Abstract
Hypersensitivity reactions are common adverse drug reactions (ADRs) associated with antiepileptics. Carbamazepine is one of the routinely prescribed drugs for the treatment of epilepsy and neuropathic pain. ADRs due to carbamazepine range from mild maculopapular rash to severe anticonvulsant hypersensitivity syndrome (AHS). AHS is the triad of fever, rash, and internal organ involvement occurring 1-8 weeks after exposure to an anticonvulsant (1 in 1,000 to 10,000 exposures). Spontaneously reported three cases of AHS-drug hypersensitivity reactions induced by carbamazepine are discussed here. Seven to ten days after starting therapy, patients developed maculopapular skin rashes, fever and liver or kidney involvement. The causal relationship between drug and ADR was found to be ‘certain’ in one case and ‘probable’ in other two cases with both WHO-UMC and Naranjo causality assessment scale. All the three cases show category 4a according to Hartwig's severity scale as ADR was the cause for hospital admission. On assessing preventability of ADRs by modified Schumock and Thorntons’ scale, one case was falling into category of ‘definitely preventable’ and other two were ‘not preventable’. AHS is rare but serious reaction with carbamazepine which requires vigilant monitoring by physicians to avoid major consequences.
Keywords: Adverse drug reaction, anticonvulsant hypersensitivity syndrome, carbamazepine, causality assessment, maculopapular rash
INTRODUCTION
Hypersensitivity reactions are common adverse drug reactions (ADRs) associated with antiepileptics.[1] Carbamazepine is one of the routinely prescribed drugs for the treatment of epilepsy. Neuropathic or deafferentiation pain which occurs in post-herpetic or trigeminal neuralgia, also responds to antiepileptics such as phenytoin, carbamazepine, and gabapentine.
Aromatic anticonvulsants [phenytoin, phenobarbital (phenobarbitone) and carbamazepine] are the most frequently involved drugs for cutaneous ADRs. Cutaneous eruptions occur in 3% of individuals who receive carbamazepine and include diffuse erythema, exanthematous rash, urticaria, purpuric petechiae, or a mucocutaneous syndrome any of which can occur from day 8 to day 16 after treatment initiation.[2] Other dermatological adverse effects of carbamazepine include drug hypersensitivity, exfoliative dermatitis, erythroderma, erythema multiforme, and toxic epidermal necrolysis. Rare reactions include dermatomyositis, lupus erythematosis-like syndrome, eczema, photosensitivity, pustules, psoriasiform, and lichenoid reactions and Anticonvulsant hypersensitivity syndrome (AHS). AHS was first described in 1950 but confusion still abounds regarding the syndrome. The triad of fever, rash and internal organ involvement occurring 1-8 weeks after exposure to an anticonvulsant heralds this rare (1 in 1,000 to 10,000 exposures) but serious reaction. Fever, in conjunction with malaise and pharyngitis, is often the first sign. This is followed by a rash which can range from a simple exanthema (mild form) to toxic epidermal necrolysis (severe form). Internal organ involvement usually involves the liver, although other organs such as the kidney, CNS or lungs may be involved.[2] We report three interesting cases of AHS-drug hypersensitivity reactions induced by carbamazepine given to patients with neuralgia. The salient features of the presentation, diagnosis and management of this condition are discussed below.
CASE REPORTS
Case 1
A 30-year-old male patient was suffering from herpes labialis, for which he consulted local physician and was prescribed tablet carbamazepine 200 mg two times a day, tablet acyclovir dispersible 200 mg five times a day, tablet pregabaline 75 mg twice a day and tablet methylcobalamine 500 μg thrice a day. He improved initially from herpes labialis lesions but after 10 days, developed lesions in oral mucosa which spread to the face, upper extremities, trunk and ultimately all over the body for which he consulted the same doctor. He was treated with steroids, cetirizine, and erythromycin and the initial treatment was continued as such. He was suspected to be having reactivation of the herpes virus or drug reaction. But the severity of the lesion was pointing more toward drug allergic reaction. Moreover he was continuing the tablet acyclovir in routine prescribed doses, so possibility of virus reactivation was very minimal. The condition of the patient worsened after 5 days and was referred to dermatology department of Dhiraj Hospital, a tertiary care teaching rural hospital. On cutaneous examination, multiple hyper-pigmented lesions and some target lesions were present all over the body, creamy-curdy lesions over the tongue, oral mucosa, and palate [Figure 1]. On ophthalmic examination, multiple scaly lesions in lids, gross lid swelling, purulent discharge, congested conjunctiva, ulceration in conjunctiva, and lower fornix. The patient was suspected of having AHS due to drug allergic reaction. Blood investigations revealed WBC count 6800/mm3, neutrophils 78%, lymphocytes 15%, urine albumin 4+ and SGPT 66 IU/l, suggestive of liver and kidney involvement.
Figure 1.
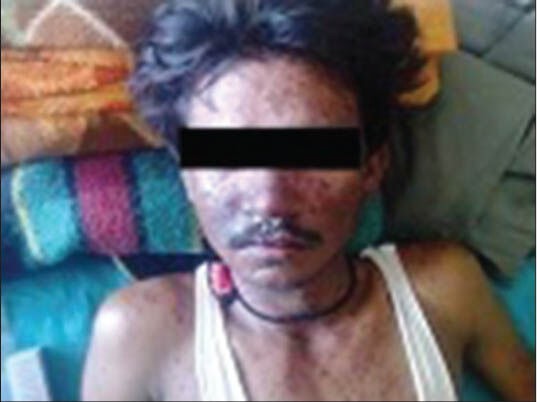
Case 1: Maculopapular rashes over trunk, face and forearm
Carbamazepine was stopped and the patient was treated with injection dexamethasone 8 mg and injection pheniramine maleate 4 mg intramuscularly once a day for 3 days, tablet azithromycin 500 mg once a day for 6 days, injection ranitidine i.v. two times a day. He improved after 5 days of treatment and his SGPT levels returned to normal.
Case 2
A 41-year-old male patient had complaints of skin lesions over the trunk with shooting pain at the back, radiating to the front. He was diagnosed as having herpes zoster by a local physician and treated with tablet acyclovir 800 mg five times a day, tablet mecobalamine 500 μg thrice a day, tablet folic acid 5 mg once a day and tablet carbamazepine 300 mg once a day.
The lesions of herpes zoster subsided within 5 days of treatment but on the 7th day, he developed new reddish skin lesions over the trunk. He discontinued all medications and the new lesions subsided within 2 days. The patient had again consulted the doctor for persisting shooting pain and was prescribed tablet carbamazepine 300 mg once a day, tablet fexofenadine 60 mg once a day, tablet clonazepam 0.5 mg once a day and tablet aceclofenac 100 mg daily. But after 9 days, reddish-colored lesions reappeared over the trunk spreading to upper and lower limbs so he was referred to department of skin and venereal diseases of Dhiraj Hospital, Piparia, Vadodara. On examination, the lesions were flat, reddish in color with well-demarcated margin present all over the body [Figure 2]. Laboratory investigations were sent immediately to rule out internal organ involvement. Investigations showed hemoglobin (Hb) 13.4 gm%, total leukocyte count (TLC) 5300/mm3, neutrophils 86%, lymphocytes 09%, monocytes 02%, eosinophil 03%, basophil 00%, random blood glucose (RBS) 128 mg%, blood urea 21mg%, serum creatinine 0.9 mg%, SGPT 50 IU/L, SGOT 23 IU/L and serum bilirubin 0.6 mg%. The level of SGPT was borderline high suggesting of mild liver involvement. Carbamazepine was discontinued and he was given injection dexamethasone 8 mg and injection pheniramine maleate 4 mg intramuscularly once a day for 3 days. After 4 days of treatment, lesions subsided without any sequelae and liver enzymes came back to normal.
Figure 2.
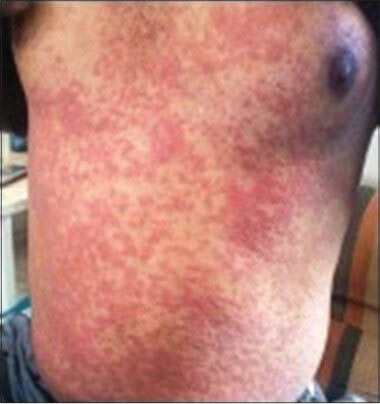
Case 2: Maculopapular rashes over trunk
Case 3
A 50-year-old male patient was suffering from trigeminal neuralgia and was on tablet carbamazepine 300 mg once a day for last 1 week. He developed reddish-colored skin lesions all over the body, spreading into oral cavity with fever after 8 days of starting the treatment. He was suspected of having drug reaction and referred to department of skin and venereal diseases of Dhiraj hospital, Piparia. On clinical examination, skin lesions were present all over the body with itching and fever [Figure 3]. The lesion appeared first in oral cavity and spread to the trunk and extremities. He was diagnosed as having maculopapular eruptions due to carbamazepine. Blood investigations revealed WBC count 6300/mm3, neutrophils 78%, lymphocytes 15%, urine albumin 3+ and SGPT 60 IU/l, suggestive of liver and kidney involvement. Tablet carbamazepine was stopped immediately and he was given oral prednisolone 30 mg once a day for 1 week and tapered by 5 mg per week along with injection pheniramine maleate 4 mg intramuscularly. He was on regular follow-up with good improvement in his clinical condition.
Figure 3.
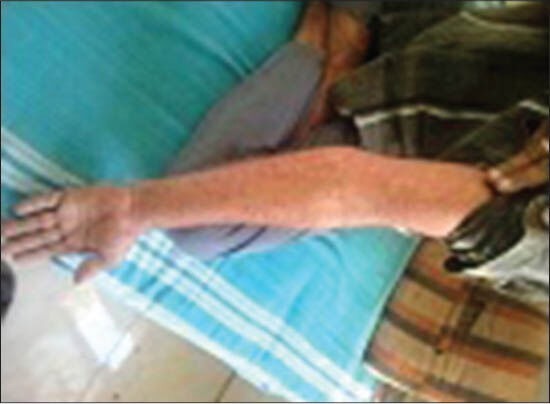
Case 3: Maculopapular rashes over forearm
Analysis of ADR
All the ADRs were analyzed for the causality, severity, and the preventability assessments using WHO-UMC scale, Naranjo algorithm,[3] Hartwig scale[4] and Modified Schumock and Thornton scales,[5] respectively as shown in Table 1.
Table 1.
Analysis of ADRs
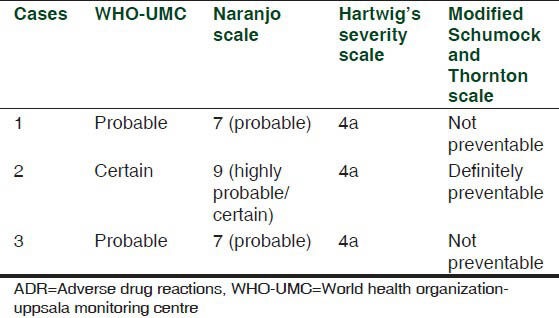
The causal relationship between drug and ADR was found to be certain in case 2 with WHO-UMC causality categories as it has plausible association with time frame, and positive proven rechallenge. While in case 1 and case 3 due to lack of rechallenge, causality association was ‘probable’. Causality assessment with Naranjo scale shows certain association between drug and ADR for case 2 while it shows probable association for case 1 and 3. All of the three cases show category 4a according to Hartwig's severity scale as ADR was the cause for hospital admission. On assessing preventability of ADRs by modified Schumock and Thorntons’ scale, case 2 was falling into category of ‘definitely preventable’ and case 1 and 3 were ‘not preventable’.
DISCUSSION
Spontaneously reported three cases of anticonvulsant hypersensitivity syndrome within a short time span of 3 months are discussed here which requires attention of all health care professionals. Cutaneous adverse reactions to drugs occur in up to 8% of the global general population and in 2-3% of hospitalized patients. Cutaneous eruptions occur in 3% of individuals who receive anticonvulsants.[6] The most common cutaneous adverse reactions to medications include generalized morbilliform rashes (50-95%) and urticaria (5-22%).[2] Development of rash may be an early warning of bone marrow toxicity. Abnormal liver function and bone marrow suppression with deaths due to aplastic anemia have also been reported with carbamazepine. Maculopapular eruptions usually appear within 3-20 days of treatment initiation and spontaneously abate within a similar timeframe when medication is discontinued.[6] In this study also all the patients started reaction within 7-10 days of initiating treatment with carbamazepine and which subsided within 5-7 days of discontinuation of treatment. Mild cutaneous adverse drug reactions are very common with anticonvulsants but AHS is less frequently reported.
AHS is a triad of fever, rash and internal organ involvement.[2] In our study, case 1 had liver and kidney involvement suggested by elevated SGPT and proteinuria +4, while case 2 had liver involvement suggested by elevated liver enzymes. All the three components of the triad in the patients confirm definite diagnosis of AHS. In case 3, due to early attention in the hospital, offending drug was discontinued and patient was prevented from going into the severe stage of the reaction with internal organ involvement.
The exact mechanism of this reaction is unknown. However, suggested mechanism of adverse drug reaction (ADR) is allergic hypersensitivity. The aromatic anticonvulsants (phenytoin, phenobarbital/phenobarbitone, and carbamazepine) are metabolised to hydroxylated aromatic compounds, such as arene oxides. If detoxification of this toxic metabolite is insufficient, the toxic metabolite may bind to cellular macromolecules causing cell necrosis or a secondary immunological response. Cross-reactivity among the aromatic anticonvulsants may be as high as 75%. In addition, there is a familial tendency to hypersensitivity to anticonvulsants.[3]
Discontinuation of the anticonvulsant is essential in patients who develop symptoms compatible with anticonvulsant hypersensitivity syndrome. Eruptions do not always recur after drug rechallenge. However, in our study one patient (case 2) was accidentally re-administered carbamazepine and developed more marked eruptions which is pointing toward positive rechallenge.
Development of a rash during treatment warrants withdrawal of the drug and if no alternative exists, desensitization can be considered.[6] Treatments include antihistamines (H1-receptor blockers), epinephrine, glucocorticords, anabolic steroids, antigonadotropic agents, and airway management, depending on the severity of the condition.[4] All the three patients received steroids and antihistamines. None of the patients required ICU admission or major intervention. All the patients improved with given antihistamine and steroid treatment.
We also found similar case reports of angioedema and maculopapular eruptions associated with carbamazepine by Elias et al.,[2,6] a case of drug induced hypersensitivity syndrome due to carbamazepine by Morimoto et al.[5] The presence of the HLA-A*3101 allele was associated with carbamazepine-induced hypersensitivity reactions among subjects of Northern European ancestry. The presence of the allele increased the risk of hypersensitivity reactions from 5.0% to 26.0%. Due to lack of pharmacogenetic laboratory setup, we could not genotype our patient for the HLA-A*3101.[7]
On analyzing the ADR for preventability using modified Schumock and Throntons’ scale, one of the cases was falling in category of ‘definitely preventable’. This case points towards a preventable medication error. Proper history taking about drug reaction would have prevented the represcribing of carbamazepine and therefore this reaction. As maculopapular rash is a first sign of bone marrow depression seen with aromatic anticonvulsants, physicians should have to be vigilant regarding this type of reaction occurring during carbamazepine therapy to avoid major consequences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Edwards IR, Aronson JK. Adverse drug reaction: Definitions, diagnosis and management. Lancet. 2000;356:1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 2.Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: Incidence, prevention and management. Drug Saf. 1999;21:489–501. doi: 10.2165/00002018-199921060-00005. [DOI] [PubMed] [Google Scholar]
- 3.Havill S, Rademaker M. Anticonvulsant hypersensitivity syndrome. Prescriber Update, Ministry of Health. 1998;16:28–31. [Google Scholar]
- 4.Guy AM, Sinert RH. Overview of Angioedema Treatment. 2012. Jan 6, [Last cited on 2012 May 29]. Available from: http://emedicine.medscape.com/article/756261-overview .
- 5.Morimoto M, Watanabe Y, Arisaka T, Takada A, Tonogi M, Yamane G, et al. A case of drug-induced hypersensitivity syndrome due to carbamazepine. Bull Tokyo Dent Coll. 2011;52:135–42. doi: 10.2209/tdcpublication.52.135. [DOI] [PubMed] [Google Scholar]
- 6.Elias A, Madhusoodanan S, Pudukkadan D, Antony JT. Angioedema and maculopapular eruptions associated with carbamazepine administration. CNS Spectr. 2006;11:352–4. doi: 10.1017/s1092852900014474. [DOI] [PubMed] [Google Scholar]
- 7.McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperavičiute D, Carrington M, et al. HLA-A*3101 and Carbamazepine-Induced Hypersensitivity Reactions in Europeans. N Engl J Med. 2011;364:1134–43. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]


