Abstract
Mpl, a thermolysin-like metalloprotease, and PC-PLC, a phospholipase C, are synthesized as proenzymes by the intracellular bacterial pathogen Listeria monocytogenes. During intracellular growth, L. monocytogenes is temporarily confined in a membrane-bound vacuole whose acidification leads to Mpl autolysis and Mpl-mediated cleavage of the PC-PLC N-terminal propeptide. Mpl maturation also leads to the secretion of both Mpl and PC-PLC across the bacterial cell wall. Previously, we identified negatively charged and uncharged amino acid residues within the N terminus of the PC-PLC propeptide that influence the ability of Mpl to mediate the maturation of PC-PLC, suggesting that these residues promote the interaction of the PC-PLC propeptide with Mpl. In the present study, we identified a non-catalytic histidine residue (H226) that influences Mpl secretion across the cell wall and its ability to process PC-PLC. Our results suggest that a positive charge at position 226 is required for Mpl functions other than autolysis. Based on the charge requirement at this position, we hypothesize that this residue contributes to the interaction of Mpl with the PC-PLC propeptide.
Introduction
Metalloproteases are important factors for bacterial pathogens. They can target host factors to assist in colonization, invasion, dissemination and cell damage (Miyoshi & Shinoda, 2000). These proteases can also assist in the activation of other virulence factors. One such metalloprotease is that of the Gram-positive bacterium Listeria monocytogenes (Mpl) (Mengaud et al., 1991).
L. monocytogenes is a facultative intracellular pathogen that causes the food-borne disease listeriosis. This bacterium begins its intracellular life cycle within a membrane-bound vacuole from which it must escape to survive (Tilney & Portnoy, 1989). Once into the host cytosol, L. monocytogenes multiplies and spreads from cell to cell using an actin-based mechanism of motility. This spreading event results in the formation of a double-membrane vacuole from which the bacterium escapes to perpetuate the intracellular life cycle.
Two virulence factors that contribute to escape from vacuoles are Mpl and a broad-range phospholipase C known as PC-PLC (Smith et al., 1995). Both Mpl and PC-PLC are secreted as inactive proenzymes, each with an N-terminal propeptide (Mengaud et al., 1991; Raveneau et al., 1992). Translocation of these two proenzymes across the bacterial membrane is faster than their rate of secretion across the cell wall, resulting in an accumulation of PC-PLC and Mpl presumably at the inner wall zone of the bacterium (Forster & Marquis, 2012; Marquis & Hager, 2000; Snyder & Marquis, 2003). As the vacuolar pH decreases, Mpl undergoes maturation by intramolecular proteolysis, and the mature Mpl enzyme activates PC-PLC by cleaving the N-terminal propeptide of the phospholipase (Bitar et al., 2008; Forster et al., 2011). Mpl autolysis also leads to the rapid secretion of Mpl and PC-PLC across the cell wall into the vacuolar space, enabling PC-PLC to access its substrates at the vacuolar membrane.
One mechanism by which pH regulates protease activity is by influencing the charge of specific amino acid residues that act as pH sensors (Srivastava et al., 2007). To be involved in pH sensing, the amino acid must contain a titratable group on its side chain or reside at the amino or carboxyl terminus of the protein. Depending on the side group pKa, these amino acids can be either protonated or deprotonated upon a change in pH. Amino acids acting as pH sensors can affect protein conformation (Dawson et al., 2009; Fritz et al., 2008), protein stability (Schuerch et al., 2005) and proenzyme maturation (Feliciangeli et al., 2006).
In this study, we wished to identify amino acids, other than those involved in catalysis, that influence the pH-dependent nature of Mpl’s activity. We focused on titratable amino acids in mature Mpl that would become protonated upon a decrease in pH, and identified a histidine residue (H226) that influences Mpl functions other than autolysis. Our results suggest that there is a charge requirement for histidine residue 226. A positive charge at this position enables the efficient processing of PC-PLC and secretion of mature Mpl across the bacterial cell wall, whereas a hydrophobic or polar residue at this position does not, despite the fact that autolysis is not affected.
Methods
Bacterial strains and growth conditions.
All L. monocytogenes strains used in this study are listed in Table 1. L. monocytogenes strains harbouring pKSV7-derived plasmids were cultured in brain–heart infusion medium supplemented with chloramphenicol (10 µg ml−1). Escherichia coli strains containing pKSV7-derived plasmids were cultured in Luria–Bertani medium supplemented with ampicillin (100 µg ml−1).
Table 1. L. monocytogenes strains used in this study.
| Strain | Genotype and relevant features | Source/reference |
| NF-L943 | L. monocytogenes prfA G155S | Shetron-Rama et al. (2003) |
| HEL-469 | Internal in-frame deletion of mpl in NF-L943 background | Bitar et al. (2008) |
| HEL-981 | NF-L943 mpl-FlagN-cat | Forster et al. (2011) |
| HEL-987 | NF-L943 mpl H226T | This study |
| HEL-1004 | NF-L943 mpl H226F | This study |
| HEL-1007 | NF-L943 mpl H226R | This study |
| HEL-1195 | NF-L943 mpl H226F-FlagN-cat | This study |
| HEL-1235 | NF-L943 mpl H226R-FlagN-cat | This study |
Generation of Mpl point mutants.
clustal w alignment of Mpl to other bacterial metalloproteases was performed using the ConSeq server (http://conseq.bioinfo.tau.ac.il/) (Berezin et al., 2004) to assist in determining what mutation should be made at Mpl histidine residue 226. These mutations and the addition of a Flag tag (DYKDDDDK) at the N terminus of the catalytic domain of Mpl (mpl-FlagN-cat) were generated by allelic exchange (Camilli et al., 1993) using the shuttle vector pKSV7 (Smith & Youngman, 1992). Site-directed mutagenesis with overlap extension PCR (SOEing PCR) (Ho et al., 1989) was used to construct single point mutations in the mpl sequence using DNA from NF-L943 or HEL-981 as templates for PCR. SOEing PCR products were digested with restriction enzymes and ligated into pKSV7, thus creating a series of plasmids (Table 2). Each plasmid was sequenced, then electroporated into NF-L943 to generate a series of strains listed in Table 1. Allelic exchange was verified by screening for chloramphenicol-sensitive clones and for the acquisition or loss of a restriction site within the mpl gene as designed with the SOEing primers (Table 3).
Table 2. Plasmids and oligonucleotide primers used for constructs.
| Plasmids* | Genotype | Primer pairs† | SOEing primers† | Restriction |
| pBMF985 | pKSV7 mpl H226T | 328/492, 489/493 | 328/489 | EcoRI, PstI |
| pBMF1001 | pKSV7 mpl H226F | 328/499, 489/500 | 328/489 | EcoRI, PstI |
| pBMF1002 | pKSV7 mpl H226R | 328/501, 489/502 | 328/489 | EcoRI, PstI |
| pBMF1194 | pKSV7 mpl H226F-FlagN-cat | 328/499, 489/500 | 328/489 | EcoRI, PstI |
| pBMF1234 | pKSV7 mpl H226R-FlagN-cat | 328/487, 387/488 | 328/387 | EcoRI, PstI |
Plasmids pBMF1001 through pBMF1194 carry a silent mutation at mpl V227.
Primer sequences are listed in Table 3.
Table 3. DNA oligonucleotide primer sequences.
| Name | Sequence (5′–3′) |
| Marq 328 | GTGAATTCGATTCTGTCGGGGAAGAAAA |
| Marq 387 | GCACTGCAGCAATTCAAGCCATAATGGAC |
| Marq 487 | CTTGTCATCGTCATCCTTGTAATCCTCGGAAAGCATATTTTG |
| Marq 488 | GATTACAAGGATGACGATGACAAGGTAGAACGGGCTGATA |
| Marq 489 | GATAACTGCAGGCGTTAGTTCATGACCA |
| Marq 492 | GCCACTAACTGTAATGAAAATTAATG |
| Marq 493 | CATTAATTTTCATTACAGTTAGTGGC |
| Marq 499 | CATTAATTTTCATTACAAATAGTGGC |
| Marq 500 | GCCACTATTTGTAATGAAAATTAATG |
| Marq 501 | CATTAATTTTCATTACACGTAGTGGC |
| Marq 502 | GCCACTACGTGTAATGAAAATTAATG |
Metabolic labelling and immunoprecipitation assays.
Metabolic labelling and immunoprecipitation of PC-PLC and Mpl were performed as described previously (Forster et al., 2011; Slepkov et al., 2010). Listeria-infected J774 mouse macrophage-like cells were pulse-labelled using [35S]-methionine/cysteine. Radiolabelled cells were incubated in a nigericin-supplemented potassium-containing buffer equilibrated to either physiological or acidic pH (Marquis & Hager, 2000). Chloramphenicol (20 µg ml−1) and tetracycline (50 µg ml−1) were present during this chase to inhibit further bacterial protein synthesis. Host cells were lysed on ice and lysates were separated from intracellular bacteria by centrifugation. Secreted PC-PLC and Mpl were immunoprecipitated from the cleared host cell lysates. Immunoprecipitates were resolved on a 12 % SDS-polyacrylamide gel and detected by autoradiography.
Quantification of Mpl intracellular activity.
The intracellular activity of Mpl at acidic pH was quantified by measuring the ratios of (i) Mpl mature form to propeptide and (ii) PC-PLC mature form to the total of mature and pro-form using ImageJ (http://rsbweb.nih.gov/ij/). Band intensities were normalized to the number of methionine and cysteine residues in each protein species. For wild-type Mpl, the mean ratio was 1 : 1.7 (n = 11), and the normalized ratios are reported.
Immunofluorescence microscopy.
Bacterium-associated Mpl was detected by immunofluorescence microscopy as described previously (Forster et al., 2011). Briefly, HeLa cells were infected with L. monocytogenes strains expressing Mpl-FlagN-cat mutants. Four hours post-infection, infected cells were incubated in nigericin-supplemented potassium-containing buffer equilibrated to either physiological or acidic pH. Samples were fixed and the cell wall of intracellular bacteria was partially digested using purified Ply118, a L. monocytogenes-specific phage endolysin (Loessner et al., 1996). Mpl-FlagN-cat was detected using either anti-Flag M1 or M2 antibody (Sigma), followed by a secondary antibody conjugated to fluorescein isothiocyanate. Bacterial chromosome and host cell nuclei were detected using bisbenzimide (Hoechst).
Results
Identification of H226 as a candidate amino acid important for Mpl functions
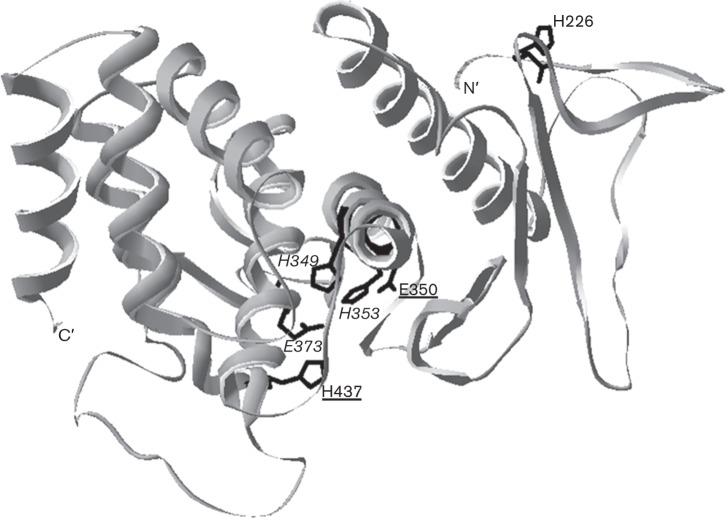
Mpl is secreted as a 55 kDa zymogen, with its propeptide composed of amino acid residues 25–200 and its catalytic domain composed of amino acid residues 201–510 (Bitar et al., 2008). Since Mpl activity is influenced by pH (Forster et al., 2011), we sought to identify non-catalytic residues that would have a titratable side group with a predicted pKa in the physiological pH range (6.0–7.4). One amino acid that can act as a pH sensor is histidine. Histidine’s imidazole ring has a pKa of approximately 6.0. Upon protonation, the imidazole ring loses its hydrophobic character, and can participate in intra- and intermolecular hydrogen bonding. To identify a candidate pH sensor, we both performed a clustal w alignment of the primary sequence of Mpl to other bacterial metalloproteases and constructed homology models of the catalytic domain of Mpl based on the crystal structures of aureolysin from Staphylococcus aureus (PDB no. 1BQB, 46 % sequence identity) and thermolysin from Bacillus thermoproteolyticus (PDB no. 2TMN, 45.2 % sequence identity) using SWISS-MODEL (Arnold et al., 2006; Guex & Peitsch, 1997; Schwede et al., 2003). We submitted the Mpl homology models to PROPKA 3.0 (http://propka.ki.ku.dk/), an online interface that determines pKa values in silico for solvent exposed titratable side groups (Li et al., 2005). One amino acid that was of interest to us from the model and alignment was histidine 226. H226, which has a calculated pKa value of 5.94, is located in a random loop at the N terminus of the catalytic domain, approximately 20 Å from the active site pocket (Fig. 1). Therefore, we chose to focus on assessing the role of H226 in Mpl activity.
Fig. 1.
Ribbon model of the catalytic domain of Mpl. A homology model of Mpl was constructed as described in Results. The N terminus (N′), C terminus (C′) and the H226 residue targeted for mutation in this study are indicated. Active site amino acids are underlined. Amino acids involved in the coordination of the Zn2+ ion are italicized.
H226 is important for Mpl functions other than autolysis
While a histidine residue is located at this position in Mpl, a threonine residue was found to be present at the same position in thermolysin and vibriolysin, whereas there was a gap at this position in aureolysin and bacillolysin (Fig. 2). Based on the alignment results, H226 was mutated to a threonine, as we rationalized that it was unlikely to influence protein folding. To assess the importance of H226 on Mpl activity, we looked at pH-dependent Mpl and PC-PLC maturation during intracellular infection since Mpl autolysis is triggered by a decrease in pH and PC-PLC maturation is mediated by mature Mpl. Cells infected with wild-type or the isogenic L. monocytogenes mutant (Mpl H226T) were pulse-labelled with [35S]-Met, followed by a chase with a potassium-containing buffer at physiological or acidic pH supplemented with nigericin to control the cytosolic pH of host cells. After the chase, Mpl and PC-PLC were immunoprecipitated from host cell lysates, as maturation leads to the rapid transportation of PC-PLC and Mpl across the bacterial cell wall into the host cell. As previously reported, neither protein is secreted from infected host cells maintained at physiological pH (Fig. 3, lane 1), whereas the catalytic domain of Mpl, the Mpl propeptide, and the mature form of PC-PLC are secreted into the cytosol of acidified infected host cells (Fig. 3, lane 2) (Forster et al., 2011; Marquis & Hager, 2000). Next, we observed that Mpl H226T was competent for autolysis, as the amount of propeptide released from host cells following a decrease in intracellular pH was similar to the amount of wild-type Mpl propeptide (Fig. 3, compare lanes 2 and 5). However, the ratio of mature Mpl to propeptide was decreased twofold compared with wild-type Mpl (Fig. 3, Table 4). This phenomenon was associated with an equivalent decrease in levels of PC-PLC maturation. Overall, these results indicated that H226 is important for Mpl functions other than autolysis.
Fig. 2.

clustal w alignment of the catalytic domain of Mpl with other metalloproteases. The primary sequence of Mpl was aligned to other bacterial metalloproteases using a BLOSUM protein matrix. Alignment includes elastase (Staphylococcus epidermidis), aureolysin (Staphylococcus aureus), bacillolysin (Bacillus subtilis subsp. amylosacchariticus), thermolysin (Bacillus thermoproteolyticus) and vibriolysin (Vibrio cholerae). Numbers on the right indicate amino acid positions. An asterisk indicates position 226 in Mpl.
Fig. 3.
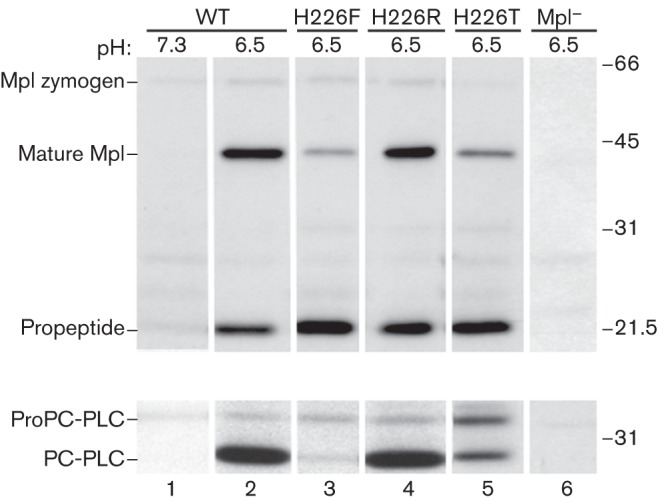
Intracellular activity of Mpl H226 mutants. Infected J774 cells were radiolabelled and chased in a nigericin-supplemented potassium-containing buffer equilibrated to either pH 7.3 or pH 6.5. The intracellular activity of wild-type Mpl (WT) and Mpl mutants was assessed by immunoprecipitating Mpl (top panels) and PC-PLC (bottom panels) from cleared host lysates. Numbers on the right indicate molecular mass markers (in kDa). Lanes: 1 and 2, NF-L943; 3, HEL-1004; 4, HEL-1007; 5, HEL-987; 6, HEL-469.
Table 4. Efficacy of mature Mpl secretion and PC-PLC maturation in infected cells.
| Mpl species | Relative ratio±se of Mpl mature form to propeptide secreted at acidic pH (n) | Ratio±se of PC-PLC maturation at acidic pH (n) |
| Wild-type | 0.97±0.06 (12) | 0.93±0.01 (7) |
| H226T | 0.46±0.07 (4) | 0.56 (1) |
| H226F | 0.09±0.02 (5) | 0.26±0.26 (2) |
| H226R | 0.95±0.13 (4) | 0.94±0.01 (2) |
A positive charge at residue 226 restores Mpl functions
To further understand how H226 contributes to the intracellular activity of Mpl, we substituted a hydrophobic and a charged amino acid residue for H226, generating mpl H226F and mpl H226R mutants to mimic the non-protonated and protonated states of histidine, respectively. The behaviour of Mpl and PC-PLC in cells infected with L. monocytogenes mpl mutants was analysed using the immunoprecipitation assay as described above. Mpl H226R behaved like wild-type in all aspects (Fig. 3, compare lanes 2 and 4; Table 4), but Mpl H226F did not. The propeptide of Mpl H226F mutant was detected in large amounts, indicating that autolysis was not affected (Fig. 3, compare lanes 2 and 3). However, the ratio of mature Mpl to propeptide was decreased 11-fold for this mutant, and PC-PLC maturation was reduced fourfold (Fig. 3, compare lanes 2 and 3; Table 4).
The decreased ratios of mature Mpl to propeptide for Mpl H226T and Mpl H226F suggested that, following autolysis, the catalytic domain was either degraded rapidly or not transported across the bacterial cell wall. Unfortunately, we have been unsuccessful at detecting bacterium-associated Mpl by the immunoprecipitation assay. Therefore, we performed an immunofluorescence assay with antibodies that can distinguish the mature form of Mpl from the zymogen, utilizing an Mpl species that has a Flag tag at the N terminus of the catalytic domain (FlagN-cat) (Forster et al., 2011). Monoclonal antibody M1 recognizes Flag only when it is located at the N terminus of a protein, whereas monoclonal antibody M2 recognizes Flag independent of its location within a protein. Consequently, M1 can only bind to the mature form of Mpl as Flag is present at the N terminus of the catalytic domain, whereas M2 can bind to the zymogen and mature form of Mpl. As previously reported, wild-type Mpl-FlagN-cat was detected as bacterium-associated at pH 7.3 only with the M2, and not the M1, antibody indicating that only the Mpl zymogen is bacterium-associated (Fig. 4a, panels 1–4) (Forster et al., 2011). Neither antibody could detect bacterium-associated Mpl-FlagN-cat in cells treated at acidic pH (Fig. 4a, panels 5−8), indicating that the protein had been secreted into the cytosol of host cells as previously determined using the immunoprecipitation assay (Fig. 3, lane 2). However, the sensitivity of the immunofluorescence assay is too low to detect the protein once it has diffused in the cytosol of host cells. Similarly, Mpl H226F-FlagN-cat was detected as bacterium-associated by the M2, but not the M1, antibody at pH 7.3 (Fig. 4b, panels 1−4). On the contrary, Mpl H226F-FlagN-cat was detected by both antibodies following a drop in pH. Considering that Mpl H226F is competent for autolysis (Fig. 3, lane 3), detection of the protein with the M1 antibody following a drop in pH indicates that the catalytic domain of Mpl H226F remained bacterium-associated (Fig. 4b, panels 5–8). Similar results were obtained with Mpl H226T (data not shown). The phenotype of Mpl H226R-FlagN-cat was the same as that of Mpl-FlagN-cat (Fig. 4a, c), indicating that the Mpl H226R did not remain bacterium-associated following autolysis.
Fig. 4.
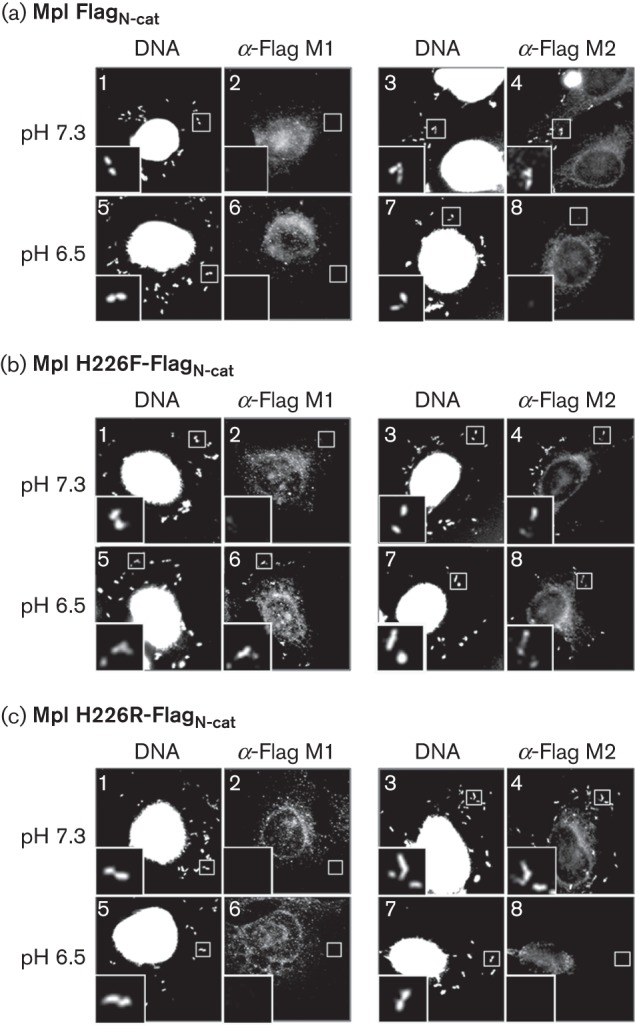
A positive charge at residue 226 is necessary for the efficient transportation of mature Mpl across the bacterial cell wall. Infected HeLa cells were incubated in a potassium-containing buffer supplemented with nigericin and equilibrated to either pH 7.3 or pH 6.5. Mpl-FlagN-cat was detected using either an α-Flag M1 antibody that detects only the mature form of Mpl, or an α-Flag M2 antibody that detects the pro- and mature forms of Mpl. Bacteria and host nuclei were detected using bisbenzimide. (a) HEL-981; (b) HEL-1195; (c) HEL-1235. 3× magnification.
Overall, these results suggested that a positive charge on the side group of H226 favours the secretion of mature Mpl across the bacterial cell wall and increases the ability of Mpl to mediate the proteolytic activation of PC-PLC.
Discussion
During its intracellular life cycle, L. monocytogenes relies on Mpl and PC-PLC to aid in its escape from double-membrane vacuoles formed upon cell-to-cell spread. Upon a decrease in vacuolar pH, Mpl undergoes autolysis and the mature form of Mpl mediates the proteolytic maturation of PC-PLC (Forster et al., 2011). Mpl maturation also leads to the transport of Mpl and PC-PLC across the cell wall (Forster et al., 2011; Yeung et al., 2005). In this study, we identified a non-catalytic histidine residue (H226) that affects Mpl functions other than autolysis and we determined that a positive charge at that location is required for transportation of the catalytic domain across the cell wall and for the ability of Mpl to mediate the proteolytic activation of PC-PLC.
Histidine 226 of Mpl localized to a random coil loop at a distance of approximately 20 Å from the opening of the Mpl catalytic pocket (Fig. 1). The protonation of Mpl H226 may promote interaction of Mpl with PC-PLC. A previous systematic study of the propeptide of PC-PLC indicated that three residues within the N terminus of the propeptide are important for Mpl to mediate PC-PLC maturation: an acidic residue at position 3 or 4 (aspartic or glutamic acid), a polar residue at position 5 (tyrosine) and a hydrophobic residue at position 6 (leucine) (Slepkov et al., 2010). The propeptide of PC-PLC comprises 24 amino acid residues. Therefore, the third amino acid residue is located 22 residues upstream of the propeptide cleavage site, suggesting that Mpl must first interact with the N terminus of the PC-PLC propeptide to align the cleavage site with its catalytic pocket. It is reasonable to speculate that PC-PLC D3 or E4 interacts with Mpl H226 to initiate the alignment of the PC-PLC cleavage site with the Mpl catalytic pocket. This type of interaction could orient the propeptide cleavage site towards the Mpl catalytic pocket, facilitating the maturation of PC-PLC. A possible model to test in the future is depicted in Fig. 5.
Fig. 5.
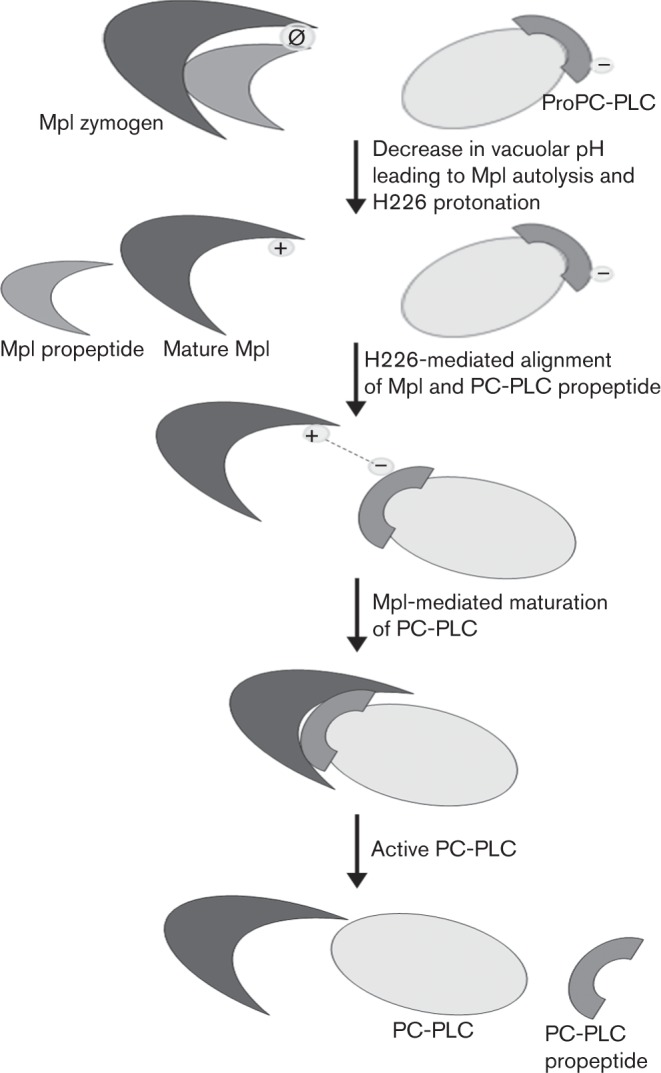
Potential model to test in the future. Prior to a decrease in pH, Mpl H226 is unlikely to be protonated (  ). Upon a decrease in pH, both Mpl autolysis and protonation of H226 occur (+). Protonated H226 orients Mpl’s catalytic pocket towards PC-PLC’s propeptide cleavage site, resulting in the processing of PC-PLC.
). Upon a decrease in pH, both Mpl autolysis and protonation of H226 occur (+). Protonated H226 orients Mpl’s catalytic pocket towards PC-PLC’s propeptide cleavage site, resulting in the processing of PC-PLC.
In conclusion, our data indicate that a positive charge at position 226 of Mpl promotes efficient processing of PC-PLC and secretion of Mpl mature form across the bacterial cell wall. Future experiments will be designed to assess whether Mpl catalytic domain residue H226 interacts with PC-PLC propeptide D3 or E4 residue to initiate the Mpl-mediated proteolytic activation of PC-PLC.
Acknowledgements
This work was supported by NIAID Public Health Service grant A152154 (H. M.). The authors have no conflict of interest concerning the work reported in this manuscript.
Abbreviations:
- SOEing PCR
site-directed mutagenesis with overlap extension PCR
References
- Arnold K., Bordoli L., Kopp J., Schwede T. (2006). The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201. 10.1093/bioinformatics/bti770 [DOI] [PubMed] [Google Scholar]
- Berezin C., Glaser F., Rosenberg J., Paz I., Pupko T., Fariselli P., Casadio R., Ben-Tal N. (2004). ConSeq: the identification of functionally and structurally important residues in protein sequences. Bioinformatics 20, 1322–1324. 10.1093/bioinformatics/bth070 [DOI] [PubMed] [Google Scholar]
- Bitar A. P., Cao M., Marquis H. (2008). The metalloprotease of Listeria monocytogenes is activated by intramolecular autocatalysis. J Bacteriol 190, 107–111. 10.1128/JB.00852-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli A., Tilney L. G., Portnoy D. A. (1993). Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol 8, 143–157. 10.1111/j.1365-2958.1993.tb01211.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J. E., Seckute J., De S., Schueler S. A., Oswald A. B., Nicholson L. K. (2009). Elucidation of a pH-folding switch in the Pseudomonas syringae effector protein AvrPto. Proc Natl Acad Sci U S A 106, 8543–8548. 10.1073/pnas.0809138106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciangeli S. F., Thomas L., Scott G. K., Subbian E., Hung C. H., Molloy S. S., Jean F., Shinde U., Thomas G. (2006). Identification of a pH sensor in the furin propeptide that regulates enzyme activation. J Biol Chem 281, 16108–16116. 10.1074/jbc.M600760200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster B. M., Marquis H. (2012). Protein transport across the cell wall of monoderm Gram-positive bacteria. Mol Microbiol 84, 405–413. 10.1111/j.1365-2958.2012.08040.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster B. M., Bitar A. P., Slepkov E. R., Kota K. J., Sondermann H., Marquis H. (2011). The metalloprotease of Listeria monocytogenes is regulated by pH. J Bacteriol 193, 5090–5097. 10.1128/JB.05134-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz R., Stiasny K., Heinz F. X. (2008). Identification of specific histidines as pH sensors in flavivirus membrane fusion. J Cell Biol 183, 353–361. 10.1083/jcb.200806081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N., Peitsch M. C. (1997). SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723. 10.1002/elps.1150181505 [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989). Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59. 10.1016/0378-1119(89)90358-2 [DOI] [PubMed] [Google Scholar]
- Li X., Hassan S. A., Mehler E. L. (2005). Long dynamics simulations of proteins using atomistic force fields and a continuum representation of solvent effects: calculation of structural and dynamic properties. Proteins 60, 464–484. 10.1002/prot.20470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loessner M. J., Schneider A., Scherer S. (1996). Modified Listeria bacteriophage lysin genes (ply) allow efficient overexpression and one-step purification of biochemically active fusion proteins. Appl Environ Microbiol 62, 3057–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis H., Hager E. J. (2000). pH-Regulated activation and release of a bacteria-associated phospholipase C during intracellular infection by Listeria monocytogenes. Mol Microbiol 35, 289–298. 10.1046/j.1365-2958.2000.01708.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengaud J., Geoffroy C., Cossart P. (1991). Identification of a new operon involved in Listeria monocytogenes virulence: its first gene encodes a protein homologous to bacterial metalloproteases. Infect Immun 59, 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi S., Shinoda S. (2000). Microbial metalloproteases and pathogenesis. Microbes Infect 2, 91–98. 10.1016/S1286-4579(00)00280-X [DOI] [PubMed] [Google Scholar]
- Raveneau J., Geoffroy C., Beretti J.-L., Gaillard J.-L., Alouf J. E., Berche P. (1992). Reduced virulence of a Listeria monocytogenes phospholipase-deficient mutant obtained by transposon insertion into the zinc metalloprotease gene. Infect Immun 60, 916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerch D. W., Wilson-Kubalek E. M., Tweten R. K. (2005). Molecular basis of listeriolysin O pH dependence. Proc Natl Acad Sci U S A 102, 12537–12542. 10.1073/pnas.0500558102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwede T., Kopp J., Guex N., Peitsch M. C. (2003). SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31, 3381–3385. 10.1093/nar/gkg520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetron-Rama L. M., Mueller K., Bravo J. M., Bouwer H. G., Way S. S., Freitag N. E. (2003). Isolation of Listeria monocytogenes mutants with high-level in vitro expression of host cytosol-induced gene products. Mol Microbiol 48, 1537–1551. 10.1046/j.1365-2958.2003.03534.x [DOI] [PubMed] [Google Scholar]
- Slepkov E. R., Pavinski Bitar A., Marquis H. (2010). Differentiation of propeptide residues regulating the compartmentalization, maturation and activity of the broad-range phospholipase C of Listeria monocytogenes. Biochem J 432, 557–563. 10.1042/BJ20100557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K., Youngman P. (1992). Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie 74, 705–711. 10.1016/0300-9084(92)90143-3 [DOI] [PubMed] [Google Scholar]
- Smith G. A., Marquis H., Jones S., Johnston N. C., Portnoy D. A., Goldfine H. (1995). The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun 63, 4231–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A., Marquis H. (2003). Restricted translocation across the cell wall regulates secretion of the broad-range phospholipase C of Listeria monocytogenes. J Bacteriol 185, 5953–5958. 10.1128/JB.185.20.5953-5958.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava J., Barber D. L., Jacobson M. P. (2007). Intracellular pH sensors: design principles and functional significance. Physiology (Bethesda) 22, 30–39. 10.1152/physiol.00035.2006 [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. (1989). Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol 109, 1597–1608. 10.1083/jcb.109.4.1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung P. S., Zagorski N., Marquis H. (2005). The metalloprotease of Listeria monocytogenes controls cell wall translocation of the broad-range phospholipase C. J Bacteriol 187, 2601–2608. 10.1128/JB.187.8.2601-2608.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]