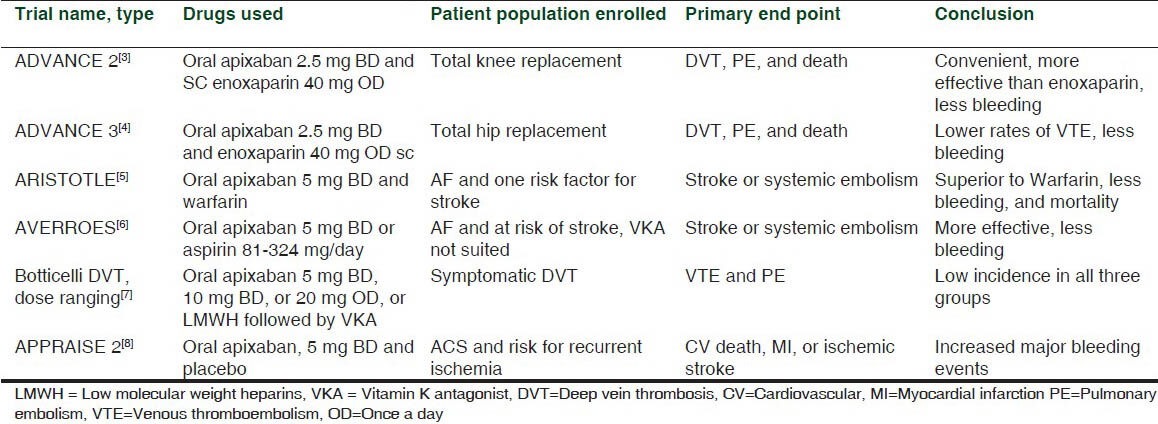
The FDA approval of apixaban for use in AF patients is based on two studies. In ARISTOTLE trial, where patients with AF were put on either apixaban or Warfarin, apixaban had a superior efficacy and lesser bleeding risks.[5] In AVERROES trial, patients with AF who either refused to take a Vitamin K antagonist (VKA) or were considered unsuitable for it were on aspirin (81 and 324 mg/day) or apixaban.[6] The relative risk reduction for stroke and systemic embolism in favor of apixaban over aspirin was 55%, with a small non-significant increase in major bleeding.[9]
In a trial comparing apixaban with LMWH, followed by warfarin, in patients with deep vein thrombosis (DVT), both the drug groups were similar in efficacy, with apixaban having lesser bleeding risk. This trial showed apixaban to be an easy and simple therapy for DVT patients unlike complex and inconvenient dosing of LMWH and VKAs.[7]
In a phase-2, double-blinded, placebo-controlled dose-ranging study of apixaban in patients with recent acute coronary syndrome (ACS), to prevent the recurrence of ACS, it was added to the ongoing anti-platelet therapy (aspirin, clopidogrel) of the patients and it was seen to reduce the ischemic events but with increase in the bleeding risk.[10] Another study done in patients with recent ACS (APPRAISE 2) also showed similar results, and had to be terminated early.[8] Further studies need to be done to establish its safety in the patients with ACS.
There have been few trials on the role of apixaban as an extended treatment in the patients, who have completed their intended treatment for DVT or pulmonary embolism; it was shown that apixaban reduces the risk without any inadvertent increase in bleeding.[11]
Pharmacokinetics of apixaban
Apixaban is mainly metabolized by CYP3A4 enzyme, hence given cautiously with inhibitors and inducers of this enzyme.[12] It is also a substrate of P-glycoprotein; hence co-administration with the inhibitors of P-glycoprotein can increase its plasma concentration.[13] The excretion of apixaban is primarily through hepatobiliary route (75%) and approximately 25% is excreted through kidneys. The half-life is 12 h and action starts approximately 3 h after the dose is given. The usual dose used is 2.5 mg, twice daily for the patients undergoing knee/hip replacement surgery. It is given as 5 mg preparation in patients with AF.[3,4,5]
Indications for use of apixaban
The main indications of apixaban are as follows:
Prevention of thrombotic complications in patients undergoing hip or knee replacement
Prevention of stroke in patients with AF.
The use of apixaban in prevention of thrombotic complications in the patients who had undergone hip or knee replacement surgery has been proved in several clinical trials.[3,4] It has also been found useful in patients with AF for prevention of stroke and thromboembolism.[5,6] It was seen that apixaban was associated with higher incidence of major bleeding, when used in patients with ACS. Hence, more studies need to be done to know the exact type of patient population of ACS where apixaban might be useful.[8,10] There are several ongoing phase 3 trials, which will give enough information regarding the efficacy and safety of apixaban in patients having acute DVT and pulmonary embolism.
The use of apixaban for extended prophylaxis in patients who were medically ill and hospitalized and at risk of venous thromboembolism, was studied in a clinical trial (ADOPT study), where it was shown that it is not superior to enoxaparin. Also, apixaban was associated with higher bleeding risk in these patients.[14]
The use of factor Xa inhibitors (apixaban, rivaroxaban, etc.) is not recommended in pregnancy, because these agents can easily cross the placental barrier due to their smaller size. However, their use in management of pulmonary embolism in pregnant females is being explored.[15,16] The studies for the usefulness of other agents of this class of factor Xa inhibitors are still going on, with the hope to find a still better drug than apixaban.
Adverse effect profile of apixaban
The most common adverse event reported for apixaban, in most of the trials is bleeding. Risk of minor and major bleeding is reported to be associated with factors Xa inhibitors, though to a lesser extent than other oral anti-coagulants.[5] Drug interactions are a concern, more for rivaroxaban compared to apixaban.[13] It should be used cautiously in patients with a deranged kidney or liver function. However, does adjustment is not needed in mild cases of hepatic or renal diseases. Other adverse events reported with apixaban, to FDA, are hyper-sensitivity reactions, syncope, nausea, dizziness, etc.[17]
One of the important issues with factor Xa inhibitors is that neither there are any available antidotes which can be effective in their case nor are the drugs dialyzable because of their high plasma protein binding. To add to the problems, the effect of apixaban lasts for 24 h after the last dose is given. For their effect to be reversed, prothrombin complex concentrates (PCCs) might prove to be useful.[18]
CONCLUSIONS
The primary advantage associated with apixaban and other drugs in its group is oral route of administration, which is convenient to the patient after the discharge. Apixaban boasts of having a pharmacokinetic profile better than other factor Xa inhibitors, as it has less drug interactions and is minimally affected by food.[12,13] However, these drugs are not completely free from problems. The principal issue is the lack of a specific antidote to them. But since these drugs have a short half-life, reversal of action does not pose much problem except where rapid or acute reversal is needed.[19] These drugs still stand the ground of being useful anti-coagulants in thromboembolic disorders and are attractive therapeutic alternatives as compared to the current treatment options. It is important to remember that these agents should be tailored to the individual patient, and management of thrombotic disorders requires multi-modal approach.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Eikelboom JW, Weitz JI. New anticoagulants. Circulation. 2010;121:1523–32. doi: 10.1161/CIRCULATIONAHA.109.853119. [DOI] [PubMed] [Google Scholar]
- 2.Augoustides JG. Breakthroughs in anticoagulation: Advent of the oral direct factor Xa inhibitors. J Cardiothorac Vasc Anesth. 2012;26:740–5. doi: 10.1053/j.jvca.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 3.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2) A randomised double-blind trial. Lancet. 2010;375:807–15. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- 4.Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–98. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 5.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 6.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 7.Botticelli Investigators, Writing Committe. Buller H, Deitchman D, Prins M, Segers A. Efficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose-ranging study. J Thromb Haemost. 2008;6:1313–8. doi: 10.1111/j.1538-7836.2008.03054.x. [DOI] [PubMed] [Google Scholar]
- 8.Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699–708. doi: 10.1056/NEJMoa1105819. [DOI] [PubMed] [Google Scholar]
- 9.Yates SW. Apixaban for stroke prevention in atrial fibrillation: A review of the clinical trial evidence. Hosp Pract (1995) 2011;39:7–16. doi: 10.3810/hp.2011.10.918. [DOI] [PubMed] [Google Scholar]
- 10.APPRAISE Steering Committee and Investigators. Alexander JH, Becker RC, Bhatt DL, Cools F, Crea F, et al. Apixaban, an oral, direct, selective factor Xa inhibitor, in combination with antiplatelet therapy after acute coronary syndrome: Results of the apixaban for prevention of acute ischemic and safety events trial. Circulation. 2009;119:2877–85. doi: 10.1161/CIRCULATIONAHA.108.832139. [DOI] [PubMed] [Google Scholar]
- 11.Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708. doi: 10.1056/NEJMoa1207541. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Zhang D, Raghavan N, Yao M, Ma L, Frost CE, et al. In vitro assessment of metabolic drug-drug interaction potential of apixaban through cytochrome P450 phenotyping, inhibition, and induction studies. Drug Metab Dispos. 2010;38:448–58. doi: 10.1124/dmd.109.029694. [DOI] [PubMed] [Google Scholar]
- 13.Nutescu E, Chuatrisorn I, Hellenbart E. Drug and dietary interactions of warfarin and novel oral anticoagulants: An update. J Thromb Thrombolysis. 2011;31:326–43. doi: 10.1007/s11239-011-0561-1. [DOI] [PubMed] [Google Scholar]
- 14.Goldhaber SZ, Leizorovicz A, Kakkar AK, Haas SK, Merli G, Knabb RM, et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167–77. doi: 10.1056/NEJMoa1110899. [DOI] [PubMed] [Google Scholar]
- 15.Greer IA. Thrombosis in pregnancy: Updates in diagnosis and management. Hematology Am Soc Hematol Educ Program 2012. 2012:203–7. doi: 10.1182/asheducation-2012.1.203. [DOI] [PubMed] [Google Scholar]
- 16.Cutts BA, Dasgupta D, Hunt BJ. New directions in the diagnosis and treatment of pulmonary embolism in pregnancy. Am J Obstet Gynecol. 2013;208:102–8. doi: 10.1016/j.ajog.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 17.California: WebMD; 2008. [Last updated 2013 Oct 01]. RxList. The internet drug index [Internet] Available from: http://www.rxlist.com/eliquis-drug.htm . [Google Scholar]
- 18.Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. 2013;34:489–98b. doi: 10.1093/eurheartj/ehs408. [DOI] [PubMed] [Google Scholar]
- 19.Perez A, Eraso LH, Merli GJ. Implications of new anticoagulants in primary practice. Int J Clin Pract. 2013;67:139–56. doi: 10.1111/ijcp.12023. [DOI] [PubMed] [Google Scholar]


