Abstract
Background:
Hypothermia is one of the problems occurring during surgery, which can happen due to thermoregulation mechanism disorders and intake of low temperature IV fluids, and may cause increase in blood pressure, heart rate, intracranial pressure, oxygen consumption, pain, and discomfort to the patient. The rate of cesarean section in our country is three times more than the global standard. As one of the responsibilities of the nurse is patient's advocacy, s/he should support them. This study aimed to investigate the effect of pre-warmed intravenous fluids on prevention of hypothermia during general anesthesia in cesarean section.
Materials and Methods:
Sixty-two women undergoing elective cesarean section by general anesthesia were randomly allocated in two groups of intervention and control. Women in the intervention group received pre-warmed serum (37°C) while those in the control group received serum at room temperature (25.5°C). The core body temperature and some hemodynamic parameters of the participants were assessed during the operation.
Results:
The mean of pulse rate, systolic blood pressure, diastolic blood pressure, and arterial O2 saturation in the two groups were not statistically significant (P > 0.05). But the mean of mothers’ core body temperature at the end of anesthesia in the intervention and control groups were 36 ± 0.5°C and 35.34 ± 0.6°C, respectively (P < 0.05).
Conclusion:
Infusion of pre-warmed serum (37°C) would prevent intraoperative hypothermia and improve the nursing care for women who undergo cesarean section by general anesthesia.
Keywords: Cesarean, hypothermia, intravenous fluids, Iran, nursing care
INTRODUCTION
Hypothermia is referred to the reduction of core body temperature to below 36°C. This phenomenon is one of the postoperative outcomes that can be caused by heat loss due to exposure to cold weather, thermoregulation mechanism disorders and, consequently, vasodilatation and loss of muscle tone as a result of anesthetics or local anesthesia.[1,2] Core body temperature ranges from 36.5°C to 37.5°C.[3] Body temperature reduction and, consequently, chills lead to an increase in heart rate, and catecholamine release, vasoconstriction, lower circulation, and metabolic acidosis.[4,5,6]
There are numerous risk factors in relation with development of intraoperative hypothermia, including aging, female gender, depth of anesthesia, type of surgery, length of anesthesia, operating room (OR) temperature, patient's low weight, and history of chronic diseases and cool fluids’ infusion.[1,2] Postoperative chills can be unpleasant and distressful, cause complications for the patients, and may lead to worsening of their postoperative pain, such that some patients indicate it as their worst hospitalization experience. Prevalence of postoperative chills has been reported to be about 40%.[4] This rate has been reported as 6.3-66% for the patients receiving general anesthesia and as an average of 56.7%,[7] ranging from 40% to 60%, for the patients receiving spinal anesthesia.[2] Prevention of hypothermia is an absolute way of preventing the postoperative chills in response to hyperthermia.
Intraoperative hypothermia can be reduced by the methods which reduce skin heat emission to the environment because of a cold OR, surgery incision evaporation, and by prevention of intravenous infusion of cold fluids. Other non-medical methods employed in various studies include warming and humidifying the air way,[4,8] warming the skin through warm covers,[9] application of patient warming system through water circulation and forced air warming system,[4,10] and infusion of warm fluids.[4,11,12]
Among the above-mentioned strategies to prevent hypothermia, warming intravenous fluids to preserve patient's body temperature in the OR can be conveniently applied, as intravenous warm fluids are available in all ORs in Iran.
One of the surgeries performed at a high rate is cesarean section (CS).[13] Exposure of these patients to anesthesia for CS leads to more temperature reduction because of the effect of anesthetics on vascular and body thermoregulation mechanisms, abdominal vast incisions, and wetting surgical covers with blood and amniotic fluid. Various studies reported controversial results in relation to application of non-medical methods in the prevention of hypothermia among the mothers undergoing CS.
Chung et al. (2012) in their study on the effect of warming of patients and infusion of pre-warmed fluids in CS on the prevention of hypothermia and postoperative chills showed that core temperature was higher in the study groups (infusion of warm fluids and forced air warming system) compared to the control group (P = 0.004). Incidence of chills was also lower in the study groups compared to the control group (P = 0.035). They concluded that forced air warming system and infusion of pre-warmed intravenous fluids prevented patients’ postoperative chills.[14]
On the contrary, Butwick et al. (2007) in a study on 30 healthy mothers who had undergone elective CS with spinal anesthesia divided into two groups of compressed air and conventional OR air conditioning showed that core temperature changes were similar in the two groups (P = 0.8), and concluded that application of forced air warming system could not prevent hypothermia among the women who had undergone CS.[15]
As patients’ support is one of the main duties of nurses, anesthesia nurses are responsible for this important issue.[16] It is essential to find preventive strategies for intraoperative hypothermia in mothers undergoing CS. Therefore, this study aimed to apply an appropriate, cost-effective, complication-free and available method of warming intravenous fluids to prevent hypothermia during CS.
MATERIALS AND METHODS
This quasi-experimental study was conducted on 62 mothers selected as candidates for elective CS under general anesthesia in Shahid Beheshti hospital in Kashan from February to September 2010. The subjects were selected through purposive sampling, and the selection was based on the inclusion criteria. From 84 mothers who assessed for eligibility, 22 mothers excluded because of not meeting inclusion criteria (n = 16) or declined to participate (n = 8). The subjects (n = 62) were randomly assigned to study (receiving pre-warmed fluid, n = 31) and control (receiving room temperature fluid, n = 31) groups. Firstly, the goal and method of study were explained to the eligible subjects and written consents were obtained from them for ethical considerations.
The inclusion criteria were: Termination of pregnancy in 37-42 weeks of gestational age; undergoing surgery with general anesthesia; having a tracheal tube; surgery length less than 1 h; not receiving corticosteroids, non-steroidal sedatives, Mg sulfate, or anti-hypertension drugs; and lack of endocrine disorders, vascular diseases, pregnancy hypertension, fever, amniotic bag rupture, polyhydramnios, and oligohydramnios. The exclusion criteria were: Having received intraoperative blood transfusion, surgery length more than 1 h, receiving medications other than conventional medication, intraoperative hypotension due to any reason leading to infusion of more fluid than calculated, or therapeutic program changes.
The candidate mothers for CS received infusion of warmed Ringer's lactate at 37°C (kept in Bon Marry serological water bath for up to 24 h before surgery) in the study group and Ringer's lactate at room temperature (25.5°C) in the control group through angiocatheter No. 16, immediately after arrival at the OR and lying on the surgical bed. OR temperature and humidity were measured and recorded using tympanic infrared thermometer (beurer medical FT55, ULM, Germany) and humidity meter (TFA Dostman/Wertheim, Germany. OR temperature and humidity were identical in both study and control groups. The required amount of fluids was calculated based on length of fasting time, maintenance fluid, amount of bleeding and urine output, and length of surgery. Anesthesia device used for both the groups was Dragger Fabious (Moislinger Allee 53-55, Germany). After they were monitored and administered primary fluids, the subjects underwent pre-operative care. After pre-oxygenation, anesthesia was induced by administering Na thiopental (5 ml/kg), and succinylcoline (1 mg/kg) was administered to facilitate intubation. Anesthesia was continued by administering isoflurane at alveolar concentration of 0.6%, a mixture of oxygen 50% and nitrous oxide 50%, and with atracurium (0.2 mg/kg) continuing the muscle relaxation. After fetal delivery, the subjects received Fentanyl (2 μg/kg). Oxytocin (20 units/L) was infused to preserve the tone of uterus. BP, pulse, and arterial O2 saturation of the subjects were measured and recorded using Saadat Monitoring device (Alborz-B model made in Iran) before and after the induction of anesthesia. Patients’ core temperature was measured and recorded using tympanic infrared thermometer at the tympana before and at 15-min intervals after the induction of anesthesia (a total of five times). After surgery, the effect of relaxants was neutralized by administering atropine (0.02 mg/kg) and neostigmine (0.04 mg/kg).
Data collection tool contained a questionnaire to record patients’ demographic information, a checklist for recording medications during anesthesia, and a checklist of the recorded intraoperative parameters. Validity of the information recording form was confirmed by content validity through references and guides of university professors. Reliability of the measurement tools was obtained by careful measurement and confirmation of their calibration and sensitivity. The devices were calibrated, while their specificity and sensitivity had been determined by the manufacturing company and the medical technologist engineer in the related hospital. The research coworkers filling the forms of data record and the subjects were blinded to the study to increase the validity of the data. After the data were collected, they were analyzed by t-test, Mann–Whitney U test, Chi-square test, and analysis of variance (ANOVA) with repeated measurements through SPSS version 16. Significance level was considered as P < 0.05.
Research limitations
Lower-volume pre-warmed serum bags (500 ml), which could be infused more quickly, were used to prevent temperature loss in the OR
Precise determination of bleeding volume is difficult in surgeries,[4] and this issue is even more difficult in CS and cases of amniotic bag rapture. To decrease this effect, the amount of bleeding was estimated by collection of amniotic fluid and by using counting and weighing surgical gauzes and long gauzes.
RESULTS
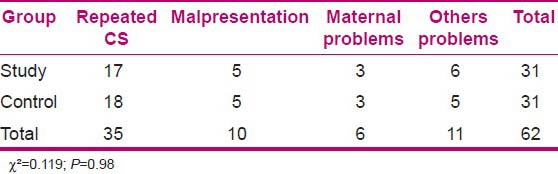
Variables of age, weight, time interval between arrival to OR and beginning of anesthesia, lengths of surgery, temperature and humidity of OR, mothers’ core temperature in the first minutes of their arrival to OR, and the amount of mothers’ fluid intake in the two groups of study and control were analyzed by statistical tests and showed no significant difference [Table 1]. Chi-square test showed no significant difference between the cause of CS in the mothers of study and control groups (P = 0.98) [Table 2].
Table 1.
Comparison of some characteristics of mothers in the study and control groups
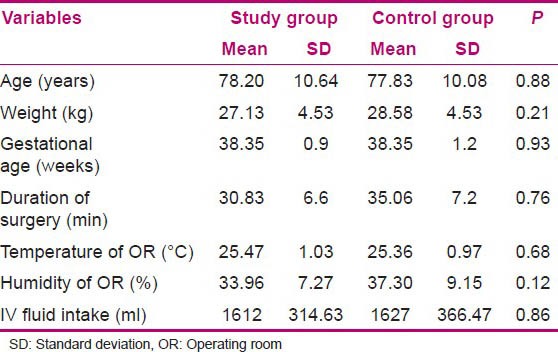
Table 2.
Comparison of causes of CS for mothers in the study and control groups
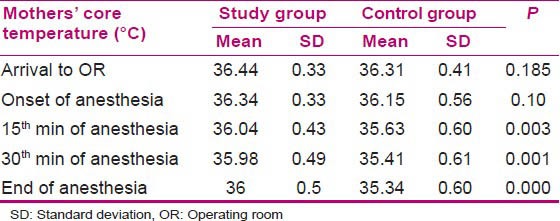
Core temperature of the mothers was measured in the first minutes of their arrival to OR until the beginning of anesthesia in the two groups at their tympana and showed no significant reduction (P = 0.10), but the measurements taken every 15 min to the end of surgery and anesthesia showed a reduction in both the groups. This reduction was more in the control group, such that at 15 min of surgery, the temperatures were 36.04 ± 0.43°C and 35.63 ± 0.6°C in the study and control groups, respectively, which showed a significant difference (P = 0.003). The core temperatures at the 30th min were 35.98 ± 0.5°C and 35.41 ± 0.6°C in the study and control groups, respectively, which showed a significant difference (P = 0.001). In addition, the core temperatures of the mothers at the end of anesthesia were 36 ± 0.5°C and 35.34 ± 0.6°C in the study and control groups, respectively, which showed a significant difference (P = 0.000) [Table 3].
Table 3.
Comparison of core temperatures of the mothers in the study and control groups during surgery
Comparison of the core temperatures of mothers in the two groups of study and control before and during anesthesia showed a significant difference by repeated-measures ANOVA (P = 0.008) [Table 4].
Table 4.
Comparison of core temperatures of the mothers in the study and control groups during surgery
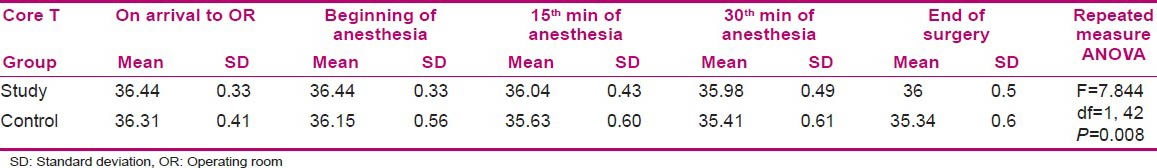
Mothers’ core temperatures showed no significant difference at the beginning of anesthesia in the two groups (P = 0.10), but the measurements taken at the 15th (P = 0.003) and 30th min of surgery (P = 0.001) and at the end of surgery (P = 0.000) showed a significant difference [Table 3]. It reveals that pre-warmed serum led to higher core temperature in the mothers of the study group compared to those of the control group [Table 4 and Figure 1].
Figure 1.
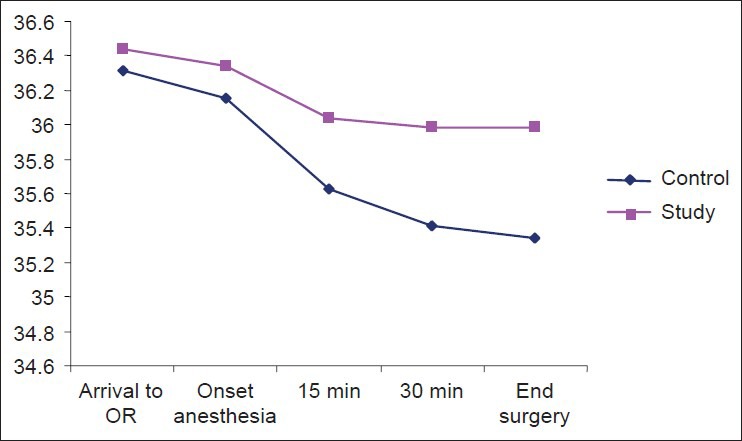
Mothers’ core temperatures during surgery
DISCUSSION
In our study, the core temperature of mothers in the study group (receiving pre-warmed fluid) was 0.5°C higher compared to those in the control group (receiving room temperature fluids). This finding is in line with that of Yokoyama et al. (2009) who studied the effect of pre-warmed intravenous fluid (38°C) among mothers as candidates for CS by spinal anesthesia on prevention of their hypothermia. In their study, the core temperature of mothers in the study group was 0.5°C higher.[17] Woolnough (2009) compared the two methods of active fluid warming (warming during surgery) and passive fluid warming (warming before surgery) in women undergoing CS and concluded that warm fluids slowed down the reduction of body temperature and increased mothers’ comfort. Finally, this study revealed the efficiency of passive fluid warming and its cost efficacy.[18]
Chung et al. (2012) studied the effect of keeping the patients warm before CS on hypothermia and postoperative chills. A total of 45 patients were randomly assigned to three groups: Receiving warm intravenous fluid (40°C), active air conditioning by compressed air, and the control group. Core temperatures of patients in the groups that received warm fluid and compressed air conditioning showed a lesser reduction than in the control group (P = 0.004). Also, the incidence of chills in these groups was less that in the control group (P = 0.035). They concluded that keeping the patient warm (with either compressed air or administering warm fluids) could prevent hypothermia and postoperative chills among the patients undergoing CS with spinal anesthesia.[14]
Goyal et al. (2011) studied the effect of warming intravenous fluids in the maintenance of core body temperature during CS under spinal anesthesia. A total of 64 patients were randomly assigned to two groups – one group received room temperature fluid (22°C) and the other warm intravenous fluid (39°C). The core temperature reduction was less among the mothers who received warm fluids compared to the other group (P < 0.01), but there was no significant difference in the incidence of chills in the two groups.[19]
Hassankhani et al. (2004) randomly assigned 60 orthopedic patients to two groups of study and control (37°C fluid and room temperature fluid, respectively). Mean differences of patients’ esophageal temperatures on the 15th, 30th, 45th, and 60th min during anesthesia were significant in both the groups. The core temperature had a descending trend from the beginning of the surgery, but this reduction and descending trend was observed more in the control group. There was also a significant difference in the mean skin temperature (P < 0.001).[12] Their findings were consistent with our findings indicating the positive effect of warm fluids’ infusion on the prevention and reduction of hypothermia during CS.
Controversial results have been reported in other studies. Butwick et al. (2007) conducted a study on 30 patients undergoing CS in two groups that received compressed air and conventional OR temperature, respectively. The results showed that the changes in core temperature were similar in the two groups (P = 0.8) and core hypothermia occurred in 8 out of 15 mothers in the compressed air group and in 10 out of 15 mothers in the conventional OP temperature group (P = 0.5). They concluded that application of compressed air could not prevent hypothermia in patients undergoing CS.[15]
Woolnough et al. (2009) showed that the incidence of chills in elective CS patients receiving warm intravenous fluids and in those receiving room temperature fluids showed no difference.[18] Other studies also showed that warming intravenous fluids could not prevent hypothermia among women undergoing elective CS.[20] These results are not consistent with ours, possibly due to low sample size of the above-mentioned study. These differences reveal the necessity of further similar studies.
Previous studies showed that even minor hypothermia might lead to serious unexpected outcomes in many of the patients, including surgery incision infection, prolonged hospitalization, increased bleeding during surgery, more demand for blood transfusion, postoperative ventricular tachycardia, postoperative chills, and prolonged recovery period, as the main complications that result from hypothermia in human beings.[21] Numerous methods have been studied to prevent hypothermia among the patients undergoing CS. The most important methods are infusion of warmed intravenous fluids and application of forced air warming system.[22]
Each liter of low-temperature infused fluid can diminish the body temperature of a person by 0.25°C.[23] Infused warmed intravenous fluids can enhance the core temperature by 0.5°C-0.7°C and reduce the risk of hypothermia.[24]
To keep the patient warm before and during surgery, the two main methods of forced air warming system and infusion of warmed intravenous fluids are used to maintain the patient's core temperature during surgery. The main advantages of body temperature preservation in normal range include low risks of incision infection, coagulation disorders, and myocardial ischemia.[25]
CONCLUSION
Based on the obtained results, it can be concluded that application of the convenient, easy, and low-cost method of warming intravenous fluids (Ringer's serum) can be helpful in prevention of hypothermia resulting from general anesthesia among mothers undergoing CS and its potential hazardous complications (prolonged hospitalization, increased bleeding during surgery, more demand for blood transfusion, postoperative ventricular tachycardia, postoperative chills, prolonged recovery period,[21] infection, and myocardial ischemia during surgery).[25] Therefore, Nurses as patients advocate should prevent the incidence of patients’ complications in OP through precisely checking the vital signs, especially temperature, and by undertaking other preventive interventions such as infusion of warmed intravenous fluids.
ACKNOWLEDGMENTS
We greatly appreciate the kind cooperation of the mothers who participated in this study, academic members of the nursing school, Vice Chancellery for Research in Hamadan University of Medical Sciences, anesthesiologists, and the staffs in Shahid Beheshti hospital in Kashan. This article has been derived from a critical care nursing MS dissertation approved by the research chancellery of Hamadan University of Medical Sciences.
Footnotes
Source of Support: Hamadan University of Medical Sciences, Hamadan. Iran
Conflict of Interest: Nil.
REFERENCES
- 1.Kiakkas P, Poulopoulou M, Papahatzi A, Souleles P. Effect of hypotermia and shivering on standard PACU monitoring of patients. AANA J. 2005;73:47–53. [PubMed] [Google Scholar]
- 2.Crowley LJ, Buggy DJ. Shivering and neurexial anesthesia. Reg Anesth Pain Med. 2008;33:241–52. doi: 10.1016/j.rapm.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Buggy DJ, Grossley AW. Thermoregulation, mild perioperative hypothermia and post anesthesia shivering. Br J Anesth. 2000;84:615–28. doi: 10.1093/bja/84.5.615. [DOI] [PubMed] [Google Scholar]
- 4.Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL. 7th ed. New York: Churchill Livingstone; 2010. Miller's Anesthesia. [Google Scholar]
- 5.Sessler DI, Kurz A. Mild perioperative hypothermia. Aneshtesiology News Special Edition. 2007:61–72. [Google Scholar]
- 6.Rostami Nezhad A, Karimi Z, Khosravi A, Chohdari AH, Ghaffarian Shirazi HR. The effect of suppository Diclofenac Na on posttperative shivering in elective ceasarean section surgery. J Armaghane-danesh, J Yasuj Univ Med Sci. 2004;9:31–7. [Google Scholar]
- 7.Bicer C, Esmaoglu A, Akin A, Boyaci A. Dexmetomidine and meperidine prevent postanesthesia shivering. Eur J Anesthesiol. 2006;23:149–53. doi: 10.1017/S0265021505002061. [DOI] [PubMed] [Google Scholar]
- 8.Hoseinkhan Z, Behzadi M. Morphine, Pethidine and Fentanyl in postoperative shivering control: A randomized clinical trial. Tehran Univ Med J. 2007;64:57–63. [Google Scholar]
- 9.Movassaghi Gh R, Palideh H. Comparison between antishivering effects of Meperidine and Methadon. Razi J Med Sci. 2002;9:107–10. [Google Scholar]
- 10.Taguchi A, Ratnaraj J, Kabon B, Sharma N, Lenhardt R, Sessler DI, et al. Effects of a circulating-water garment and forced-air warming on body heat content and core temperature. Anesthesiol Clin. 2004;100:1058–64. doi: 10.1097/00000542-200405000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behattacharya PK, Jain RK, Agarwal RC. Post anesthesia shivering (PAS): A review. Indian J Anesth. 2003;47:88–93. [Google Scholar]
- 12.Hasankhani H, Mohhammadi E, Naghizade MM, Moazzami F, Mokhtari M. The effect of warming intravenouse fluid on perioperative hemodynamic status, postoperative shivering and recovery in orthopedic surgery. Shiraz E-Med J. 2004:5. [Google Scholar]
- 13.Mohamadpourasl A, Asgharian P, Rostami F, Azizi A, Akbari G. Investigating the choice of delivery method type and it's related factors in pregnant women in Maragheh. Knowl Health Q. 2009;4:33–9. [Google Scholar]
- 14.Chung SH, Lee BS, Yang HJ, Kweon KS, Kim HH, Song J, et al. Korean J Anesthesiol. 2012;62:454–60. doi: 10.4097/kjae.2012.62.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butwick AJ, Lipman SS, Carvalho B. Intraoperative forced air-warming during cesarean delivery under spinal anesthesia does not prevent maternal hypothermia. Anesth Analg. 2007;105:1413–9. doi: 10.1213/01.ane.0000286167.96410.27. [DOI] [PubMed] [Google Scholar]
- 16.Andrzejowski J, Hoyle J, Eapen G, Turnbull D. Effect of prewarming on post- induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anesthesia. Br J Anesth. 2008;101:627–31. doi: 10.1093/bja/aen272. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama K, Suzuki M, Shimada Y, Matsushima T, Bito H, Sakamoto A. Effect of adminstration of pre-warmed intravanous fluids on the frequency of hypothermia following spinal anestesia for Cesarean delivery. J Clin Anesth. 2009;21:242–8. doi: 10.1016/j.jclinane.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Woolnough M, Allam J, Hemingway C, Cox M, Yentis SM. Intra operative fluid warming in elective caesarean section: A blinded randomised controlled trial. Int J Obstet Anesth. 2009;18:346–51. doi: 10.1016/j.ijoa.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Goyal P, Kundra S, Sharma S, Grewal A, Kaul TK, Singh MR. Efficacy of intravenous fluid warming for maintenance of core temperature during lower segment cesarean section under spinal anesthesia. J Obstet Anaesth Crit Care. 2011;1:73–7. [Google Scholar]
- 20.McCarroll SM, Cartwright P, Weeks SK, Donati F. Warming intravenous fluids and the incidence of shivering during caesarian sections under epidural anesthesia. Can Anesth Soc J. 1986;33:S72–3. [Google Scholar]
- 21.Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95:531–43. doi: 10.1097/00000542-200108000-00040. [DOI] [PubMed] [Google Scholar]
- 22.Kurz A, Kurz M, Poeschl G, Faryniak B, Redl G, Hackl W. Forced-air warming maintains intraoperative normothermia better than circulating-water mattresses. Anesth Analg. 1993;77:89–95. doi: 10.1213/00000539-199307000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Sessler DI. Consequences and treatment of perioperative hypothermia. Anesth Clin North Am. 1994;12:425–56. [Google Scholar]
- 24.Smith CE, Fisgus JR, Kan M, Lengen SK, Myles C, Jacobs D, et al. Efficacy of IV fluid warming in patients undergoing cesarean section with regional anesthesia. Am J Anesthesiol. 2000;27:84–8. [Google Scholar]
- 25.Carpenter L, Baysinger CL. Maintaining perioperative normothermia in the patient undergoing cesarean delivery. Obstet Gynecol Surv. 2012;67:436–46. doi: 10.1097/OGX.0b013e3182605ccd. [DOI] [PubMed] [Google Scholar]


