Abstract
Objectives:
To investigate the nephrotoxic effect and biochemical alterations induced by cefepime in rats.
Materials and Methods:
Cefepime was administered intramuscularly at doses of 45, 90 and 180 mg/kg b.wt. once daily for 5 consecutive days. The serum and urine samples were used for quantitative determination of urea, creatinine, glucose, total protein, calcium, sodium and potassium. The histopathological examination of kidney tissues was performed 1, 4 and 8 days after the last dose of cefepime administration.
Results:
Cefepime induced a significant increase in the total amount of urine per day, urea and creatinine concentration in the serum and urine and significant decrease in their clearance. Cefepime also caused a significant gluocosuria and proteinuria and significant decrease in their serum concentrations. The effect of cefepime on serum and urine concentrations of calcium, sodium and potassium were also determined. Cefepime injection in the three tested doses caused renal tubular, glomerular and vascular changes. The severity of these changes was dose dependent. In conclusion, these results suggest a possible contribution of cefepime in the nephrotoxicity and biochemical alterations, especially at high doses. Therefore, the renal functions should be monitored during the cefepime therapy.
Keywords: Biochemicals, cefepime, histopathology, nephrotoxicity, rats
INTRODUCTION
The kidney is a common target of toxicity of therapeutic and environmental xenobiotics, because of its high blood flow, tubular transport processes and complex metabolic activities. Beta-lactams are the largest and most rapidly growing group of antimicrobials. Because of its excretion by the kidney, it might result in nephrotoxicity, which is of major concern in the treatment of the increasingly resistant infections.[1,2]
Beta-lactams are toxic to the kidney when given singly or in doses just above the therapeutic range and even more toxic when used in combinations with other nephrotoxic medications.[3]
Cephalosporins are known to produce the acute proximal tubule necrosis in both animals and human.[4] The nephrotoxicity of cephalosporins is dependent upon renal cortical accumulation and intracellular concentration.[5] It is well-known that first-generation cephalosporins as cephaloridine and cephalothin as well as newer congeners have been associated with nephrotoxicity in both human and experimental animals.[6,7,8]
As a reminder, cephalosporins have the potential to induce nephrotoxicity. The inability to recognize this may lead to renal failure with oliguria and subject patients to an increased risk of irreversible kidney damage. With the advent of newer cephalosporins and less knowledge about its potential for nephrotoxicity, it seems important to evaluate whether it can induce kidney damage or not.
One of the recent and mostly used cephalosporins is cefepime, a parenteral fourth generation cephalosporin. Cefepime is an established and generally well-tolerated with a broad spectrum antibacterial activity.[9,10,11] Cefepime has in vitro activity against Gram-positive and Gram-negative organisms and stable against many of the common plasmid and chromosome mediated beta lactamases.[12,13]
Expanded information concerning cefepime therapy will be of benefits to both physicians and patients. Therefore, the purpose of this study was to investigate the biochemical alterations and histopathological changes of the kidney during and after cefepime administration in rats.
MATERIALS AND METHODS
Materials
Cefepime
It was obtained from Bristol Myers Squibb Company, Egypt. Its commercial name is Maxipime®.
Laboratory animal
A total of 12 albino male rats (200-250 g) were obtained from the Department of Laboratory Animal, Faculty of Veterinary Medicine, Benha University. They were fed on a balanced diet and had access to water ad libitum. Rats were kept under standard conditions of temperature 27-30°C and good ventilation. Rats were randomly divided into three groups, each of four rats and they were kept singly in metabolic cages.
Methods
Biochemical effects of cefepime in rats
Rats of the first, second and the third group were given cefepime intramuscularly in doses of 45, 90, 180 mg/kg b.wt. respectively for 5 consecutive days. Blood samples and urine voided during 24 h one day before, during and 4 days after cefepime administration were taken every other day. Serum was separated by centrifugation of blood at 1600g for 10 min and stored at − 20°C, until analysis. The serum and urine samples were used for quantitative determination of urea, creatinine, glucose, total protein, calcium, sodium and potassium.
One, 4 and 8 days after the last administration, rats from each group were sacrificed; the kidneys were collected for histopathological examination.
Histopathological examination of kidney
The preparation of kidney samples and procedures of staining were carried out according to Crossmon (1937) and Carleton et al. (1980).[14,15]
Statistical analysis
Data were expressed as mean ± standard error and were statistically analyzed using the Student's paired t-test to express the differences between groups.[16] Comparison of the mean values was performed and differences were considered statistically significant when P < 0.05.
RESULTS
Effect of cefepime on biochemical parameters
Significant (P < 0.001) biochemical alterations started after the 3rd day of cefepime injection and continued 2 days after the last administration. After that, the changes disappeared and kidney functions were recovered gradually to normal. These significant changes are recorded in Tables 1 and 2.
Table 1.
Effect of intramuscular injection of 45 (A), 90 (B) and 180 (C) mg/kg b.wt. of cefepime for 5 days on serum biochemical parameters in rats (n=4)
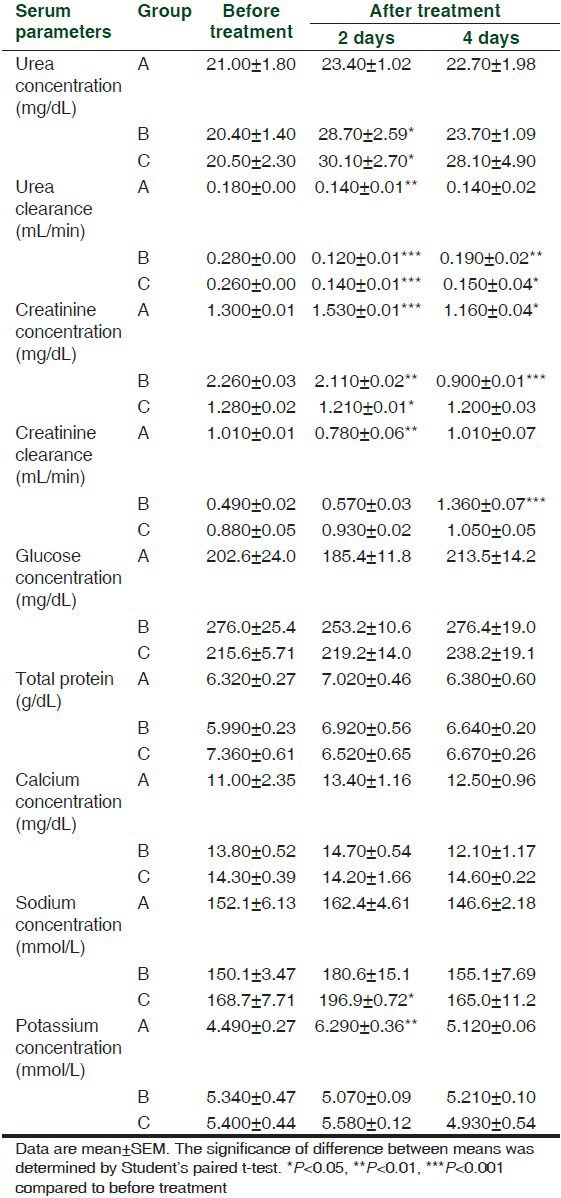
Table 2.
Effect of intramuscular injection of 45 (A), 90 (B) and 180 (C) mg/kg b.wt. of cefepime for 5 days on urine biochemical parameters in rats (n=4)
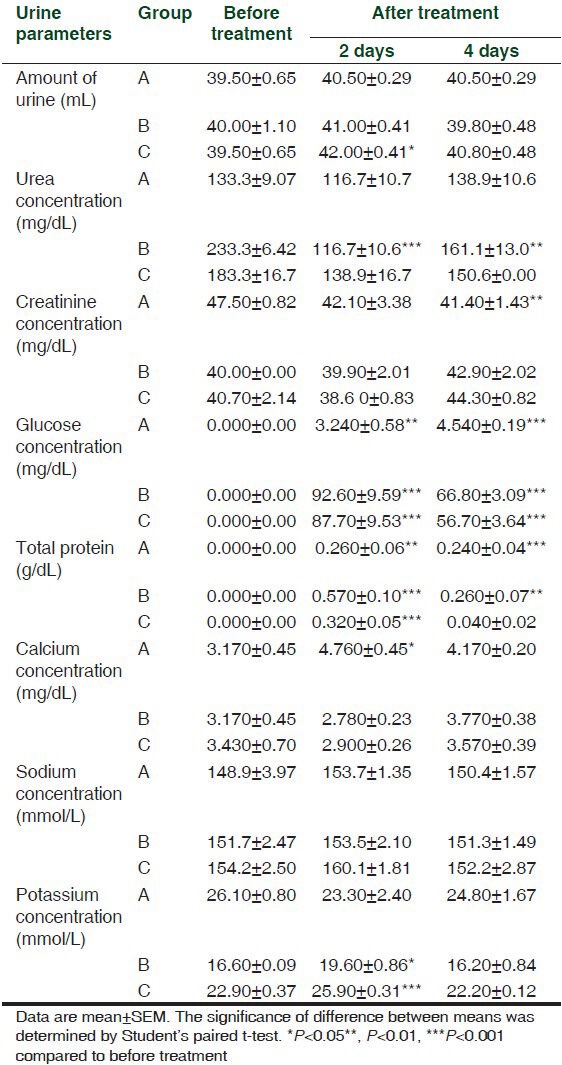
The intramuscular injection of 45 mg cefepime/kg b.wt. caused a significant (P < 0.001) increase in a total amount of urine per day at the 3rd day of administration. Intramuscular injection of 90 mg cefepime/kg b.wt. significantly (P < 0.001) decreased urea concentration in urine and its clearance at the 3rd, 5th and 7th day of administration. Serum concentration of urea was significantly (P < 0.001) increased at 180 mg cefepime/kg b.wt. on the 5th day of administration.
Cefepime at the three administered doses induced significant (P < 0.001) increase in creatinine concentration in serum together with significant (P < 0.001) decrease in its urine concentration as well as significant (P < 0.001) decrease in its clearance from the 1st day until the 9th day of the experiment.
Significant glucosuria (P < 0.001) were obtained after intramuscular injection of 45, 90 and 180 mg/kg b.wt. of cefepime in all days of the experiment. Significant proteinuria (P < 0.001) were induced in the 1st day of the experiment at doses of 90 and 180 mg/kg b.wt. while at the 5th day, it was induced only at 180 mg/kg b.wt. and in the 9th day at 45 mg/kg b.wt. of the cefepime.
Calcium concentration in urine was significantly (P < 0.001) decreased in the 1st day after intramuscular injection of cefepime at 90 mg/kg b.wt. Significant (P < 0.001) increase in the serum concentration of sodium was achieved after cefepime injection at 180 mg/kg b.wt. on the 3rd, 5th and 7th day of administration. Cefepime in the three tested doses caused significant (P < 0.001) increases in the potassium concentration of serum and urine.
Histopathological findings
The histopathological changes in the kidney after intramuscular injection of cefepime for 5 consecutive days in rats were investigated. The changes were dose dependent. Mild degenerative changes and cystic dilatation of the renal tubules with the presence of eosinophilic debris in their lumina were observed 4 days after the last intramuscular injection of 45 mg/kg. b.wt. [Figure 1]. Four days after the last intramuscular injection of 90 mg/kg. b.wt. for 5 days, mild congestion of the renal blood vessels and intertubular blood capillaries were observed and some groups of renal tubules showed cloudy swelling [Figure 2a]. Granular eosinophilic materials in the lumen of some renal tubules were seen 8 days after the last intramuscular injection of 90 mg/kg b.wt. for 5 days [Figure 2b].
Figure 1.
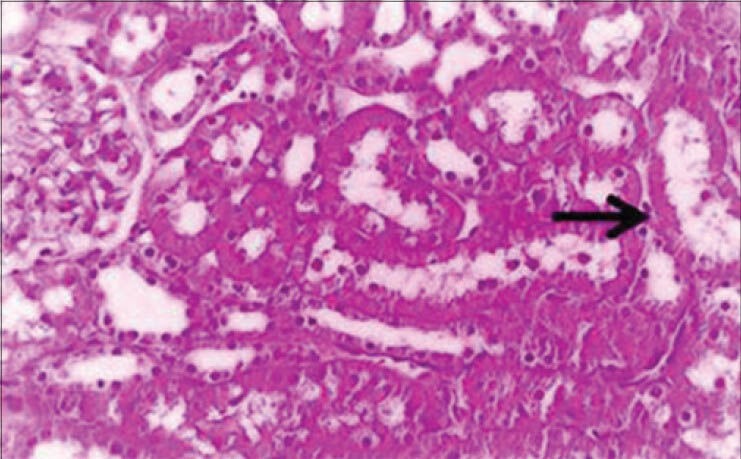
Kidney of rat administered 45 mg cefepime/kg b.wt. for 5 days showing cystic dilatation of renal tubules and eosinophilic debris in its lumen (H and E, ×250)
Figure 2.
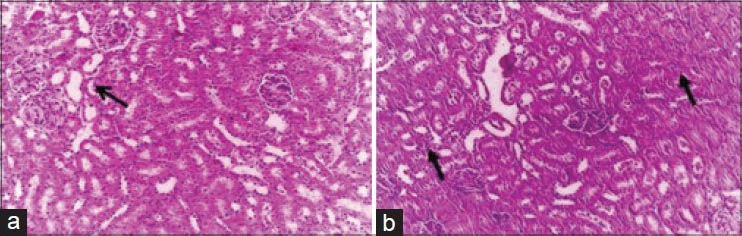
Kidney of rat administered 90 mg cefepime/kg b.wt. for 5 days showing: (a) Mild cystic dilatation of some collecting renal tubules (H and E, ×200). (b) Granular eosinophilic materials in the lumen of some renal tubules and mild degenerative changes in the form of cloudy swelling in the epithelial cells lining the convoluted tubules (H and E, ×200)
Eight days after the last intramuscular injection of 180 mg/kg b.wt. for 5 days, The renal tubules showed the presence of homogenous eosinophilic masses and suffered from pressure atrophy [Figure 3a]. Desquamation of epithelial cells of some renal tubules together with hyaline casts in the collecting tubules was observed [Figure 3b].
Figure 3.
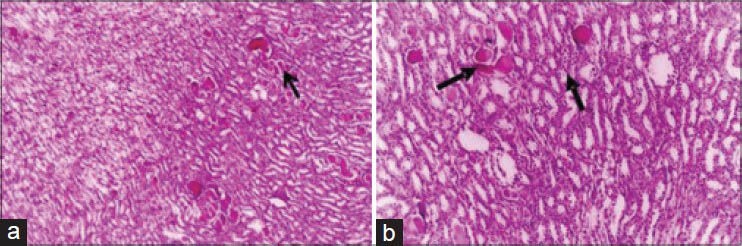
Kidney of rat administered 180 mg cefepime/kg b.wt. for 5 days showing: (a) Mild cystic dilatation of some renal tubules and homogenous eosinophilic masses in their lumen. Some renal tubules suffered from pressure atrophy (H and E, ×100). (b) Desquamation of epithelial cells of some renal tubules together with hyaline casts in the collecting tubules and cystic dilatation (H and E, ×200)
DISCUSSION
Regulation of the internal environment of body cells is maintained mainly by the kidney through glomerular filtration, selective reabsorption and secretion by tubules as well as exchange of hydrogen ions and reduction of ammonia for conservation of base. Threshold substances as urea, creatinine, protein, electrolytes and glucose are almost completely reabsorbed by the tubules when their concentrations in the plasma are within the normal level, but appear in the urine when their plasma level exceeds and/or due to defect in renal tubules as a result of nephrotoxicity. This fact is good predictive in the correlation between their serum and urine levels as demonstrated in the present study.
Cefepime caused a significant increase in the total amount of urine per day at the 3rd day of cefepime administration. This diuretic like effect might be explained on the basis of increased renal blood flow due to vasodilatation of the renal artery and failure of the tubular reabsorption of water due to renal tubular damage manifested histopathologically by degenerative changes in the form of cloudy swelling. These result was consistent with that an increase in the urine volume after administration of ceftizoxime in dogs[17] and cefamandole in rats.[18] These data was inconsistent with that cefadroxil and cefprozil respectively decreased the urine volume in rats in the dose-dependent manner.[19,20] Cefteram pivoxil had no effect on urine volume at intravenous doses of 250-1000 mg/kg and after oral doses of 500-2000 mg/kg b.wt.[21]
In the present study, urea concentration was significantly increased in serum and significantly decreased in urine. The urea clearance was significantly decreased and the degree of significance was dose dependent. This indicated that cefepime impaired ability of the kidney to excrete urea in urine. This might be attributed to damage and occlusion of renal tubules by casts and decreased the glomerular filtration rate due to the proliferation of the glomerular tuft, which became nearly filled the Bowman's space as reported in these histopathological results. These results were similar with that serum urea nitrogen increased over the control values after intravenous injection of cefodizime sodium (600 and 1800 mg/kg) and also after administration of cefpirome sulphate and cefazoline sodium in rabbits.[22,23] Cephaloridine treatment (500 mg/kg subcutaneously) elevated the blood urea nitrogen.[24] Cefamandole significantly increased urea in serum and significantly decreased it in urine and also urea clearance was also decreased.[18] Cefmetaline slightly increased urea nitrogen after oral dosing of 500 mg/kg b.wt. in rabbits.[25] The increase in serum urea and incidence of renal failure had been reported in some patients treated with cefepime during the post marketing experience.[26]
The elevation in serum creatinine and the decrease of it in urine after administration of cefepime were reported in the present study. The cause and explanation was discussed before as in urea. This result was agreeable with that cefodizime increased serum creatinine[22] and after administration of cefpirome in rabbits.[23] Cefamandole significantly increased serum creatinine and decreased it in urine and also creatinine clearance was decreased.[18] Cefepime elevated serum creatinine and caused interstitial nephritis.[27]
Cefepime administration caused glucosuria and significantly decreased serum glucose. This could be explained on the basisof lack of glucose filtration due to the proliferation of the glomerular tuft, which became nearly filled the Bowman's space and decreased tubular reabsorption after renal tubular damage. This manifested histopathologically by degenerative changes in the form of cloudy swelling or even completes destruction of the renal tubules. This result was similar with that; cefminox slightly decreased serum glucose in male rats at the doses more than 200 mg/kg/day intramuscularly[28] as well as after administration of cefpirome in rabbits[23] and after intramuscular injection of cefamandole in doses of 202.5 and 405 mg/kg. b.wt. in rats.[18]
Cefepime caused proteinuria and decreased serum proteins level. This might be attributed to the progressive cellular and tubular dysfunction manifested histopathologically in the present work. This was consistent with that recorded in rabbits following administration of both cefpirome[23] and after oral dosing of cefmtaline, urinary protein was detected.[25]
Cefepime significantly declined urinary calcium and elevated its serum level. This might be attributed to the failure of the kidney to excrete calcium in the urine. This result was agreeable with the decrease of urinary excretion of electrolytes following administration of cefprozil in dose-dependent manners in rats.[20] Cefamandole also induced hypercalcemia and decrease in urinary calcium.[18] In other way, cefteram had no effects on electrolytes excretion at oral doses of 500-2000 mg/kg b.wt. in rats.[21]
Serum and urine sodium level was significantly increased, especially after cefepime administration in a dose of 180 mg/kg b.wt. A Parallel increases in the serum and urine potassium following administration of cefepime in doses of 90 and 180 mg/kg b. w. This elevation might be due to the diuretic like effect of cefepime, decrease of the glomerular filtration and decrease of tubular reabsorption of sodium and potassium in the renal tubules. The obtained results were consistent with an increase in sodium and potassium excretion after administration of ceftizoxime to female mongrel dogs[17] and after administration of cefminox in rats.[28] Moreover, cefamandole increased the sodium and potassium concentration in serum and urine.[18] In another way, cefadroxil decreased the excretion of electrolytes (sodium, potassium and chloride) in first 3 h in rats[19] and cefprozil decreased urine volume and urinary excretion of electrolytes in dose-dependent manners in rats.[20]
The mechanism of tubular and glomerular changes in kidney might be related to use of a large dose of the cefepime, which accumulated in the epithelial cells in concentrations sufficient to exert a direct cytotoxic effect.[29] The inflammatory changes and tubular necrosis obtained in this study were consistent with those obtained after the administration of cefamandole.[30]
The most common findings in the renal tubules were manifested by the presence of eosinophilic casts, which appeared as homogenous pinkish materials nearly taken the shape of renal tubules was similar with that cefodizime caused renal proximal tubular changes such as necrosis, hyaline cast and calcification, suggesting renal disorders.[22] Interstitial nephritis associated with cefepime had been recorded.[27]
CONCLUSION
These results suggest a possible contribution of cefepime in the nephrotoxicity and biochemical alterations, especially at high doses. Therefore, the patient renal functions should be monitored during the cefepime therapy as well as cefepime doses should be adjusted to those who suffer from kidney malfunctions.
ACKNOWLEDGMENT
I would like to express my sincere thanks to other members of Pharmacology Department, Faculty of Veterinary Medicine, Benha University, Egypt for their help to accomplish this work.
Footnotes
Source of Support: My research project was fully sponsored by Faculty of Veterinary Medicine, Benha University, Egypt, as I am working as assistant lecturer in this university
Conflict of Interest: None declared.
REFERENCES
- 1.Kamiya A, Okumura K, Hori R. Quantitative investigation on renal handling of drugs in rabbits, dogs, and humans. J Pharm Sci. 1983;72:440–3. doi: 10.1002/jps.2600720429. [DOI] [PubMed] [Google Scholar]
- 2.Tune BM. Nephrotoxicity of beta-lactam antibiotics: Mechanisms and strategies for prevention. Pediatr Nephrol. 1997;11:768–72. doi: 10.1007/s004670050386. [DOI] [PubMed] [Google Scholar]
- 3.Servais H, Mingeot-Leclercq M, Tulkins BM. Antibiotics induced nephrotoxicity. In: Tarlof JB, Lash HL, editors. Target Organ Toxocology Series, Toxicology of the Kidney. 3rd ed. USA: CRC Press; 2005. pp. 654–62. [Google Scholar]
- 4.Kuo CH, Hook JB. Depletion of renal glutathione content and nephrotoxicity of cephaloridine in rabbits, rats, and mice. Toxicol Appl Pharmacol. 1982;63:292–302. doi: 10.1016/0041-008x(82)90052-7. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein RS, Pasino DA, Hook JB. Cephaloridine nephrotoxicity in aging male Fischer-344 rats. Toxicology. 1986;38:43–5. doi: 10.1016/0300-483x(86)90171-x. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein RS, Smith PF, Tarloff JB, Contardi L, Rush GF, Hook JB. Biochemical mechanisms of cephaloridine nephrotoxicity. Life Sci. 1988;42:1809–16. doi: 10.1016/0024-3205(88)90018-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhanel GG. Cephalosporin-induced nephrotoxicity: Does it exist? DICP. 1990;24:262–5. doi: 10.1177/106002809002400311. [DOI] [PubMed] [Google Scholar]
- 8.Lash LH, Tokarz JJ, Woods EB. Renal cell type specificity of cephalosporin-induced cytotoxicity in suspensions of isolated proximal tubular and distal tubular cells. Toxicology. 1994;94:97–118. doi: 10.1016/0300-483x(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 9.Huang SS, Lee SC, Lee N, See LC, Tsai MH, Shieh WB. Comparison of in vitro activities of levofloxacin, ciprofloxacin, ceftazidime, cefepime, imipenem, and piperacillin-tazobactam against aerobic bacterial pathogens from patients with nosocomial infections. J Microbiol Immunol Infect. 2007;40:134–40. [PubMed] [Google Scholar]
- 10.Zobell JT, Young DC, Waters CD, Ampofo K, Cash J, Marshall BC, et al. A survey of the utilization of anti-pseudomonal beta-lactam therapy in cystic fibrosis patients. Pediatr Pulmonol. 2011;46:987–90. doi: 10.1002/ppul.21467. [DOI] [PubMed] [Google Scholar]
- 11.Lacy CF, Armstrong LL, Goldman MP, Lance LL. 21st ed. Hudson, Ohio: Lexi-Comp Inc; 2012. Drug Information Handbook: A Comprehensive Resource for All Clinicians and Healthcare Professionals; p. 306. [Google Scholar]
- 12.Sanders CC. Cefepime: The next generation? Clin Infect Dis. 1993;17:369–79. [PubMed] [Google Scholar]
- 13.Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: A systematic review and meta-analysis. Lancet Infect Dis. 2007;7:338–48. doi: 10.1016/S1473-3099(07)70109-3. [DOI] [PubMed] [Google Scholar]
- 14.Crossmon G. Modification of Mallory's connective tissue stain with a discussion of the principles involved. Anat Rec. 1937;69:33–83. [Google Scholar]
- 15.Carleton HM. In: Carleton's Histopathological Technique. 5th ed. Carleton HM, Drury RA, Wallington EA, editors. New York: Oxford University Press; 1980. pp. 33–48. [Google Scholar]
- 16.Snedecor GW, Cochran WG. 7th ed. Ames Iowa: Iowa State University Press; 1980. Statistical Methods; pp. 39–63. [Google Scholar]
- 17.Honda F, Ono T, Itoh N, Mori J, Ohtsuka M, Kamitani T. General pharmacology of ceftizoxime sodium. Arzneimittelforschung. 1980;30:1680–7. [PubMed] [Google Scholar]
- 18.el-Sayed MG, Hassanin MR, Hafez MH, el-Komy AA, Mohamed A. Some pharmacodynamic and biochemical aspects of cefamandole. Dtsch Tierarztl Wochenschr. 1997;104:481–7. [PubMed] [Google Scholar]
- 19.Hasegawa Y, Muto N, Morita M. General pharmacology of cefadroxil (author's transl) Jpn J Antibiot. 1979;32:1356–71. [PubMed] [Google Scholar]
- 20.Goto A, Amano M, Sakai A, Hara M, Takahashi N. General pharmacology of BMY-28100. Jpn J Antibiot. 1990;43:1289–309. [PubMed] [Google Scholar]
- 21.Hirai S, Kodama T, Hiraiwa T, Abe N, Arai H, Ono S, et al. General pharmacology of T-2588, a new oral cephem antibiotic. Jpn J Antibiot. 1986;39:958–78. [PubMed] [Google Scholar]
- 22.Morioka H, Yajima R, Inazu M, Kobayashi T, Sakaguchi T. Effect of cefodizime sodium on the kidney function in male rabbits - Single and 7-day repeated intravenous administration. J Toxicol Sci. 1988;13(Suppl 1):329–60. doi: 10.2131/jts.13.supplementi_329. [DOI] [PubMed] [Google Scholar]
- 23.Deki T, Matsuoka A, Marutani K, Nakagawa T, Masuda K, Matsuzawa T, et al. Nephrotoxicity of cefpirome sulfate in rabbits – Single and multiple intravenous administration. J Toxicol Sci. 1990;15(Suppl 3):173–200. doi: 10.2131/jts.15.supplementiii_173. [DOI] [PubMed] [Google Scholar]
- 24.Rush GF, Heim RA, Ponsler GD, Engelhardt J. Cephaloridine-induced renal pathological and biochemical changes in female rabbits and isolated proximal tubules in suspension. Toxicol Pathol. 1992;20:155–68. doi: 10.1177/019262339202000203. [DOI] [PubMed] [Google Scholar]
- 25.Kato I, Ogawa M, Ueno M, Nishimura K, Sato K, Kii Y, et al. Toxicity study of cefmatilen hydrochloride hydrate (S-1090) (8) – Nephrotoxicity study in rabbits by single oral administration. J Toxicol Sci. 2001;26(Suppl 1):149–56. [PubMed] [Google Scholar]
- 26.Princeton, NJ: Bristol-Myers Squibb Company; 2003. Bristol-Myers Squibb Company. Maxipime prescribing information. [Google Scholar]
- 27.Zanetti G, Bally F, Greub G, Garbino J, Kinge T, Lew D, et al. Cefepime versus imipenem-cilastatin for treatment of nosocomial pneumonia in intensive care unit patients: A multicenter, evaluator-blind, prospective, randomized study. Antimicrob Agents Chemother. 2003;47:3442–7. doi: 10.1128/AAC.47.11.3442-3447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurebe M, Yokota M, Yuda Y, Sasaki H, Niizato T, Watanabe H, et al. Toxicological studies of a new cephamycin, MT-141. II. Its subacute toxicity in rats. Jpn J Antibiot. 1984;37:855–89. [PubMed] [Google Scholar]
- 29.Tune BM, Fravert D. Cephalosporin nephrotoxicity. Transport, cytotoxicity and mitochondrial toxicity of cephaloglycin. J Pharmacol Exp Ther. 1980;215:186–90. [PubMed] [Google Scholar]
- 30.Wold JS, Welles JS, Owen NV, Gibson WR, Morton DM. Toxicologic evaluation of cefamandole nafate in laboratory animals. J Infect Dis. 1978 May;137(Suppl):S51–9. doi: 10.1093/infdis/137.supplement.s51. [DOI] [PubMed] [Google Scholar]


