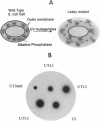
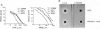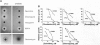Abstract
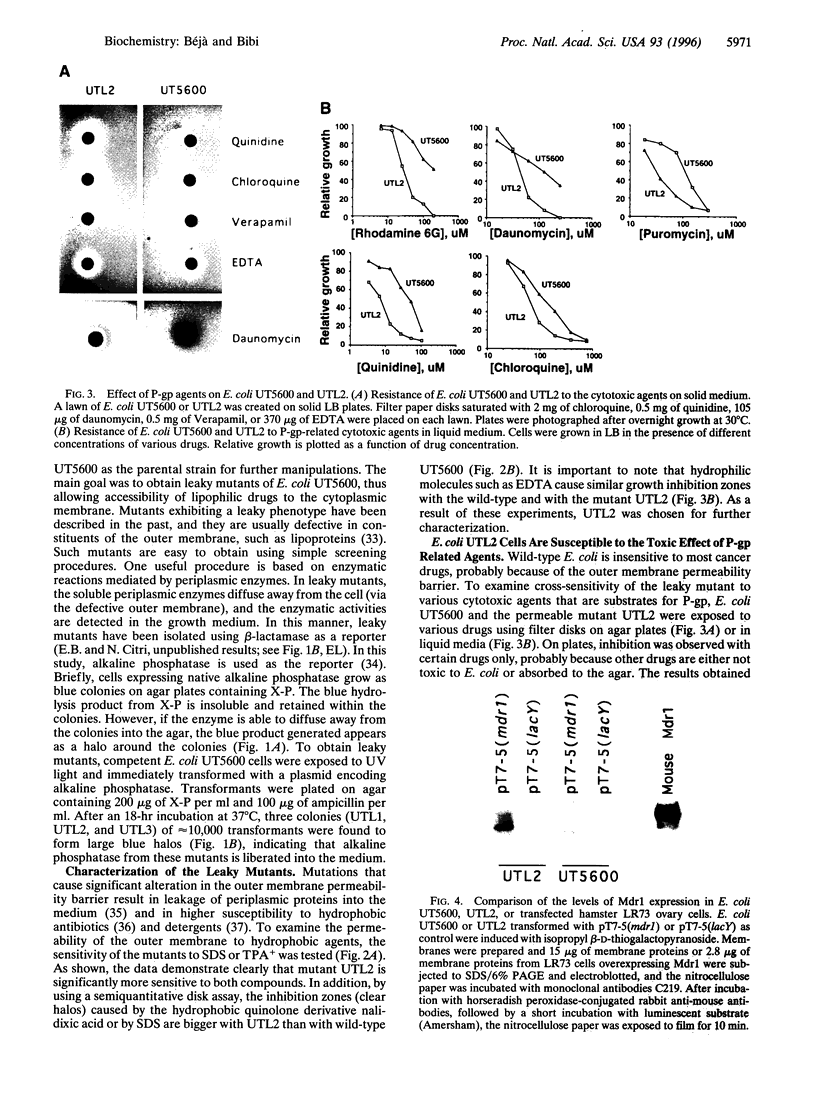
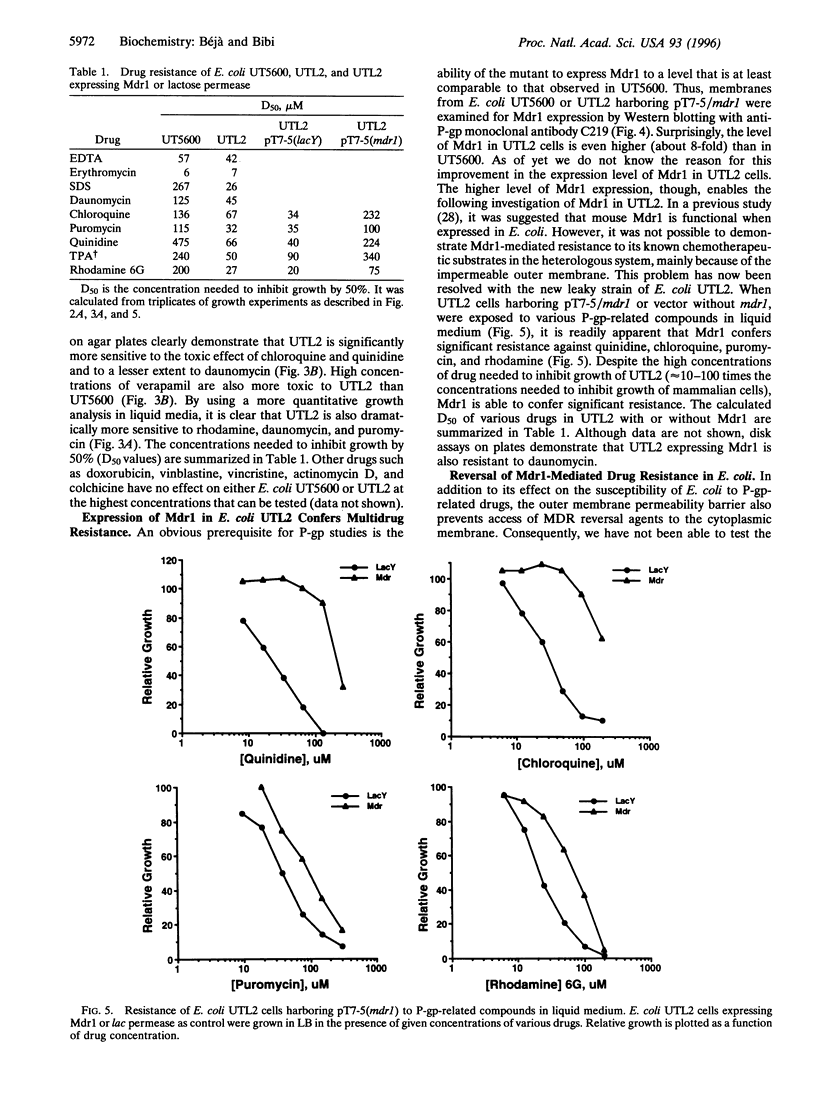
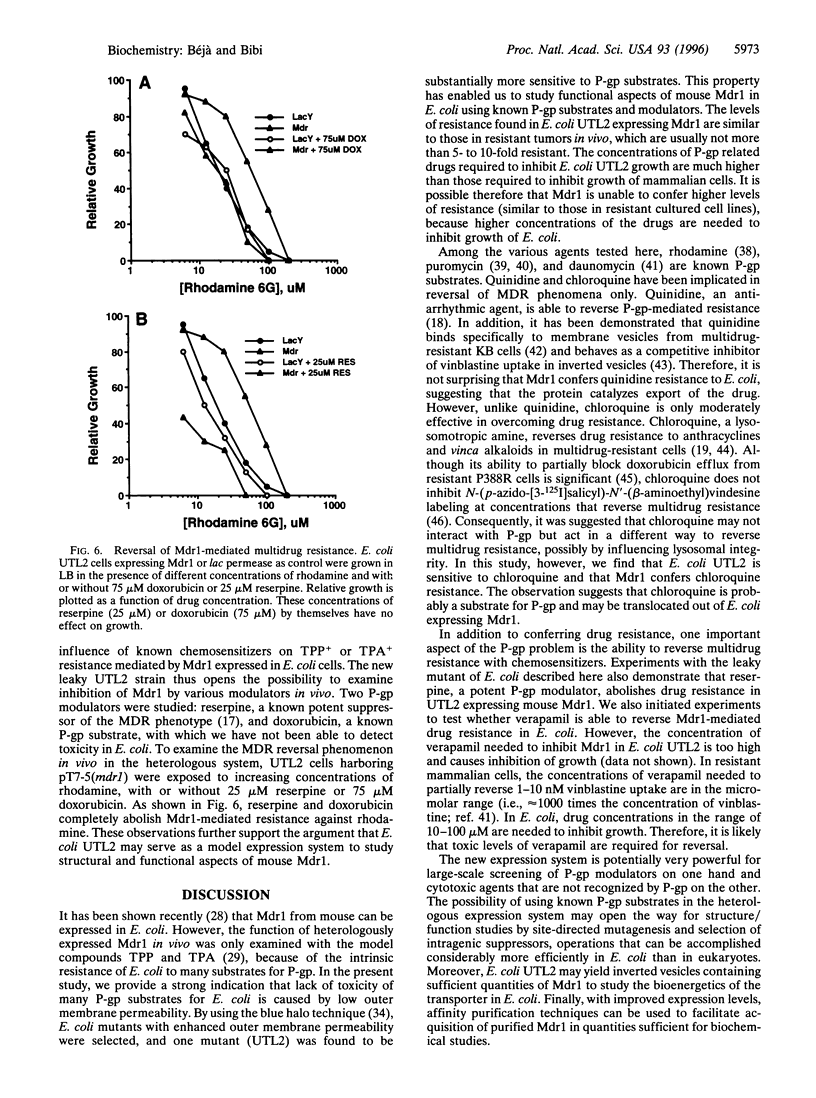
Functional expression of the multidrug resistance protein P-glycoprotein (P-gp) in Escherichia coli is providing an appropriate system for structure/function studies and might provide an invaluable tool to screen potential P-gp substrates and inhibitors. The major problem encountered in such studies, however, is the impermeability of the outer membrane of Gram-negative bacteria, which protects microorganisms against the cytotoxic effects of many lipophilic cancer drugs and blocks accessibility of P-gp reversal agents. In the present study we have constructed, by mutagenesis, a "leaky" (containing a permeable outer membrane) strain of E. coli, which is significantly more susceptible to the toxic effect of known P-gp substrates and cytotoxic agents. Expression of mouse Mdr1 in the mutant confers cross-resistance to daunomycin, quinidine, chloroquine, rhodamine 6G, and puromycin. Most importantly, reserpine and doxorubicin completely abolish Mdr1-mediated rhodamine resistance. The results provide strong support for previous observations, suggesting that Mdr1 can be expressed functionally in E. coli and indicate that the leaky mutant will be useful for further structure/function studies of the heterologously expressed eukaryotic drug efflux protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S., Cornwell M. M., Kuwano M., Pastan I., Gottesman M. M. Most drugs that reverse multidrug resistance also inhibit photoaffinity labeling of P-glycoprotein by a vinblastine analog. Mol Pharmacol. 1988 Feb;33(2):144–147. [PubMed] [Google Scholar]
- Benson S. A., Occi J. L., Sampson B. A. Mutations that alter the pore function of the OmpF porin of Escherichia coli K12. J Mol Biol. 1988 Oct 20;203(4):961–970. doi: 10.1016/0022-2836(88)90121-0. [DOI] [PubMed] [Google Scholar]
- Bibi E., Béjà O. Membrane topology of multidrug resistance protein expressed in Escherichia coli. N-terminal domain. J Biol Chem. 1994 Aug 5;269(31):19910–19915. [PubMed] [Google Scholar]
- Bibi E., Gros P., Kaback H. R. Functional expression of mouse mdr1 in Escherichia coli. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9209–9213. doi: 10.1073/pnas.90.19.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béjà O., Bibi E. Multidrug resistance protein (Mdr)-alkaline phosphatase hybrids in Escherichia coli suggest a major revision in the topology of the C-terminal half of Mdr. J Biol Chem. 1995 May 26;270(21):12351–12354. doi: 10.1074/jbc.270.21.12351. [DOI] [PubMed] [Google Scholar]
- Cornwell M. M., Pastan I., Gottesman M. M. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem. 1987 Feb 15;262(5):2166–2170. [PubMed] [Google Scholar]
- Cornwell M. M., Safa A. R., Felsted R. L., Gottesman M. M., Pastan I. Membrane vesicles from multidrug-resistant human cancer cells contain a specific 150- to 170-kDa protein detected by photoaffinity labeling. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3847–3850. doi: 10.1073/pnas.83.11.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell M. M., Tsuruo T., Gottesman M. M., Pastan I. ATP-binding properties of P glycoprotein from multidrug-resistant KB cells. FASEB J. 1987 Jul;1(1):51–54. doi: 10.1096/fasebj.1.1.2886389. [DOI] [PubMed] [Google Scholar]
- Efferth T., Löhrke H., Volm M. Reciprocal correlation between expression of P-glycoprotein and accumulation of rhodamine 123 in human tumors. Anticancer Res. 1989 Nov-Dec;9(6):1633–1637. [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Evans G. L., Ni B., Hrycyna C. A., Chen D., Ambudkar S. V., Pastan I., Germann U. A., Gottesman M. M. Heterologous expression systems for P-glycoprotein: E. coli, yeast, and baculovirus. J Bioenerg Biomembr. 1995 Feb;27(1):43–52. doi: 10.1007/BF02110330. [DOI] [PubMed] [Google Scholar]
- Fralick J. A., Burns-Keliher L. L. Additive effect of tolC and rfa mutations on the hydrophobic barrier of the outer membrane of Escherichia coli K-12. J Bacteriol. 1994 Oct;176(20):6404–6406. doi: 10.1128/jb.176.20.6404-6406.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann U. A., Willingham M. C., Pastan I., Gottesman M. M. Expression of the human multidrug transporter in insect cells by a recombinant baculovirus. Biochemistry. 1990 Mar 6;29(9):2295–2303. doi: 10.1021/bi00461a013. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gros P., Ben Neriah Y. B., Croop J. M., Housman D. E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986 Oct 23;323(6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Gros P., Buschman E. The mouse multidrug resistance gene family: structural and functional analysis. Int Rev Cytol. 1993;137C:169–197. [PubMed] [Google Scholar]
- Gros P., Talbot F., Tang-Wai D., Bibi E., Kaback H. R. Lipophilic cations: a group of model substrates for the multidrug-resistance transporter. Biochemistry. 1992 Feb 25;31(7):1992–1998. doi: 10.1021/bi00122a014. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio M., Lovelace E., Pastan I., Gottesman M. M. Agents which reverse multidrug-resistance are inhibitors of [3H]vinblastine transport by isolated vesicles. Biochim Biophys Acta. 1991 Jan 9;1061(1):106–110. doi: 10.1016/0005-2736(91)90274-c. [DOI] [PubMed] [Google Scholar]
- Hyde S. C., Emsley P., Hartshorn M. J., Mimmack M. M., Gileadi U., Pearce S. R., Gallagher M. P., Gill D. R., Hubbard R. E., Higgins C. F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990 Jul 26;346(6282):362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Kartner N., Shales M., Riordan J. R., Ling V. Daunorubicin-resistant Chinese hamster ovary cells expressing multidrug resistance and a cell-surface P-glycoprotein. Cancer Res. 1983 Sep;43(9):4413–4419. [PubMed] [Google Scholar]
- Klohs W. D., Steinkampf R. W. The effect of lysosomotropic agents and secretory inhibitors on anthracycline retention and activity in multiple drug-resistant cells. Mol Pharmacol. 1988 Aug;34(2):180–185. [PubMed] [Google Scholar]
- Kuchler K., Thorner J. Functional expression of human mdr1 in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2302–2306. doi: 10.1073/pnas.89.6.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura C. S., Holbrook S. R., Ames G. F. Structural model of the nucleotide-binding conserved component of periplasmic permeases. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):84–88. doi: 10.1073/pnas.88.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirski S. E., Gerlach J. H., Cole S. P. Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res. 1987 May 15;47(10):2594–2598. [PubMed] [Google Scholar]
- Pastan I., Gottesman M. M., Ueda K., Lovelace E., Rutherford A. V., Willingham M. C. A retrovirus carrying an MDR1 cDNA confers multidrug resistance and polarized expression of P-glycoprotein in MDCK cells. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4486–4490. doi: 10.1073/pnas.85.12.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M., Gros P., Whiteway M., Thomas D. Y. Functional complementation of yeast ste6 by a mammalian multidrug resistance mdr gene. Science. 1992 Apr 10;256(5054):232–234. doi: 10.1126/science.1348873. [DOI] [PubMed] [Google Scholar]
- Ruetz S., Gros P. Functional expression of P-glycoproteins in secretory vesicles. J Biol Chem. 1994 Apr 22;269(16):12277–12284. [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Meyers M. B., Biedler J. L., Felsted R. L. Vinblastine photoaffinity labeling of a high molecular weight surface membrane glycoprotein specific for multidrug-resistant cells. J Biol Chem. 1986 May 15;261(14):6137–6140. [PubMed] [Google Scholar]
- Sarkadi B., Price E. M., Boucher R. C., Germann U. A., Scarborough G. A. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem. 1992 Mar 5;267(7):4854–4858. [PubMed] [Google Scholar]
- Schurr E., Raymond M., Bell J. C., Gros P. Characterization of the multidrug resistance protein expressed in cell clones stably transfected with the mouse mdr1 cDNA. Cancer Res. 1989 May 15;49(10):2729–2733. [PubMed] [Google Scholar]
- Shapiro A. B., Ling V. ATPase activity of purified and reconstituted P-glycoprotein from Chinese hamster ovary cells. J Biol Chem. 1994 Feb 4;269(5):3745–3754. [PubMed] [Google Scholar]
- Shapiro A. B., Ling V. Reconstitution of drug transport by purified P-glycoprotein. J Biol Chem. 1995 Jul 7;270(27):16167–16175. doi: 10.1074/jbc.270.27.16167. [DOI] [PubMed] [Google Scholar]
- Sharom F. J., Yu X., Doige C. A. Functional reconstitution of drug transport and ATPase activity in proteoliposomes containing partially purified P-glycoprotein. J Biol Chem. 1993 Nov 15;268(32):24197–24202. [PubMed] [Google Scholar]
- Shiraishi N., Akiyama S., Kobayashi M., Kuwano M. Lysosomotropic agents reverse multiple drug resistance in human cancer cells. Cancer Lett. 1986 Mar;30(3):251–259. doi: 10.1016/0304-3835(86)90049-2. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Schindler M. Cell biological mechanisms of multidrug resistance in tumors. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3497–3504. doi: 10.1073/pnas.91.9.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater L. M., Sweet P., Stupecky M., Gupta S. Cyclosporin A reverses vincristine and daunorubicin resistance in acute lymphatic leukemia in vitro. J Clin Invest. 1986 Apr;77(4):1405–1408. doi: 10.1172/JCI112450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Kawabata H., Tsukagoshi S., Sakurai Y. High calcium content of pleiotropic drug-resistant P388 and K562 leukemia and Chinese hamster ovary cells. Cancer Res. 1984 Nov;44(11):5095–5099. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981 May;41(5):1967–1972. [PubMed] [Google Scholar]
- Vuorio R., Vaara M. Mutants carrying conditionally lethal mutations in outer membrane genes omsA and firA (ssc) are phenotypically similar, and omsA is allelic to firA. J Bacteriol. 1992 Nov;174(22):7090–7097. doi: 10.1128/jb.174.22.7090-7097.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora J. M., Beck W. T. Chloroquine enhancement of anticancer drug cytotoxicity in multiple drug resistant human leukemic cells. Biochem Pharmacol. 1986 Dec 1;35(23):4303–4310. doi: 10.1016/0006-2952(86)90710-0. [DOI] [PubMed] [Google Scholar]
- Zamora J. M., Pearce H. L., Beck W. T. Physical-chemical properties shared by compounds that modulate multidrug resistance in human leukemic cells. Mol Pharmacol. 1988 Apr;33(4):454–462. [PubMed] [Google Scholar]
- al-Shawi M. K., Senior A. E. Characterization of the adenosine triphosphatase activity of Chinese hamster P-glycoprotein. J Biol Chem. 1993 Feb 25;268(6):4197–4206. [PubMed] [Google Scholar]