Abstract
Background:
The reporting of adverse drug reactions (ADRs) by nurses in hospitals is very important.
Aims:
This study was aimed at investigating the impact of an educational intervention to improve ADR reporting and whether trained nurses had better knowledge, attitude, and practice toward ADR reporting.
Materials and Methods:
A total of 300 nurses in a tertiary care teaching hospital in Tehran, Iran were evaluated with a knowledge, attitude, and practice (KAP) questionnaire regarding ADR reporting in March 2010. After this, an educational program about ADR was provided to nurses. Then the nurses were re-evaluated by the same questionnaire. Comparisons were made of the attitude and knowledge within nurses, before and after education. Data were analyzed using SPSS software. P < 0.05 was considered as significant level. Independent-sample t-test was used to measure the intervention effect.
Results:
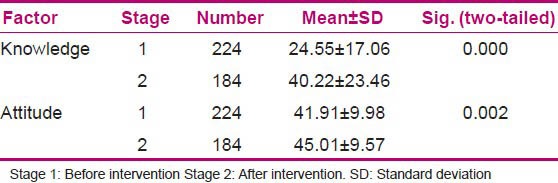
The response rate was 61.3% (N = 184). Knowledge of nurses before the intervention was significantly less than the knowledge after the intervention (P = 0.001). Also, there was a significant effect on attitude (P = 0.002). During the follow-up period of 4 months after the intervention, 26 spontaneous reports were received.
Conclusion:
Continuous ADR educational program, training, and integration of ADRs’ reporting into the activities of the nurses would likely improve ADR reporting.
Keywords: Adverse drug reaction, educational assessment, nursing personnel
INTRODUCTION
World Health Organization's (WHO's) definition of an adverse drug reaction (ADR) is “a response to a drug that is noxious and unintended and occurs at doses normally used in man for the prophylaxis, diagnosis or treatment of a disease, or for modification of a physiological function.”[1] ADRs are one of the leading causes of morbidity and mortality. It has been estimated that around 2.9-5.6% of all hospital admissions are due to ADRs and as many as 35% of hospitalized patients experience an ADR during their hospitalization.[2] Fatal ADRs rank among the most common causes of death. The economic burden of ADRs is also considerable; for example, in the United States, the cost of drug-related morbidity and mortality exceeded $177.4 billion in 2000.[3]
Spontaneous reporting of ADRs is one of the basic methods for post-marketing surveillance and is a method to generate signals of unrecognized ADRs.[4] It remains the cornerstone of pharmacovigilance and is important in maintaining patient safety. However, reporting of serious ADRs rarely exceeds 10%.[5]
Thalidomide disaster was one of the biggest triggers for the initiation of systematic collection of data on ADRs in 1960s.[6] In Iran, a national ADR reporting system was established by the Ministry of Health in June 1998. Despite the existence of the national ADR reporting system in Iran, a major problem of voluntary surveillance by healthcare professionals has been the high level of underreporting.[2] According to the WHO standards, countries with the best reporting rates generate over 200 reports per 1,000,000 inhabitants per year. Therefore, in Iran, with a population of over 60 million, it is expected to receive at least 12,000 reports per year. Unfortunately this is not so, considering the fact that only 2330 reports were sent to the Iranian Pharmacovigilance Center (IPC) in 2006.[7]
The reporting of ADRs in hospitals is very important because severe ADRs are most likely to be seen in hospitals; ADRs can be detected early on, and spontaneous reports can be more accurate.[8] Nurses, in their unique position as drug administrators who record the observed effects of drug use, have been found to have an important role in ADR reporting and constitute a potentially valuable source for spontaneous ADR reports in hospitals, Because they are close to the patients and have a good knowledge of patient's health status, symptoms, drugs, and ADRs. They are often the sources in alerting the responsible prescriber about the possible ADRs. There is thus a logical reason to involve nurses and encourage them to contribute in ADR reporting system.[9,10] In this study, we assessed the role of an educational intervention in ADR reporting. We investigated whether trained nurses had better knowledge, attitude, and practice (KAP) toward the reporting of ADRs in comparison with the same group before training.
MATERIALS AND METHODS
Dr. Shariati hospital is a 598-bedded tertiary care teaching hospital in Tehran, Iran. Prior to 2009, no ADR reporting system was in place. The hospital's pharmacovigilance unit was established in February 2010 to report ADRs.
In our previous study in November 2009, we chose an observational-descriptive research in which a questionnaire was prepared to investigate the KAP of nurses regarding ADR.[11] The questionnaire included the issues addressed in previous studies examining the KAP of medical practitioners to ADR reporting,[7] but were modified to take into account the national basis of the current investigation. For the purpose of the study, the KAP questionnaire was designed by the researchers of the pharmacovigilance in pharmaceutical care department in the hospital through searching in related internet websites. This KAP questionnaire consisted of a total of 17 questions. Among these questions, six were related to the knowledge, seven to the attitude, and the remaining four questions were related to the practice aspects.
Knowledge- and practice-related questions were designed as multiple choices. Knowledge-related questions consisted of easy, moderate, and difficult ones (in equal portions). Attitude-related questions were developed in five choices Likert scale. Content validity (systematic examination of the test content to determine whether it covers a representative sample of the behavior domain to be measured) of the questionnaire was evaluated by expert pharmacists. The initial draft was circulated to the members of the research team and modifications were carried out as per the suggestions. Upon receiving the responses from healthcare professionals, its internal consistency reliability was tested by finding the Cronbach's alpha coefficient on a sample consisting of 20 randomly selected hospital nurses. Test-related reliability was tested by finding the intra-cluster correlation on the same sample after a week. After this modification, the finalized questionnaire was employed, in order to collect data from the major sample.
We are aware of some studies that evaluated the effectiveness of different interventions aimed at increasing ADR reporting rate among healthcare professionals. However, the design and purposes of these studies vary.[9,12,13,14,15,16,17] In March 2010, we developed an educational intervention consisting of a 3 h presentation, undertaken by a clinical pharmacologist and a pharmacist specialized in pharmacovigilance, describing the definitions of pharmacovigilance and ADRs; giving a review of international studies on drug-related morbidity and mortality, hospital admissions due to ADRs, and economic importance of ADRs; describing the methods used in pharmacosurveillance and in spontaneous reporting systems, explaining that underreporting constituted the system's major limitation; and conducting a discussion about the most important cases of ADRs reported to the Iranian Pharmacovigilance Center. We also explained the main parts of the Yellow Card and how to complete it while facing a suspected ADR. We encouraged nurses to report all suspected ADRs including those that were mild or anticipated through ADR-reporting Yellow Cards available at all the nursing stations. All nurses of the hospital were invited to participate in this seminar. One month after the educational intervention, the nurses were re-evaluated using the same questionnaire. Comparisons were made of the attitude and knowledge within nurses, before and after the educational program. There was also a follow-up period that began immediately after the intervention. The outcome assessed included an indicator of reporting quantity (total number of records).
Statistical analysis
The filled KAP questionnaires were analyzed by producing descriptive statistics using the Statistical Package for Social Sciences (SPSS) for MS windows version 17. Score one was assigned to the correct answers to knowledge and practice questions and zero was assigned to the wrong answers. The numerical variables (e.g. the number of working hours per month) were described numerically. The answers to attitude questions were ranked from one to five accordingly; so, score five represents the best attitude. In order to determine the effective factors on knowledge (the summary variable of knowledge), the independent variables with entry into the regression model were used. Accordingly, in order to determine the effective factors on attitude, the summary variable of knowledge was added to the series of independent variables, and to determine the effective factors on practice, both knowledge and attitude variables were added to the series of independent variables. The statistical significance level was considered as 0.05 (P < 0.05). Independent-sample t-test was used to measure the intervention effect on nurses’ attitude and knowledge before and after the educational program. As it was impossible to compare two samples as person to person, we chose independent-sample t-test; otherwise, paired-sample t-test is more reliable than independent-sample t-test in evaluating an intervention effect.
RESULTS
Demographics
Of the 300 nurses who received the questionnaires, 184 (61.3%) completed the questionnaires. Among these respondents, 94.4% were females. Nearly 95% of all respondents were graduated with Bachelor of Science degree in nursing. The demographic data are shown in Table 1.
Table 1.
Age, years from graduation, working hours per month, length of practice (N=184)
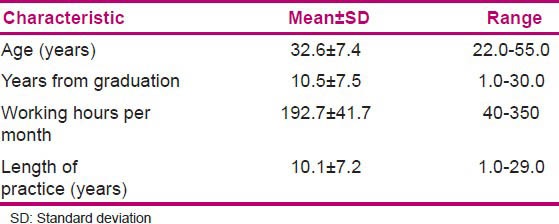
Internal reliability for KAP variables was tested by finding the Cronbach's alpha coefficient, which was 0.739, 0.513, and 0.754, respectively. Omission of none of the items in attitude questions could increase the Cronbach's alpha coefficient appreciably.
Knowledge about ADR reporting
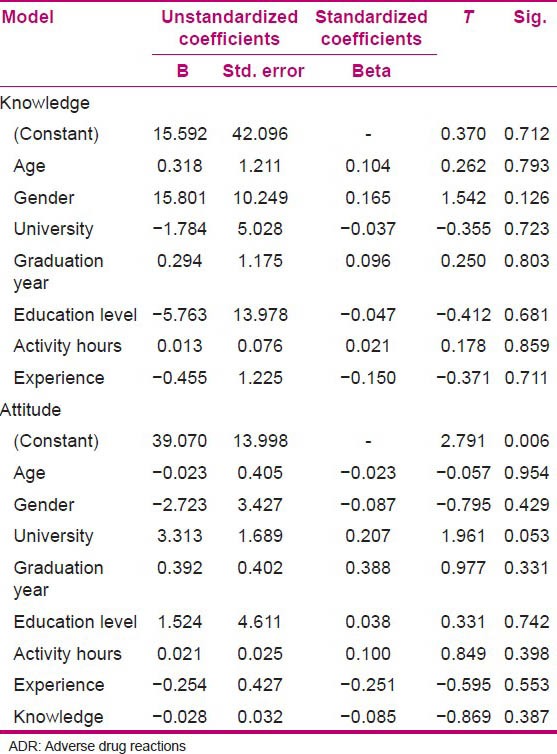
Nurses’ knowledge toward the ADR reporting was evaluated by using six pharmacovigilance-related questions. The results are shown in Table 2. There was no statistically significant relation between independent variables in linear regression analysis and knowledge of the evaluated population [Table 3].
Table 2.
Nurses’ knowledge about ADR reporting
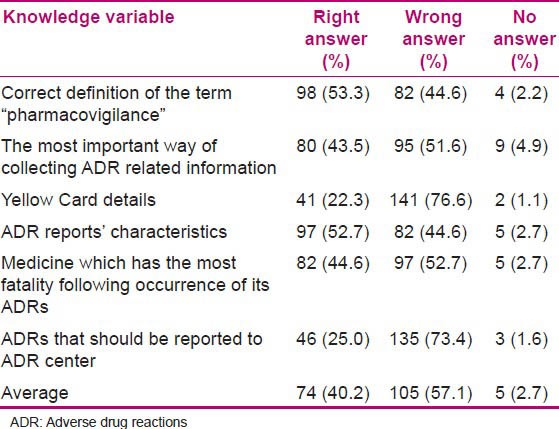
Table 3.
Effective factors on knowledge and attitude of nurses regarding ADR
Attitudes toward ADR reporting
To explore the nurses’ attitudes to pharmacovigilance, seven questions were designed. The descriptive results are presented in Table 4. Also, the independent variables which were entered into the regression analysis did not show any correlation with attitude of the evaluated population. The mean and other statistics of the distribution of knowledge and attitude variables is shown in Table 5.
Table 4.
Nurses’ attitudes toward ADR reporting
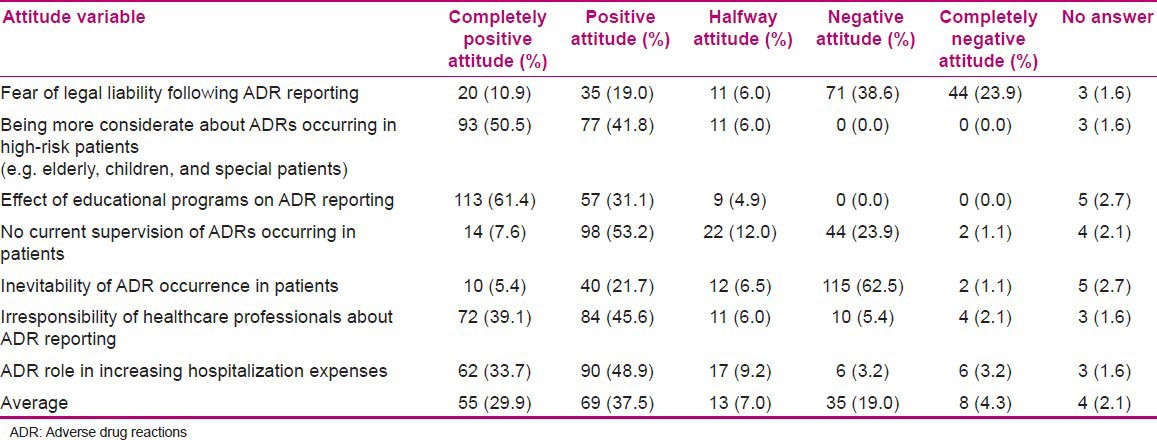
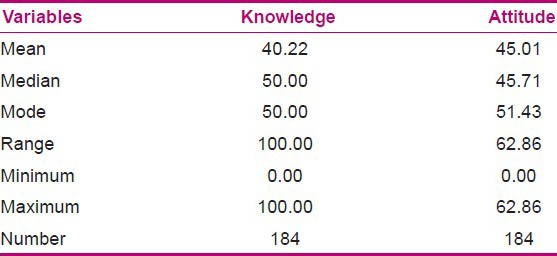
Table 5.
Distribution of knowledge and attitude variables (out of 100)
Practice variables
Nearly 12% of the respondents had experienced ADR reporting. Of this group, 90.7% used Yellow Cards for reporting the ADRs, 9.3% used phone, and nobody used internet or fax for submitting the report. One of the questions was regarding the nurses’ reactions while facing an ADR. More than 89% (148 out of 165) of the respondents to this question reported that they would prefer to announce the ADRs to the physicians in the ward. Figure 1 shows the distribution of their answers to this question.
Figure 1.
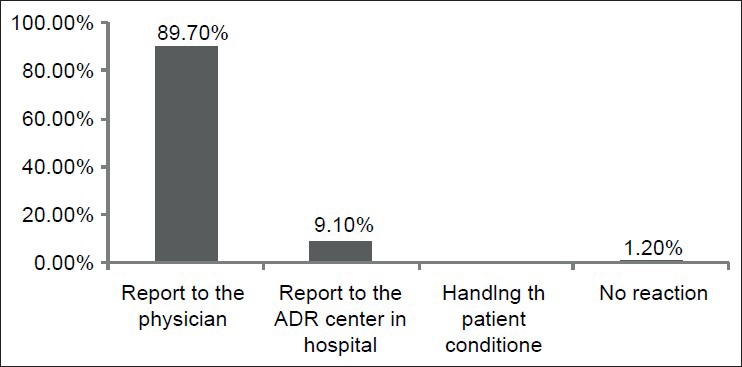
Distribution of the nurses’ reactions while facing an ADR
Comparison of attitudes and knowledge of the nurses before and after educational intervention
To evaluate the changes in knowledge and attitudes of the evaluated population, the mean scores of the knowledge and attitudes before and after the intervention were compared using independent-sample t-test. The results demonstrated that the mean scores of knowledge and attitudes changed significantly after the educational intervention. In other words, it resulted in considerable positive changes in both knowledge and attitudes among the nurses [Table 6].
Table 6.
Comparing the mean knowledge and attitudes scores before and after intervention
DISCUSSION
The most important finding in the present study was that an educational intervention resulted in considerable positive changes in both knowledge and attitude among nurses. The maximum knowledge level was about correct definition of the term “pharmacovigilance,” ADR reports’ characteristics, the medicine which has the most fatality following occurrence of its ADRs, and the knowledge about the most important way of collecting ADR-related information; and the least knowledge level was on the Yellow Card details. Similar trend was shown in our previous study in assessing nurses’ knowledge before the educational intervention. It was observed from a study conducted in Taleqani Hospital in Iran that the knowledge of nurses before the educational intervention was significantly less than the knowledge after the intervention, but there was no significant effect on the attitude.[18]
The results of our study also demonstrated that the attitude level toward the effect of educational programs on ADR reporting and more consideration about ADRs occurring in high-risk patients (e.g., elderly, children, and special patients) was at the highest. It is similar to the nurses’ attitude before the intervention in our previous study.
More than 89% of the respondents to the question regarding their reactions while encountering an ADR stated that they would prefer to report the ADRs to the physicians in the ward. While in a study by Qing et al., 22.8% of nurses reported ADRs primarily to the hospital pharmacy[19] and 56% of nurses in a similar study by Hajebi et al. used to send their reports to physicians in the ward.[18]
We must not lose sight of the sobering fact that about half of the cases of drug-related injury are from potentially avoidable ADRs.[1] The incidence of ADRs reported by physicians and nurses is found to be low.[5,8,10,12,16,20] However, there might be some exceptions. For instance, 41% (n = 40/97) of an Indian hospital's doctors had reported suspected ADRs to the pharmacovigilance centers. Also, 89% of the responders were aware of the existence of ADR reporting and monitoring system at their hospital.[21] In our study, ADR reporting over the follow-up period which began immediately after the intervention was constant, maybe with a slight increase in the reporting rate 2 weeks after the educational program. Despite encouraging the nurses to report all suspected ADRs through ADR-reporting Yellow Cards available at all the nursing stations, during the period of 4 months, only 26 spontaneous reports were received. Similar results were obtained in a study conducted in Sweden during which in a 12-month period, only 18 ADR reports were submitted by nurses who had received special instruction regarding ADR reporting.[22] In contrast to this, in a study by Tabali et al., an initial 148% increase in the number of ADR reports was observed after the educational intervention.[23] Although the actual number of reported ADRs in our study was small, it was an absolute increase in reporting, considering the fact that in comparison, in the years prior to our intervention, only 12% of the respondents had experienced ADR reporting. In other words, whilst all spontaneous reporting systems are affected by underreporting,[24] we expect more reports because the aim and methods of the reporting and monitoring ADRs was extensively explained to the nurses in advance. The underreporting in the follow-up period may be explained by the extensive workload of nurses. ADR reporting is an activity which may take some time to become fully integrated into nurses’ daily routines.
Some studies have used interventions such as mailings, newsletters, oral presentations, verbal reminders, articles in staff newsletters, advertisements, and coordination between healthcare professionals and the hospital pharmacists to increase ADR reporting rate.[12,13,14,15] The pharmacists’ comments to an open question regarding improving ADR reporting in Green et al., study included: Education and training, spending more time on the wards with patients, more feedback and reminders, encouragement from managers, and increased accessibility of Yellow Cards.[5] Also, doctors in a study by Oshikoya[25] and community pharmacists in a study by Bawazir[26] mentioned education and training as the most recognized means of improving ADR reporting. Educational intervention has been shown to improve ADR reporting.[14,16] Also, we showed that education is a positive predictor in influencing nurses to report ADRs. Similarly, Green et al. showed that pharmacists who had received pharmacovigilance training mostly through internal departmental meetings were more likely to report ADRs and knew more about the purposes of the Yellow Card scheme.[5] The effect of educational interventions may diminish over time. So, periodically repeating educational programs maybe would have a booster effect on ADR reporting. We are planning to conduct monthly educational programs for the nurses. It would be logical to extend this study to other teaching hospitals in Iran to enable us generalize our findings.
CONCLUSION
Hospital reporting programs are necessary to increase awareness of ADR reporting among the physicians and nurses. Enhanced reporting rate will improve overall drug assessment and accelerate earlier detection of serious reactions. Our program was an initiation to create a culture of ADR reporting among the healthcare professionals in the hospital. We showed in our study that nurses can respond to educational strategies designed to meet their training needs and can increase ADR reporting. We are planning for further improvement of our ADR-reporting system by increasing the physicians’ and nurses’ awareness of ADR monitoring and reporting, interactions between the physicians and nurses with the pharmacovigilance center in the hospital, inclusion of pharmacovigilance into pre- and post graduate continuing education programs, and sending feedback information to reporters.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support provided by the Tehran University of Medical Sciences (TUMS). Also, we wish to thank the nursing staff of Dr. Shariati Hospital who kindly participated in this study.
Footnotes
Source of Support: Tehran University of Medical Sciences
Conflict of Interest: Nil.
REFERENCES
- 1.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 2.Baniasadi Sh, Fahimi F, Shalviri G. Developing an adverse drug reaction reporting system at a teaching hospital. Basic Clin Pharmacol Toxicol. 2008;102:408–11. doi: 10.1111/j.1742-7843.2008.00217.x. [DOI] [PubMed] [Google Scholar]
- 3.Ernest FR, Grizzle AJ. Drug-related morbidity and mortality: Updating the cost-of-illness model. J Am Pharm Assoc. 2001;41:192–9. doi: 10.1016/s1086-5802(16)31229-3. [DOI] [PubMed] [Google Scholar]
- 4.Ekman E, Backstrom M. Attitudes among hospital physicians to the reporting of adverse drug reactions in Sweden. Eur J Clin Pharmacol. 2009;65:43–6. doi: 10.1007/s00228-008-0564-9. [DOI] [PubMed] [Google Scholar]
- 5.Green CF, Mottram DR, Rowe PH, Pirmohamed M. Attitudes and knowledge of hospital pharmacists to adverse drug reaction reporting. Br J Clin Pharmacol. 2001;51:81–6. doi: 10.1046/j.1365-2125.2001.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grootheest KV, Olsson S, Couper M, de Jong-van den Berg L. Pharmacists’ role in reporting adverse drug reactions in an international perspective. Pharmacoepidemiol Drug Saf. 2004;13:457–64. doi: 10.1002/pds.897. [DOI] [PubMed] [Google Scholar]
- 7.Vessal G, Mardani Z, Mollai M. Knowledge, attitude, and perceptions of pharmacists to adverse drug reaction reporting in Iran. Pharm World Sci. 2009;31:183–7. doi: 10.1007/s11096-008-9276-6. [DOI] [PubMed] [Google Scholar]
- 8.Vallano A, Cereza G, Pedros C, Agusti A, Danes I, Aguilera C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol. 2005;60:653–8. doi: 10.1111/j.1365-2125.2005.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall M, McCormack P, Arthurs N, Feely J. The spontaneous reporting of adverse drug reactions by nurses. Br J Clin Pharmacol. 1995;40:173–5. doi: 10.1111/j.1365-2125.1995.tb05774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Backstrom M, Ekman E, Mjorndal T. Adverse drug reaction reporting by nurses in Sweden. Eur J Clin Pharmacol. 2007;63:613–8. doi: 10.1007/s00228-007-0274-8. [DOI] [PubMed] [Google Scholar]
- 11.Hanafi S, Torkamandi H, Hayatshahi A, Gholami KH, Javadi MR. Knowledge, attitudes and practice of nurses regarding adverse drug reaction reporting. Iran J Nurs Midwifery Res. 2012;17:1–7. [PMC free article] [PubMed] [Google Scholar]
- 12.McGettigan P, Golden J, Conroy RM, Arthur N, Feely J. Reporting of adverse drug reactions by hospital doctors and the response to intervention. Br J Clin Pharmacol. 1997;44:98–100. doi: 10.1046/j.1365-2125.1997.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nazario M, Feliu JF, Rivera GC. Adverse drug reactions: The San Juan Department of Veterans Affairs Medical Center experience. Hosp Pharm. 1994;29:244. [PubMed] [Google Scholar]
- 14.Scott HD, Teacher-Renshaw A, Rosenbaum SE, Wa- ters WJ, Green M, Andrews LG, et al. Physician reporting of adverse drug reactions. Results of the Rhode Island adverse drug reaction reporting project. JAMA. 1990;263:1785–8. [PubMed] [Google Scholar]
- 15.Fincham JA. Statewide program to stimulate reporting of adverse drug reactions. J Pharm Pract. 1989;2:239–44. [Google Scholar]
- 16.Figueiras A, Herdeiro MT, Polonia J, Gestal-Otero JJ. An educational intervention to improve physician reporting of adverse drug reactions. A cluster-randomized controlled trial. JAMA. 2006;296:1086–93. doi: 10.1001/jama.296.9.1086. [DOI] [PubMed] [Google Scholar]
- 17.Feely J, Moriarty S, O’Connor P. Stimulating reporting of adverse drug reactions by using a fee. Br Med J. 1990;300:22–3. doi: 10.1136/bmj.300.6716.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajebi G, Mortazavi SA, Salamzadeh J, Zian AZ. A survey of knowledge, attitude and practice of nurses towards pharmacovigilance in Taleqani Hospital. Iran J Pharm Res. 2010;9:199–206. [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Zhang SM, Chen HT, Fang SP, Yu X, Liu D, et al. Awareness and attitudes of health care professionals in Wuhan, China to the reporting of adverse drug reactions. Chin Med J. 2004;117:856–61. [PubMed] [Google Scholar]
- 20.Granas AG, Buajordet M, Stenberg-Nilsen H, Harg P, Horn AM. Pharmacists’ attitudes towards the reporting of suspected adverse drug reactions in Norway. Pharmacoepidemiol Drug Saf. 2007;16:429–34. doi: 10.1002/pds.1298. [DOI] [PubMed] [Google Scholar]
- 21.Ramesh M, Parthasarathi G. Adverse drug reactions reporting: Attitudes and perceptions of medical practitioners. Asian J Pharm Clin Res. 2009;2:10–4. [Google Scholar]
- 22.Backstrom M, Mjorndal T, Dahlqvist R. Spontaneous reporting of adverse drug reactions by nurses. Pharmacoepidemiol Drug Saf. 2002;11:647–50. doi: 10.1002/pds.753. [DOI] [PubMed] [Google Scholar]
- 23.Tabali M, Jeschke E, Bockelbrink A, Witt CM, Willich SN, Ostermann T, et al. Educational intervention to improve physician reporting of adverse drug reactions (ADRs) in a primary care setting in complementary and alternative medicine. BMC Public Health J. 2009;9:274. doi: 10.1186/1471-2458-9-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiessard F, Roux E, Miremont-Salame G, Fourrier- Reglat A, Haramburu F, Tubert- Bitter P, et al. Trends in spontaneous adverse drug reaction reports to the French pharmacovigilance system (1986-2001) Drug Saf. 2005;28:731–40. doi: 10.2165/00002018-200528080-00007. [DOI] [PubMed] [Google Scholar]
- 25.Oshikoya KA, Awobusuyi JO. Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. BMC Clin Pharmacol. 2009;9:14. doi: 10.1186/1472-6904-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bawazir SA. Attitude of community pharmacists in Saudi Arabia towards adverse drug reaction reporting. Saudi Pharm J. 2006;14:75–83. [Google Scholar]


