Essential medicines play an important role in improving access to medicines for the majority of the world's population. The concept of a carefully selected list of medicines of assured quality that meet the majority of healthcare needs of a community has proved to be an effective and affordable solution for the treatment of common ailments. The concept gained ground in the 1970s and 1980s and most countries today have a national list of essential medicines based on the model list created by the World Health Organization (WHO). These periodically updated lists provide a number of advantages to health managers, policy makers, prescribers, dispensers, and others in terms of procurement, dispensing, and rational usage. Modules based on the essential medicine concept coupled with standard treatment guidelines (STGs) are an effective way of teaching rational pharmacotherapy to students of health sciences. Information about essential medicines is provided by a number of organizations; ranging from WHO, national health organizations, drug regulators, and nongovernmental organizations (NGOs). Easy access to this extensive pool is often a challenge as there is no single authoritative source.
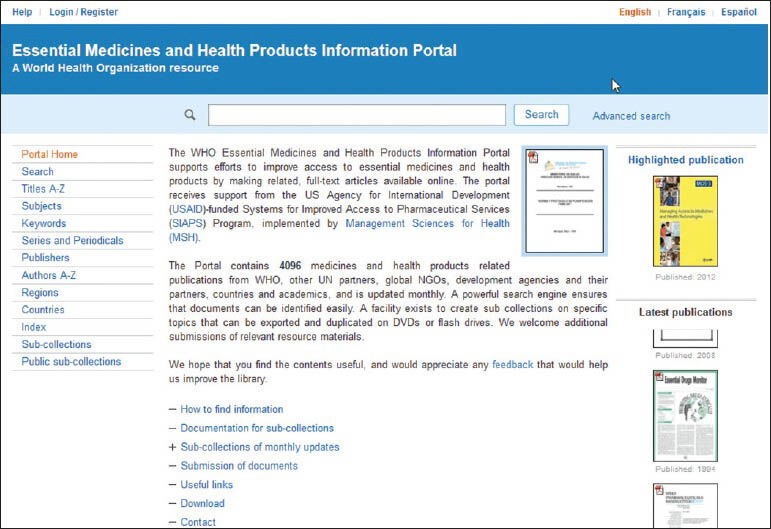
The essential medicines and health products information portal makes available full text articles and publications related to this important area. The portal lists more than 3500 medicines and health products-related publications from WHO, other United Nations (UN) partners, global NGOs, development agencies and their partners, countries, and academics, and is updated monthly.
The portal has a powerful search engine, which enables readers to find publications easily and is available in three languages, English, French, and Spanish and the home page features a highlighted publication and also displays the latest of the series. The subsection on how to find information and publications on the portal will be useful for readers. There are a number of methods to access information on the portal; among these are searching for particular words, accessing publications by title, subject, key word, publisher, author, region, country, and key phrase.
The titles section provides access to publications from the WHO, partner organizations and non-WHO publications and publications related to essential medicines and health products in a number of languages. The collection can be browsed alphabetically and consist of publications in 17 subjects ranging from medicine information and evidence for policy, medicine access, and rational use, to e-health and injection safety. Each subject has subcategories providing easy access and each category and subcategory provides information on the number of publications available, which makes it easy to plan your access. The key words are comprehensive and arranged alphabetically.
The list of periodicals available through the portal range from the Bulletin of the World Health Organization, to selected BiomedCentral (BMC) periodicals, BMJ, INRUD News, Nature, SEARO Technical publication series, to the Journal of Pharmacology and Pharmacotherapeutics. The list of authors and organizations is arranged alphabetically. Publications can also be searched according to WHO regions and countries. At present only 42 countries are available and this list needs expansion. The list of index terms is arranged alphabetically and has been well chosen.
A number of national essential medicines lists, formularies, and STGs are available through the portal. I came across STGs from Zimbabwe, Ghana, Malawi, Lesotho, Vanuatu, Namibia, Somalia, South Africa, Tanzania, Tonga, Ethiopia, Seychelles and Zanzibar. Many STGs are available in combination with essential medicine lists. From India the national essential medicine lists, drug policies of different states and studies dealing with availability and affordability of medicines are available. Drug formularies are available from a number of countries. Among them are Barbados, Belize, Bahrain, Eastern Cape Province of South Africa, Egypt, Ethiopia, Fiji, Jordan, Kenya, Mercy Ships formulary, Saudi Arabia, Malaysia, Philippines, Thailand, and the WHO model formulary.
The sub-collections feature enables users to create customized sub-collections of articles and publications from the library according to personal needs and interest and export the same. The public sub-collections section contains a national medicines policies sub-collection, which brings together documents dealing with this important area. The portal contains links to other WHO sites and the Help section provides useful information on how to use this powerful resource.
The portal provides users with the option of registering at the site and obtaining a user name and password, which would enable them to create customized sub-collections. All content on the portal is freely available for download and use and can be transferred to pen drives and CDs. The portal provides users the facility to download the new version of the WHO Medicines bookshelf and brings together under a single roof a variety of resources related to essential medicines and health products and would be of interest to academicians, students, health professionals, and all those interested in medicines, health products, and their rational use.
About the portal: Essential medicines and health products information portal, a WHO resource. Available from: http://www.apps.who.int/medicinedocs/en/[Last accessed on 2013 October 28].
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.


