Abstract
Imaging forms an integral component for diagnosis of dental and in specific periodontal diseases. To date, intra-oral radiographic techniques are the main non-invasive diagnostic aids for the detection and assessment of internal changes in mineralized periodontal tissues like alveolar bone. These analog radiographic techniques suffer from inherent limitations like: Two dimensional projection, magnification, distortion, superimposition and misrepresentation of anatomic structures. The evolution of novel imaging modalities, namely cone beam computed tomography, tuned aperture CT empowered dental researchers to visualize the periodontium three dimensionally. This improves interpretation of structural and biophysical changes, ensures densitometric assessments of dentoalveolar structures including variations in alveolar bone density, and peri-implant bone healing more precisely. This detailed review, highlights current leading edge concepts, envisions a wide range of imaging modalities which pave the way for better understanding and early intervention of periodontal diseases.
Keywords: Cross-sectional imaging, digital imaging, implant imaging
INTRODUCTION
The emerging concept in the etiopathogenesis of periodontal disease precludes clinicians to examine periodontal structures more accurately in all dimensions. Analog two dimensional (2D) imaging used as an adjunct to periodontal manifestations could not contribute to this completely. Novel imaging modalities, available to date revealed the periodontium, three dimensionally thereby enabling periodontists for early diagnosis and better intervention. However, the dilemma of choosing the appropriate diagnostic aid is yet to be revealed. In order to select a specific radiographic view, clinician must first identify the region of interest (ROI). Some periodontal ROI include the amount of bone loss, mandibular canal to implant distance etc., At all times, clinician should be guided by the principle of using the least invasive technique with the lowest risk of radiation exposure.[1]
The traditional principle of ALARA, “ As Low As Reasonably Achievable” now modified to the acronym ALARP “As Low As Reasonably Practicable” helps to minimize radiation exposure. Based on this principle there are four major ways to reduce radiation exposure:
Shielding: With the use of proper barriers that block or minimize ionizing radiation
Time: Minimize the time spent in radiation fields
Distance: Maintaining adequate distance between radioactive sources and subjects
Amount: Minimizing the usage of radioactive material.[2]
CONVENTIONAL 2D IMAGING TECHNIQUES
Traditional analog imaging modalities are 2D systems that use image receptors like radiographic films or intensifying screens. These include periapical views, panoramic, occlusal and cephalometric radiography. A digital 2D image is described by an image matrix that has individual picture elements called pixels. Each pixel has discrete digital value that describes image intensity at a particular point.[3]
Periapical views, as illustrated in Table 1 as the term implies are routinely used to visualize the root apex. They also depict severe periodontal bone loss. However, periapical views distort the location of bone height along the tooth root do not reveal the osseous crest precisely, thereby cannot detect moderate periodontal bone loss. In contrary, vertical bitewings can be used to assess bone height in patients with moderate to severe bone loss.[4,5,6] Panoramic radiography is a commonly used imaging modality, by most periodontists. It provides an overall view of the periodontium, thereby minimizes the radiation exposure. However, its main drawback is image distortion that limits its usefulness.[7]
Table 1.
Analog two dimensional imaging techniques

ADVANCED 2D IMAGING TECHNIQUES
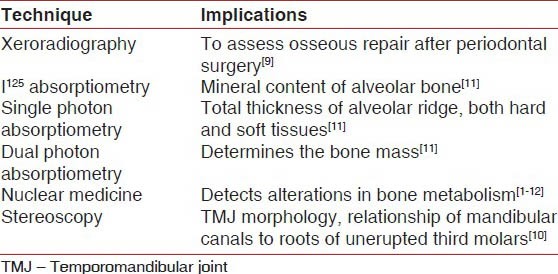
The limitations of traditional 2D imaging techniques could be overcome with the evolution of advanced 2D imaging techniques as illustrated in Table 2 like: Microradiography, xeroradiography, stereoscopy, scanography, I125 absorptiometry and nuclear medicine.[8,9,10]
Table 2.
Novel two dimensional imaging techniques
Microradiography is primarily indicated for the quantitative assessment of structural features in mineralized tissues. It is likely to produce a true radiographic image across the total thickness of the specimen. The two types of microradiography include: Conventional contact microradiography and parallel beam microradiography which analyses the degree of mineralization of dental tissues like dentinal tubules. However, their inherent limitations like long exposure time and need for high intensity X-ray sources precludes its use.[8]
Xeroradiography is a promising imaging technique first introduced by Carbon in 1938. In 1963, Stronezak first used it in dentistry. It accomplishes the property of edge enhancement by which small structures and areas of minimal density differences are better visualized. So, it is an excellent aid in evaluating initial osseous changes, assessment of osseous repair after periodontal therapy, and to clearly visualize the crestal heights.[9]
Stereoscopy is a technique introduced by MacKenzie Davidson in 1988. It is currently used for examining temporomandibular joint morphology, evaluation of bony pockets, determination of root configuration needing endodontic treatment, assessment of relationship of mandibular canals to roots of unerupted third molars, and to determine the bone contour during dental implants placement. Despite its wide applications, stereoscopy is overlooked due to the need for long exposure time.[10]
Scanography (soredex scanora) is a commercially available X-ray unit capable of performing both rotational and linear scanography. It is capable of both posterioanterior and lateral linear scanning of the maxillofacial complex. The rotational scanography technique was found to be effective in the assessment of periodontal disease and in detection of periapical lesions.[10]
I125 absorptiometry was introduced into dentistry by Hausmann et al. in 1962. In 1982 Ortman used this method to measure the mineral content of alveolar bone. It is the most sensitive technique for analyzing periodontal bone changes and can be used as a standard for comparing the sensitivity of other techniques. Other variants of this technique include single photon absorptiometry that measures the total thickness of the alveolar ridge (hard and soft tissue) and dual photon absorptiometry that determines the bone mass.[11]
Nuclear medicine colloquially termed “bone scanning” is used to study alterations in bone metabolism using radio labeled bone seeking radiopharmaceutical like 99 m-technitium.[12] Nuclear medicine depicts changes that indicate bony metastases, primary bone tumors, metabolic bone diseases, and stress fractures.[13,14,15] Nuclear medicine is useful in dentistry for the early detection of periapical pathologies and growth disorders.[16,17,18,19] Today nuclear medicine has three categories of imaging devices: Those used for planar nuclear imaging, single-photon emission computed tomography (SPECT), and for positron emission tomography (PET).
Planar nuclear imaging
This technique efficiently images large anatomical areas from a wide variety of directions. It is used to view areas of the alveolar process in the laboratory and clinical studies of periodontitis.[20,21,22,23,24] SPECT is an enhancement of planar imaging with improved image resolution. PET: Clinical applications of PET scanning include cardiac imaging and tumor diagnosis.[12]
The main difficulty encountered during radiographic interpretation is demarcating the pathology from the normal anatomic background. The critical component of interpretation is the elimination of irrelevant structures (noise). This was made possible with the emergence of subtraction radiography.
Subtraction radiography is a technique introduced by Grondahl in 1920. A subtraction image, subtracts the background features, minimizes background complexity, and thereby amplifies even minor density differences by superimposing serial images obtained at consecutive intervals. Thus, it is a sensitive and accurate evaluation method. Color coding the subtraction images further improves detection of bone loss or gain, depicted as shades of red and green respectively. This method can also be used to monitor peri-implant bone stability.[25] The application of subtraction radiography in dentistry was facilitated by the development of computer allowing conventional radiographs to be digitalized and subtracted.[26]
Advantages of digital subtraction radiography over conventional radiography
Digital subtraction radiography (DSR) detects early alveolar bone changes when only 1-5% per unit volume of bone is lost. Significant differences in crestal bone height of 0.78 mm and minor defects (0.49 mm) depth can also be detected in cortical bone. This has proved to be advantageous over conventional radiography, which requires a change in mineralization of 30-60%. In addition, conventional radiography does not detect lesions in cancellous bone. In implantology, DSR is useful in assessment of bone at all phases of therapy.[12,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] DSR has also been used to evaluate the progression, arrest, or regression of carious lesions. However, the extent of ill-defined carious radiolucencies, could not be evaluated with conventional radiography.[42,43,44,45] DSR has certain limitations like need for accurate alignment during sequential radiographic exposures.[27]
Limitations of DSR
For a successful DSR, reproducible exposure geometry, and identical contrast and density of the serial radiographs, are essential prerequisites. This technique is very sensitive to any physical noise occurring between radiographs[46,47] and even minor changes leads to large errors in the results.[48] Differences in image contrast and intensity between the base line and the follow-up images can hamper the detection task and make the quantitative measurements unreliable.[49] Despite all these efforts, there is no definite and accurate simple solution to control projection geometry and correct the discrepancies. So this technique is not frequently used in the dental profession.[50] Recently, a new image subtraction method called “diagnostic subtraction radiography” has been introduced. This includes the combination of positioning device during film exposure with specialized software designed for digital image subtraction using conventional personal computers in dental offices. This image analysis software system applies an algorithm that corrects for the effects of angular alignment discrepancies and provides some degree of flexibility in the imaging procedure.[51]
Computer assisted densitometric image analysis (CADIA) as introduced by Brägger et al. (1992) is one form of subtraction radiography. CADIA is an excellent method for the assessment of longitudinal changes in bone density in therapeutic trials. CADIA is based on the comparison of two serial images that are acquired with standardized projection geometry and equalized for the density differences in the images. It is more sophisticated version of subtraction radiography that allows the investigator to quantify changes by comparing the radiographic density in a predetermined ROI between the baseline and follow-up radiographs. The area of change and the depth of lesion in a buccolingual plane can be measured. In vitro; studies demonstrated the ability of CADIA to detect changes in bone volume to the extent of detecting the crestal density loss before the height of the crest is reduced.[52] CADIA can also be used for assessing the outcome of guided tissue regeneration procedures.[53,54]
CROSS-SECTIONAL IMAGING TECHNIQUES
A next leap of technology will be in building a third dimension by assembling many layers of information. This pursuit for obtaining cross-sectional information in all planes of interest has focused light towards novel cross-sectional imaging modalities as illustrated in Table 3 like CT and its other variants namely cone beam computed tomography (CBCT), quantitative computed tomography (QCT), tuned aperture computed tomography (TACT), micro focus CT.
Table 3.
Crossectional imaging techniques
In 1972, Fuhrmann announced the invention of a revolutionary imaging technique, referred as “computerized axial transverse scanning.” CT is a specialized radiographic technique that portrays cross sectional image of an object without superimposition of structures in the plane parallel to the X-ray beam. It is claimed to be 100 times more sensitive than conventional X-ray systems. It demonstrates differences between various soft tissues. The ability of CT system to distinguish between objects of similar density (contrast resolution) and capturing the data in digital form for subsequent analysis and reformatting precludes its use as an advanced diagnostic aid in periodontitis. Studies[55] have shown that CT assessment of alveolar bone height and intrabony pockets is precise. However, the increased radiation exposure limits its use in periodontics.[56]
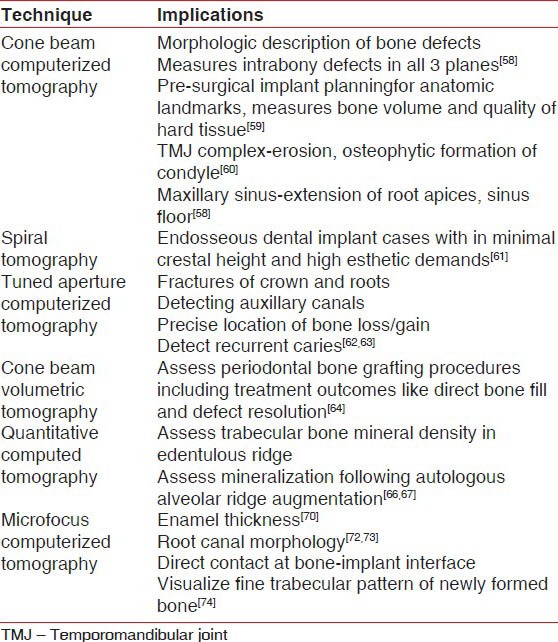
CBCT is an alternative imaging technology introduced for acquiring three dimensional (3D) data for diagnostic tasks such as implant treatment planning. Its inherent advantages being less expensive components and lower patient exposure than CT. Currently, five CBCT systems are used namely the Newtom 3G (quantitative radiology), i-CAT (Imaging Sciences International), CB Mercuray (Hitachi Medical Corporation), 3D Accuitomo (J. Morita Manufacturing), and the ILUMA (IMTEC Imaging). The Newtom 9000 was the first CBCT device introduced.[57] It is as accurate as direct measurements with a periodontal probe and as reliable as radiographs for interproximal areas, including buccal and lingual defects. Vandenberghe et al. compared periodontal bone architecture using 2D charged coupled device and 3D full volume CBCT based imaging modalities. They concluded that CBCT images provide more bone details and have a greater potential in measuring intrabony defects in all three planes.[58]
In implantology CBCT aids in pre-surgical implant planning by measuring bone volume and quality precisely. It also guides in locating the anatomic obstacles to be avoided during implant placement. as illustrated in Table 4.[59] In addition, a clear view of TMJ complex, demonstrating erosion, osteophytic formation of the condyle or both is obtained without interference from surrounding dense temporal bone.[60] At spacial resolution of 300 μm and less, CBCT images the position of root apices of maxillary teeth extending to the nasal cavity and maxillary sinus, as well as cortical border erosion of these structures resulting from apical rarefying osteitis.[58]
Table 4.
Imaging techniques for the implant patient
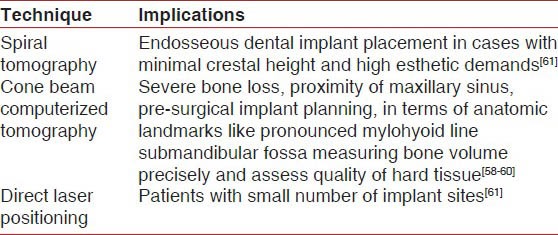
The thirst for accuracy has brought to light other variables of cross-sectional imaging. Spiral tomography is used as a valuable adjunct in the treatment planning of endosseous dental implants as illustrated in Table 4. In specific, it is useful in cases requiring optimal implant angulation due to minimal crestal width and high esthetic demands. Spiral tomography is diagnostically superior to CT in terms of reducing artefacts, blurred images and decreasing the radiation exposure to vital structures of head and neck by 47-71% compared to CT.[61]
One promising novel advance in 3D imaging, developed by Dr. Richard Webber is TACT that produces a holographic image. The “tune aperture” refers to the varying viewing angles at which 2D base images are recorded. It can be used to produce 3D views of teeth, pathology, and other areas of interest. TACT aids in diagnosing fractures of crowns and roots detects auxiliary canals. In addition, it detects the location of periodontal bone loss or gain, TMJ bony changes, and alveolar contours.[62] Nair et al. proposed that TACT is extremely effective in detection of recurrent caries.[63]
Another variant of CT is cone beam volumetric tomography (CBVT), in which 3D images are obtained. This obviates the necessity for surgical re-entry to assess the outcome of periodontal bone grafting. It produces images that have high resolution and accuracy for measuring regenerative therapy outcomes like direct bone fill and defect resolution.[64]
QCT is relatively sensitive technique that has intermediate precision and valuable accuracy of 5-20%. QCT offers precise 3D anatomic localization of bone density measurements in Hounsfield units.[65] This permits easy differentiation between cortical and cancellous bone and helps to avoid extra-osseous, but potentially confounding structures such as walls of mineralized vessels. QCT is an established method for measurement of the trabecular bone mineral density in the alveolar process of edentulous regions in post-menopausal women. In addition, it assesses the extent of mineralization following autologous alveolar ridge augmentation.[66,67]
Micro focus CT is a new type of imaging, with a spacial resolution of <10 μm (Nittetsu Elex, Kanagawa) to study trabecular bone structure[68,69] enamel thickness,[70] calcification of human teeth[71] as well as dental root canal morphology.[72,73] It enables identification of bone resorption, bone to implant interface, and visualization of fine trabecular pattern of newly formed bone.[74] Advantages of micro focus CT are ease of reconstruction of 3D images within a short time, minimizing the risk of artefacts. Kochi et al. in 2010[75] compared the efficacy of micro focus CT with histomorphometry in assessing bone augmentation. Micro focus CT analysis enables highly quantitative and qualitative measurement of bone augmentation. Thus, it is a dynamic non-invasive method for measuring bone regeneration.
IMAGING TECHNIQUES FOR IMPLANT PLACEMENT
Proper diagnosis and treatment planning are critical steps in implant therapy. The objective is to place implants in alveolar sites that are favorable for achieving osseointegration. An anatomic variation in jaw morphology makes imaging an integral component of implant planning.[76] The choice of implant imaging should be based on clinical demands like, the need for portrayal of anatomic or topographic conditions (dependent to a great extent on the experience of the surgeon), ease of image production information expected from the image, biologic risk for the patient and financial considerations.[77]
In patients in whom determination of the bone width is possible by clinical findings, these imaging techniques may remain the only radiographs necessary for treatment planning. With conventional tomography, it is possible to obtain cross sectional images that can be used to determine bone width. With both conventional and CT, it is possible to obtain information about the width, height, and inclination of the alveolar process anatomic and topographic structures and to some extent, the trabecular architecture.
Generally, the radiographic evaluation of implant patients should be carried out according to the following 3 axioms:
General considerations: Prior to implant placement, it seems appropriate to consider panoramic radiography as a standard radiographic examination for referred patients, It provides an accurate means of determining implant length in both the maxilla and mandible with low biologic risk. Periapical radiographs may be indicated in regions not sharply depicted in the panoramic radiograph
Applications for cross-sectional imaging: In the maxilla cross-sectional imaging should be advised: (1) In patients with severe bone loss in the alveolar process, together with signs of enlargement of the incisive canal in the periapical radiograph, for single implants in the incisor region or multiple implants in the incisor and canine region, (2) in sites with severe bone loss and close proximity of the maxillary sinus and, (3) in patients planning for fixed prosthesis in the completely edentulous maxilla is planned. In the mandible, cross-sectional imaging should be used when a fixed prosthesis is planned
Optimal applications for cross sectional imaging in both the maxilla and mandible: Conventional or CT is advised in conditions where unfavorable soft tissues hinder assessment of bone volume. In the mandible, it can be employed for patients with a pronounced mylohyoid line and submandibular fossa or when interforaminal implantation is planned for atrophy corresponding to Cawood and Howell level V/VI. From a radiobiologist point of view, conventional tomography should be preferred whenever possible for single tooth gaps and extended edentulous spaces up to a quadrant.[78,79,80,81,82,83,84]
At present, conventional cross-sectional tomography is recommended by the American Academy of Oral and Maxillofacial Radiology for most patients receiving implants. Tomography is recommended for the evaluation of individual implant sites, especially located in both maxilla and the mandible. Conversely, CT can be applied to the evaluation of multiple adjacent implant sites.[85]
The direct laser positioning system (DLP system) introduced by Naitoh et al.[86] was developed using a panoramic X-ray machine with a linear tomographic function. The measurement accuracy is improved compared to other tomographic machines and the reformatted CT. The DLP system allows the adjustment of the angle of the objective plane to the angulation of each designed implant site by tilting the occlusal plate with the accessory tool. The DLP system is considered useful, especially for patients with a small number of implant sites (Tyndall and Brooks 2000)[87] as illustrated in Table 4.
The recently developed limited cone beam X-ray CT (LCBCT) by Arai et al. in (1999)[88] makes use of cone beam and a 2D X-ray sensor. It is reported to be useful in pre-operative treatment planning for dental implant placement (Ito et al. 2001). The integral absorbed dose of radiation using LCBCT was approximately 1/15th that of spiral CT (SCT). It is a reliable tool for pre-operative evaluation before dental implant surgery because of its high resolution and decreased radiation. In the mandible, the inter foramina region is chosen because of its favorable anatomic conditions and the rarity of life threatening complications. LCBCT can be used to measure the distance between two points in mandibular bone more accurately than SCT.[89]
Thus, imaging the potential implant site to obtain a 3D view guides the clinician in all stages of implant therapy from diagnosis, placement to success. The choice of the appropriate radiographic aid is thus one of the prime steps in implant planning and therapy.
SUMMARY
Recent advances in imaging sciences have enabled periodontists to visualize structural and biophysical changes in the periodontium more accurately. However the choice of the appropriate modality pertinent to the case still remains a dilemma. The well-known 2D approaches though cost effective have limitations like image distortion and decreased resolution. The paradigm shift from 2D to 3D imaging techniques like CBCT, QCT, CBVT, and microfocus CT enabled to overcome these limitations. These novel advances have periodontal implications both for diagnosis and for evaluation of therapeutic outcomes. To summarize from a diagnostic point of view CBCT gives a mirror image of intrabony defects in all three planes. TACT and DSR precisely locates bone loss or gain and aids in pre-surgical implant planning. QCT quantifies trabecular bone mineral density in edentulous ridge. CADIA detects changes in the bone volume. This era of evidence based dentistry precludes the need to assess therapeutic outcomes. Regenerative therapy and implantology are the two fields of periodontology that need attention in this aspect. CBVT assess the outcome of bone grafting procedures like bone fill. QCT detects mineralization following autologous alveolar ridge augmentation.
CONCLUSION
This review attempts to summarize novel imaging advances in terms of their principles, periodontal implications, simplifying the choice of appropriate radiographic aid for early diagnosis and better periodontal interventions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Newmann TG, Kornmann KS, Newmann MG, Jeffcoat M. Qiuntessence Publication; 1992. Imaging techniques for the periodontium. Advances in Periodontics; pp. 42–55. [Google Scholar]
- 2.London: Health and Safety Executive; Health and Safety at Work etc., Act 1974: Risk management: ALARP at a glance. [Retrieved on 13 Feb 2011]. “‘ALARP’ is short for ‘as low as reasonably practicable’”. [Google Scholar]
- 3.Hausmann E. Radiographic and digital imaging in periodontal practice. J Periodontol. 2000;71:497–503. doi: 10.1902/jop.2000.71.3.497. [DOI] [PubMed] [Google Scholar]
- 4.Hausmann E. A contemporary perspective on techniques for the clinical assessment of alveolar bone. J Periodontol. 1990;61:149–56. doi: 10.1902/jop.1990.61.3.149. [DOI] [PubMed] [Google Scholar]
- 5.Hausmann E, Allen K, Christersson L, Genco RJ. Effect of x-ray beam vertical angulation on radiographic alveolar crest level measurement. J Periodontal Res. 1989;24:8–19. doi: 10.1111/j.1600-0765.1989.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 6.Jeffcoat MK, Wang IC, Reddy MS. Radiographic diagnosis in periodontics. Periodontol 2000. 1995;7:54–68. doi: 10.1111/j.1600-0757.1995.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 7.Eley BM, Cox SW. Advances in periodontal diagnosis. 1. Traditional clinical methods of diagnosis. Br Dent J. 1998;184:12–6. doi: 10.1038/sj.bdj.4809529. [DOI] [PubMed] [Google Scholar]
- 8.Takagi S, Chow LC, Brown WE, Dobbyn RC, Kuriyama M. Application of an x-ray image magnifier to the microradiography of dental specimens. J Dent Res. 1985;64:866–9. doi: 10.1177/00220345850640060101. [DOI] [PubMed] [Google Scholar]
- 9.Lopez J., Jr Xeroradiography in dentistry. J Am Dent Assoc. 1976;92:106–10. doi: 10.14219/jada.archive.1976.0300. [DOI] [PubMed] [Google Scholar]
- 10.White SC, Pharaoh MJ. 5th ed. St. Louis, Missouri: Mosby Publication; 2004. Oral Radiology Principles and Interpretation; pp. 248–50. [Google Scholar]
- 11.Hausmann E, Ortman LF, McHenry K, Fallon J. Relationship between alveolar bone measured by 125I absorptiometry with analysis of standardized radiographs: 1. Magiscan. J Periodontol. 1982;53:307–10. doi: 10.1902/jop.1982.53.5.307. [DOI] [PubMed] [Google Scholar]
- 12.Matteson SR, Deahl ST, Alder ME, Nummikoski PV. Advanced imaging methods. Crit Rev Oral Biol Med. 1996;7:346–95. doi: 10.1177/10454411960070040401. [DOI] [PubMed] [Google Scholar]
- 13.Bell EG. Nuclear medicine and skeletal disease. Hosp Pract. 1972;8:49–60. [Google Scholar]
- 14.Jacobsson S, Hollender L, Lindberg S, Larsson A. Chronic sclerosing osteomyelitis of the mandible. Scintigraphic and radiographic findings. Oral Surg Oral Med Oral Pathol. 1978;45:167–74. doi: 10.1016/0030-4220(78)90080-4. [DOI] [PubMed] [Google Scholar]
- 15.Mettler FA, Guiberteau MJ. New York: Grune and Stratton; 1983. Essentials of Nuclear Medicine Imaging; pp. 214–47. [Google Scholar]
- 16.Garcia DA, Jansons D, Kapur KK. Bone-imaging and semiconductor probe measurements of technetium-99m-polyphosphate in the detection of periapical pathology in the dog. Arch Oral Biol. 1976;21:167–74. doi: 10.1016/0003-9969(76)90126-6. [DOI] [PubMed] [Google Scholar]
- 17.Donoff RB, Jeffcoat MK, Kaplan ML. Use of a miniaturized detector in facial bone scanning. Int J Oral Surg. 1978;7:482–7. doi: 10.1016/s0300-9785(78)80041-6. [DOI] [PubMed] [Google Scholar]
- 18.Kaban LB, Cisneros GJ, Heyman S, Treves S. Assessment of mandibular growth by skeletal scintigraphy. J Oral Maxillofac Surg. 1982;40:18–22. doi: 10.1016/s0278-2391(82)80010-4. [DOI] [PubMed] [Google Scholar]
- 19.Cisneros GJ, Jeffcoat MK, Kaban LB. Bone-seeking radiopharmaceutical uptake as an indicator of mandibular growth in rats. Angle Orthod. 1985;55:336–44. doi: 10.1043/0003-3219(1985)055<0336:BRU>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Garcia DA, Entine G, Tow DE. Detection of small bone abscesses with a high-resolution cadmium telluride probe. J Nucl Med. 1974;15:892–5. [PubMed] [Google Scholar]
- 21.Garcia DA, Sullivan TM, Jungman C, O’Neil DM. An experimental evaluation of endodontic procedures and filling materials. Oral Surg Oral Med Oral Pathol. 1981;52:641–7. doi: 10.1016/0030-4220(81)90084-0. [DOI] [PubMed] [Google Scholar]
- 22.Jeffcoat MK, Kaplan ML, Weinstein M, Goldhaber P. Semiconductor probe measurements in beagle pups during deciduous tooth development. J Dent Res. 1978;57:743–7. doi: 10.1177/00220345780570051701. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan ML, Garcia DA, Goldhaber P, Davis MA, Adelstein SJ. Uptake of 99mTe-Sn-EHDP in beagles with advanced periodontal disease. Calcif Tissue Res. 1975;19:91–8. doi: 10.1007/BF02563994. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan ML, Davis MA, Aschaffenburg PH, Adelstein SJ, Goldhaber P. Clinical, radiographic and scintigraphic findings in experimental periodontal disease in dogs. Arch Oral Biol. 1978;23:273–8. doi: 10.1016/0003-9969(78)90018-3. [DOI] [PubMed] [Google Scholar]
- 25.Gröndahl HG, Gröndahl K. Subtraction radiography for the diagnosis of periodontal bone lesions. Oral Surg Oral Med Oral Pathol. 1983;55:208–13. doi: 10.1016/0030-4220(83)90180-9. [DOI] [PubMed] [Google Scholar]
- 26.Gröndahl HG, Gröndahl K, Webber RL. A digital subtraction technique for dental radiography. Oral Surg Oral Med Oral Pathol. 1983;55:96–102. doi: 10.1016/0030-4220(83)90314-6. [DOI] [PubMed] [Google Scholar]
- 27.Fidler A, Likar B, Pernus F, Skaleric U. Influence of developer exhaustion on accuracy of quantitative digital subtraction radiography: An in vitro study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:233–9. doi: 10.1067/moe.2000.107362. [DOI] [PubMed] [Google Scholar]
- 28.Southard KA, Southard TE. Detection of simulated osteoporosis in human anterior maxillary alveolar bone with digital subtraction. Oral Surg Oral Med Oral Pathol. 1994;78:655–61. doi: 10.1016/0030-4220(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 29.Southard KA, Southard TE. Detection of simulated osteoporosis in dog alveolar bone with the use of digital subtraction. Oral Surg Oral Med Oral Pathol. 1994;77:412–8. doi: 10.1016/0030-4220(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 30.Delano EO, Ludlow JB, Ørstavik D, Tyndall D, Trope M. Comparison between PAI and quantitative digital radiographic assessment of apical healing after endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:108–15. doi: 10.1067/moe.2001.115466. [DOI] [PubMed] [Google Scholar]
- 31.Lindh C, Petersson A, Klinge B, Nilsson M. Trabecular bone volume and bone mineral density in the mandible. Dentomaxillofac Radiol. 1997;26:101–6. doi: 10.1038/sj.dmfr.4600217. [DOI] [PubMed] [Google Scholar]
- 32.Ramadan AB, Mitchell DF. A roentgenographic study of experimental bone destruction. Oral Surg Oral Med Oral Pathol. 1962;15:934–43. doi: 10.1016/0030-4220(62)90087-7. [DOI] [PubMed] [Google Scholar]
- 33.Bender IB, Seltzer S. Roentgeographic and direct observation of experimental lesions inbone. J Am Dent Assoc. 1961;62:152–60. [Google Scholar]
- 34.Wengraf A. Radiologically occult bone cavities: An experimental study and study and review. Br Dent J. 1964;117:532–6. [Google Scholar]
- 35.Nicopoulou-Karayianni K, Bragger U, Patrikiou A, Stassinakis A, Lang NP. Image processing for enhanced observer agreement in the evaluation of periapical bone changes. Int Endod J. 2002;35:615–22. doi: 10.1046/j.1365-2591.2002.00526.x. [DOI] [PubMed] [Google Scholar]
- 36.Gröndahl K, Kullendorff B, Strid KG, Gröndahl HG, Henrikson CO. Detectability of artificial marginal bone lesions as a function of lesion depth. A comparison between subtraction radiography and conventional radiographic technique. J Clin Periodontol. 1988;15:156–62. doi: 10.1111/j.1600-051x.1988.tb01562.x. [DOI] [PubMed] [Google Scholar]
- 37.Ortman LF, Dunford R, McHenry K, Hausmann E. Subtraction radiography and computer assisted densitometric analyses of standardized radiographs. A comparison study with I125 absorptiometry. J Periodontal Res. 1985;20:644–51. doi: 10.1111/j.1600-0765.1985.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 38.Sanz M, Newman MG. Advanced diagnostic techniques. In: Newman MG, Takei HH, Carranza FA, editors. Clinical Periodontology. 9th ed. Philadelphia: W.B. Saunders; 2002. pp. 487–502. [Google Scholar]
- 39.Christgau M, Hiller KA, Schmalz G, Kolbeck C, Wenzel A. Quantitative digital subtraction radiography for the determination of small changes in bone thickness: An in vitro study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:462–72. doi: 10.1016/s1079-2104(98)90076-2. [DOI] [PubMed] [Google Scholar]
- 40.Reddy MS, Wang IC. Radiographic determinants of implant performance. Adv Dent Res. 1999;13:136–45. doi: 10.1177/08959374990130010301. [DOI] [PubMed] [Google Scholar]
- 41.Nummikoski PV, Steffensen B, Hamilton K, Dove SB. Clinical validation of a new subtraction radiography technique for periodontal bone loss detection. J Periodontol. 2000;71:598–605. doi: 10.1902/jop.2000.71.4.598. [DOI] [PubMed] [Google Scholar]
- 42.Wenzel A, Anthonisen PN, Juul MB. Reproducibility in the assessment of caries lesion behaviour: A comparison between conventional film and subtraction radiography. Caries Res. 2000;34:214–8. doi: 10.1159/000016593. [DOI] [PubMed] [Google Scholar]
- 43.Eberhard J, Hartman B, Lenhard M, Mayer T, Kocher T, Eickholz P. Digital subtraction radiography for monitoring dental demineralization. An in vitro study. Caries Res. 2000;34:219–24. doi: 10.1159/000016594. [DOI] [PubMed] [Google Scholar]
- 44.Schmidlin PR, Tepper SA, Scriba H, Lutz F. In vitro assessment of incipient approximal carious lesions using computer-assisted densitometric image analysis. J Dent. 2002;30:305–11. doi: 10.1016/s0300-5712(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 45.Analoui M, Stookey GK. Direct digital radiography for caries detection and analysis. Monogr Oral Sci. 2000;17:1–19. doi: 10.1159/000061634. [DOI] [PubMed] [Google Scholar]
- 46.Benn DK. Limitations of the digital image subtraction technique in assessing alveolar bone crest changes due to misalignment errors during image capture. Dentomaxillofac Radiol. 1990;19:97–104. doi: 10.1259/dmfr.19.3.2088789. [DOI] [PubMed] [Google Scholar]
- 47.Wenzel A, Sewerin I. Sources of noise in digital subtraction radiography. Oral Surg Oral Med Oral Pathol. 1991;71:503–8. doi: 10.1016/0030-4220(91)90441-e. [DOI] [PubMed] [Google Scholar]
- 48.Kornman KS. Nature of periodontal diseases: Assessment and diagnosis. J Periodontal Res. 1987;22:192–204. doi: 10.1111/j.1600-0765.1987.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 49.White SC, Pharoah MJ. 5th ed. St. Louis: Mosby; 2004. Oral Radiology, Principles and Interpretation; pp. 225–45. [Google Scholar]
- 50.Hekmatian E, Sharif S, Khodaian N. Isfahan, Iran: Abstract of the articles in the 5th Congress of Dental Students’ Researches; 2001. Digital Subtraction Radiography in Dentistry. [Google Scholar]
- 51.Gröndahl K, Gröndahl HG, Webber RL. Influence of variations in projection geometry on the detectability of periodontal bone lesions. A comparison between subtraction radiography and conventional radiographic technique. J Clin Periodontol. 1984;11:411–20. doi: 10.1111/j.1600-051x.1984.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 52.Brägger U, Pasquali L, Rylander H, Carnes D, Kornman KS. Computer-assisted densitometric image analysis in periodontal radiography. A methodological study. J Clin Periodontol. 1988;15:27–3. doi: 10.1111/j.1600-051x.1988.tb01551.x. [DOI] [PubMed] [Google Scholar]
- 53.Brägger U, Hämmerle CH, Mombelli A, Bürgin W, Lang NP. Remodelling of periodontal tissues adjacent to sites treated according to the principles of guided tissue regeneration (GTR) J Clin Periodontol. 1992;19:615–24. doi: 10.1111/j.1600-051x.1992.tb01708.x. [DOI] [PubMed] [Google Scholar]
- 54.Guillemin MR, Mellonig JT, Brunsvold MA, Steffensen B. Healing in periodontal defects treated by decalcified freeze-dried bone allografts in combination with ePTFE membranes. Assessment by computerized densitometric analysis. J Clin Periodontol. 1993;20:520–7. doi: 10.1111/j.1600-051x.1993.tb00401.x. [DOI] [PubMed] [Google Scholar]
- 55.Fuhrmann RA, Bücker A, Diedrich PR. Assessment of alveolar bone loss with high resolution computed tomography. J Periodontal Res. 1995;30:258–63. doi: 10.1111/j.1600-0765.1995.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 56.Scarfe WC, Farman AG. What is cone-beam CT and how does it work? Dent Clin North Am. 2008;52:707–30. doi: 10.1016/j.cden.2008.05.005. v. [DOI] [PubMed] [Google Scholar]
- 57.Howerton WB, Jr, Mora MA. Advancements in digital imaging: What is new and on the horizon? J Am Dent Assoc. 2008;139(Suppl):20S–4. doi: 10.14219/jada.archive.2008.0354. [DOI] [PubMed] [Google Scholar]
- 58.Vandenberghe B, Jacobs R, Yang J. Diagnostic validity (or acuity) of 2D CCD versus 3D CBCT-images for assessing periodontal breakdown. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:395–401. doi: 10.1016/j.tripleo.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 59.Lee S, Gantes B, Riggs M, Crigger M. Bone density assessments of dental implant sites: 3. Bone quality evaluation during osteotomy and implant placement. Int J Oral Maxillofac Implants. 2007;22:208–12. [PubMed] [Google Scholar]
- 60.Honda K, Arai Y, Kashima M, Takano Y, Sawada K, Ejima K, et al. Evaluation of the usefulness of the limited cone-beam CT (3DX) in the assessment of the thickness of the roof of the glenoid fossa of the temporomandibular joint. Dentomaxillofac Radiol. 2004;33:391–5. doi: 10.1259/dmfr/54316470. [DOI] [PubMed] [Google Scholar]
- 61.Bianchi J, Goggins W, Rudolph M. In vivo, thyroid and lens surface exposure with spiral and conventional computed tomography in dental implant radiography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:249–53. doi: 10.1067/moe.2000.107354. [DOI] [PubMed] [Google Scholar]
- 62.Webber RL, Horton RA, Tyndall DA, Ludlow JB. Tuned-aperture computed tomography (TACT). Theory and application for three-dimensional dento-alveolar imaging. Dentomaxillofac Radiol. 1997;26:53–62. doi: 10.1038/sj.dmfr.4600201. [DOI] [PubMed] [Google Scholar]
- 63.Nair MK, Seyedain A, Agarwal S, Webber RL, Nair UP, Piesco NP, et al. Tuned aperture computed tomography to evaluate osseous healing. J Dent Res. 2001;80:1621–4. doi: 10.1177/00220345010800070501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grimard BA, Hoidal MJ, Mills MP, Mellonig JT, Nummikoski PV, Mealey BL. Comparison of clinical, periapical radiograph, and cone-beam volume tomography measurement techniques for assessing bone level changes following regenerative periodontal therapy. J Periodontol. 2009;80:48–55. doi: 10.1902/jop.2009.080289. [DOI] [PubMed] [Google Scholar]
- 65.Shahlaie M, Gantes B, Schulz E, Riggs M, Crigger M. Bone density assessments of dental implant sites: 1. Quantitative computed tomography. Int J Oral Maxillofac Implants. 2003;18:224–31. [PubMed] [Google Scholar]
- 66.Genant HK, Steiger P, Block JE, Glueer CC, Ettinger B, Harris ST. Quantitative computed tomography: Update 1987. Calcif Tissue Int. 1987;41:179–86. doi: 10.1007/BF02555236. [DOI] [PubMed] [Google Scholar]
- 67.Cann CE. Quantitative CT for determination of bone mineral density: A review. Radiology. 1988;166:509–22. doi: 10.1148/radiology.166.2.3275985. [DOI] [PubMed] [Google Scholar]
- 68.Rüegsegger P, Koller B, Müller R. A microtomographic system for the nondestructive evaluation of bone architecture. Calcif Tissue Int. 1996;58:24–9. doi: 10.1007/BF02509542. [DOI] [PubMed] [Google Scholar]
- 69.Ohta T, Harada Y, Yamada N, Takagi H, Azuma Y, Komoriya K. Three dimensional analysis of bone structure by microfocus X-ray computed tomography. J Soc Bone Morphometry. 1999;9:97–101. [Google Scholar]
- 70.Hara T, Hashimoto H, Ide Y. Application of micro-CT to the measurement of enamel thickness. J Oral Biol. 1999;41:303–6. [Google Scholar]
- 71.Hayakawa T, Mishima H, Yokota I, Sakae T, Kozawa Y, Nemoto K. Application of high resolution microfocus X-ray CT for the observation of human tooth. Dent Mater J. 2000;19:87–95. doi: 10.4012/dmj.19.87. [DOI] [PubMed] [Google Scholar]
- 72.Bjørndal L, Carlsen O, Thuesen G, Darvann T, Kreiborg S. External and internal macromorphology in 3D-reconstructed maxillary molars using computerized X-ray microtomography. Int Endod J. 1999;32:3–9. doi: 10.1046/j.1365-2591.1999.00172.x. [DOI] [PubMed] [Google Scholar]
- 73.Sakae T, Hayakawa T, Ohrigome K. Potential application of micro-CT for study of bone-Ti implant interface. J Hard Tissue Biol. 2000;9:15–17. [Google Scholar]
- 74.Kiba H, Hayakawa T, Oba S, Kuwabara M, Habata I, Yamamoto H. Potential application of high-resolution microfocus X-ray techniques for observation of bone structure and bone-implant interface. Int J Oral Maxillofac Implants. 2003;18:279–85. [PubMed] [Google Scholar]
- 75.Kochi G, Sato S, Fukuyama T, Morita C, Honda K, Arai Y, et al. Analysis on the guided bone augmentation in the rat calvarium using a microfocus computerized tomography analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:e42–8. doi: 10.1016/j.tripleo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 76.Aranyarachkul P, Caruso J, Gantes B, Schulz E, Riggs M, Dus I, et al. Bone density assessments of dental implant sites: 2. Quantitative cone-beam computerized tomography. Int J Oral Maxillofac Implants. 2005;20:416–24. [PubMed] [Google Scholar]
- 77.Dula K, Mini R, van der Stelt PF, Buser D. The radiographic assessment of implant patients: Decision-making criteria. Int J Oral Maxillofac Implants. 2001;16:80–9. [PubMed] [Google Scholar]
- 78.Rothman SL, Chaftez N, Rhodes ML, Schwarz MS. CT in the preoperative assessment of the mandible and maxilla for endosseous implant surgery. Work in progress. Radiology. 1988;168:171–5. doi: 10.1148/radiology.168.1.3380955. [DOI] [PubMed] [Google Scholar]
- 79.Wishan MS, Bahat O, Krane M. Computed tomography as an adjunct in dental implant surgery. Int J Periodontics Restorative Dent. 1988;8:30–47. [PubMed] [Google Scholar]
- 80.Tal H, Moses O. A comparison of panoramic radiography with computed tomography in the planning of implant surgery. Dentomaxillofac Radiol. 1991;20:40–2. doi: 10.1259/dmfr.20.1.1884852. [DOI] [PubMed] [Google Scholar]
- 81.Dandrau JP, Pharaboz G, Bellavoir A. Dentascan in dental implantation. Rev Stomatol Chir Maxillofac. 1992;93:263–6. [PubMed] [Google Scholar]
- 82.Abrahams JJ. The role of diagnostic imaging in dental implantology. Radiol Clin North Am. 1993;31:163–80. [PubMed] [Google Scholar]
- 83.DelBalso AM, Greiner FG, Licata M. Role of diagnostic imaging in evaluation of the dental implant patient. Radiographics. 1994;14:699–719. doi: 10.1148/radiographics.14.4.7938761. [DOI] [PubMed] [Google Scholar]
- 84.Cawood JI, Howell RA. A classification of the edentulous jaws. Int J Oral Maxillofac Surg. 1988;17:232–6. doi: 10.1016/s0901-5027(88)80047-x. [DOI] [PubMed] [Google Scholar]
- 85.Almog DM, Benson BW, Wolfgang L, Frederiksen NL, Brooks SL. Computerized tomography-based imaging and surgical guidance in oral implantology. J Oral Implantol. 2006;32:14–8. doi: 10.1563/781.1. [DOI] [PubMed] [Google Scholar]
- 86.Naitoh M, Kawamata A, Takouchi K, Ohsaki C, Ariji E. Improvements of tomography for implant treatment using panoramic X-ray machine: Development of direct laser positioning system. J Soc Oral Implantol. 2000;13:59–68. [Google Scholar]
- 87.Tyndall DA, Brooks SL. Selection criteria for dental implant site imaging: A position paper of the American Academy of Oral and Maxillofacial radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:630–7. doi: 10.1067/moe.2000.106336. [DOI] [PubMed] [Google Scholar]
- 88.Arai Y, Tammisalo E, Iwai K, Hashimoto K, Shinoda K. Development of a compact computed tomographic apparatus for dental use. Dentomaxillofac Radiol. 1999;28:245–8. doi: 10.1038/sj/dmfr/4600448. [DOI] [PubMed] [Google Scholar]
- 89.Ito K, Gomi Y, Sato S, Arai Y, Shinoda K. Clinical application of a new compact CT system to assess 3-D images for the preoperative treatment planning of implants in the posterior mandible A case report. Clin Oral Implants Res. 2001;12:539–42. doi: 10.1034/j.1600-0501.2001.120516.x. [DOI] [PubMed] [Google Scholar]


