Abstract
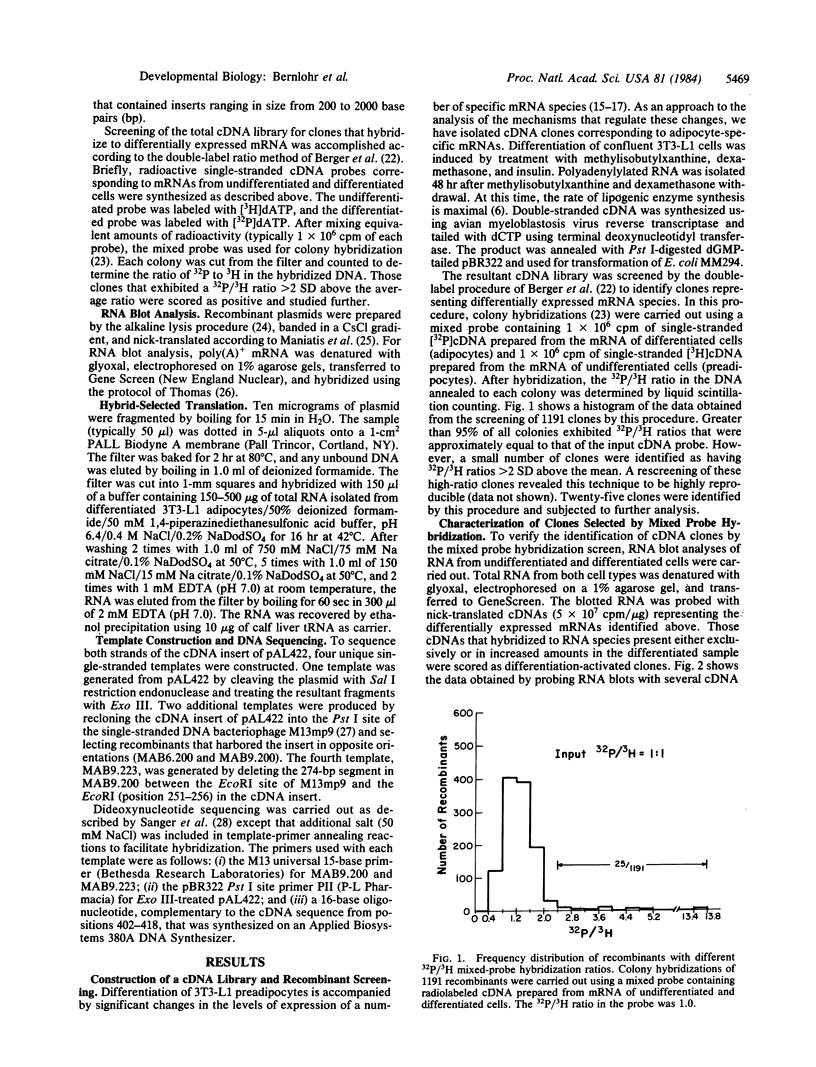
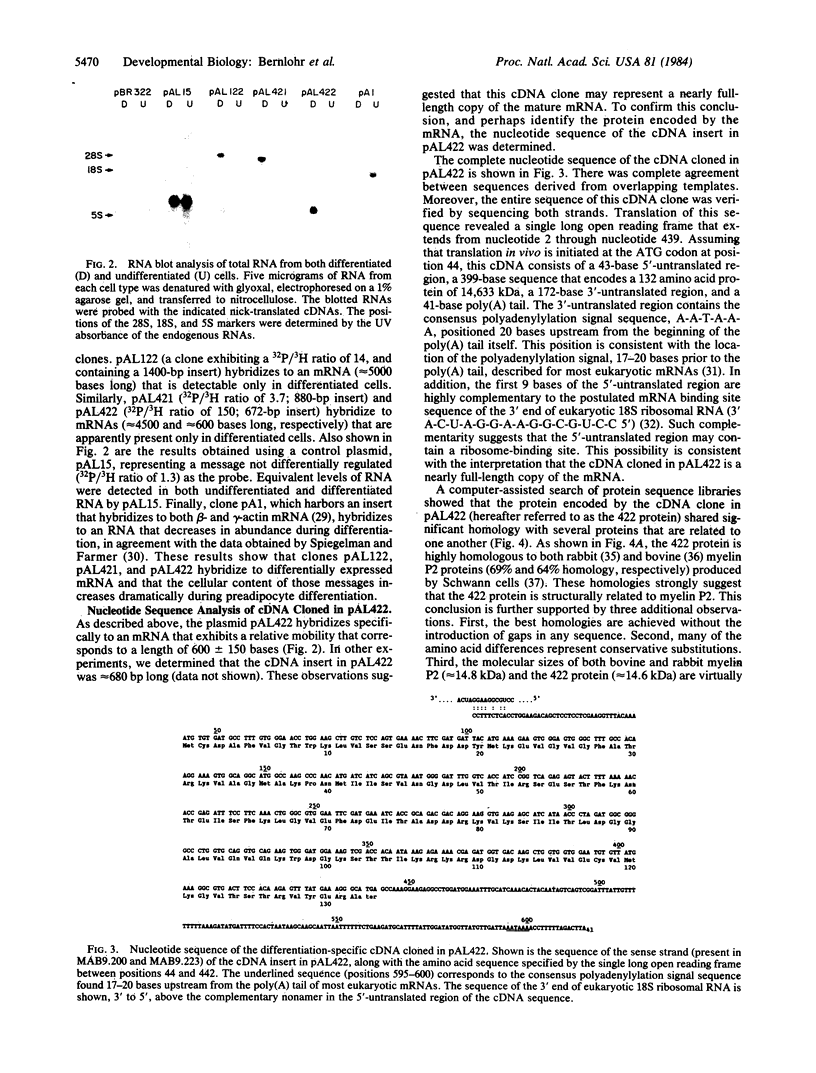
To identify and characterize specific mRNAs that increase in abundance during differentiation of mouse 3T3-L1 preadipocytes, a cDNA library was constructed from poly(A)+RNA isolated from differentiated 3T3-L1 adipocytes. Mixed probe isotope ratio selection and RNA blot analyses have identified several unique cDNA clones that represent mRNA species expressed either exclusively or at dramatically increased levels in differentiated cells. Further characterization of one such clone (pAL422) revealed that the corresponding mRNA, detectable only after differentiation, is approximately the same length (600 +/- 150 bases) as the cDNA insert (672 bases). The complete nucleotide sequence of the cDNA insert in pAL422 revealed a single long open reading frame that encodes a 132 amino acid polypeptide (the 422 protein) of 14.6 kDa. These and other results suggest that this cDNA may represent a nearly full-length copy of the mRNA. Computer-assisted analyses showed that the 422 protein shares 69% and 64% homology with myelin P2 proteins from rabbit and bovine peripheral nerves, respectively, as well as 23% and 30% homology with fatty-acid binding proteins from rat liver and intestine, respectively. Moreover, the mRNA hybrid selected by pAL422 DNA directs the in vitro translation of an approximately equal to 13 kDa polypeptide, and this protein is specifically immunoprecipitated by antiserum against bovine myelin P2. These observations strongly suggest that the 422 protein is a structural, and possibly functional, analog of myelin P2.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Strauss A. W., Ockner R. K., Bass N. M., Gordon J. I. Cloning of a cDNA encoding rat intestinal fatty acid binding protein. Proc Natl Acad Sci U S A. 1984 Jan;81(2):313–317. doi: 10.1073/pnas.81.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus C. W., Lane M. D., Rosenfeld P. J., Kelly T. J. Increase in translatable mRNA for mitochondrial pyruvate carboxylase during differentiation of 3T3 preadipocytes. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1216–1222. doi: 10.1016/0006-291x(81)90252-7. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F. G., Gross K. W., Watson G. Isolation and characterization of a DNA sequence complementary to an androgen-inducible messenger RNA from mouse kidney. J Biol Chem. 1981 Jul 10;256(13):7006–7013. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brostoff S. W., Sacks H., DiPaola C. The P2 protein of bovine root myelin: partial chemical characterization. J Neurochem. 1975 Feb;24(2):289–294. doi: 10.1111/j.1471-4159.1975.tb11878.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Reed B. C., Mackall J. C., Student A. K., Lane M. D., Bell R. M. Selective changes in microsomal enzymes of triacylglycerol phosphatidylcholine, and phosphatidylethanolamine biosynthesis during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1978 Oct 25;253(20):7256–7261. [PubMed] [Google Scholar]
- Conneely O. M., Headon D. R., Olson C. D., Ungar F., Dempsey M. E. Intramitochondrial movement of adrenal sterol carrier protein with cholesterol in response to corticotropin. Proc Natl Acad Sci U S A. 1984 May;81(10):2970–2974. doi: 10.1073/pnas.81.10.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Goad W. B., Kanehisa M. I. Pattern recognition in nucleic acid sequences. I. A general method for finding local homologies and symmetries. Nucleic Acids Res. 1982 Jan 11;10(1):247–263. doi: 10.1093/nar/10.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. I., Alpers D. H., Ockner R. K., Strauss A. W. The nucleotide sequence of rat liver fatty acid binding protein mRNA. J Biol Chem. 1983 Mar 10;258(5):3356–3363. [PubMed] [Google Scholar]
- Green H., Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974 Oct;3(2):127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Ishaque A., Hofmann T., Eylar E. H. The complete amino acid sequence of the rabbit P2 protein. J Biol Chem. 1982 Jan 25;257(2):592–595. [PubMed] [Google Scholar]
- Karlsson F. A., Grunfeld C., Kahn C. R., Roth J. Regulation of insulin receptors and insulin responsiveness in 3T3-L1 fatty fibroblasts. Endocrinology. 1979 May;104(5):1383–1392. doi: 10.1210/endo-104-5-1383. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Jensen D. F., Wancewicz E. V., Joy L. L., Khoo J. C., Steinberg D. Hormone-sensitive lipase in differentiated 3T3-L1 cells and its activation by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):732–736. doi: 10.1073/pnas.78.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Suzuki M., Suzuki A., Uyemura K. The complete amino acid sequence of the P2 protein in bovine peripheral nerve myelin. FEBS Lett. 1980 Jun 16;115(1):27–30. doi: 10.1016/0014-5793(80)80719-8. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall J. C., Lane M. D. Role of pyruvate carboxylase in fatty acid synthesis: alterations during preadipocyte differentiation. Biochem Biophys Res Commun. 1977 Dec 7;79(3):720–725. doi: 10.1016/0006-291x(77)91171-8. [DOI] [PubMed] [Google Scholar]
- Mackall J. C., Student A. K., Polakis S. E., Lane M. D. Induction of lipogenesis during differentiation in a "preadipocyte" cell line. J Biol Chem. 1976 Oct 25;251(20):6462–6464. [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Rosen O. M., Rubin C. S. Organelle relationships in cultured 3T3-L1 preadipocytes. J Cell Biol. 1980 Oct;87(1):180–196. doi: 10.1083/jcb.87.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Reed B. C., Lane M. D. Insulin receptor synthesis and turnover in differentiating 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):285–289. doi: 10.1073/pnas.77.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Lai E., Rosen O. M. Acquisition of increased hormone sensitivity during in vitro adipocyte development. J Biol Chem. 1977 May 25;252(10):3554–3557. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman B. M., Farmer S. R. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982 May;29(1):53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Farmer S. R. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982 May;29(1):53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Spooner P. M., Chernick S. S., Garrison M. M., Scow R. O. Insulin regulation of lipoprotein lipase activity and release in 3T3-L1 adipocytes. Separation and dependence of hormonal effects on hexose metabolism and synthesis of RNA and protein. J Biol Chem. 1979 Oct 25;254(20):10021–10029. [PubMed] [Google Scholar]
- Student A. K., Hsu R. Y., Lane M. D. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980 May 25;255(10):4745–4750. [PubMed] [Google Scholar]
- Takahashi K., Odani S., Ono T. A close structural relationship of rat liver Z-protein to cellular retinoid binding proteins and peripheral nerve myelin P2 protein. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1099–1105. doi: 10.1016/0006-291x(82)91225-6. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Odani S., Ono T. Primary structure of rat liver Z-protein. A low-Mr cytosol protein that binds sterols, fatty acids and other small molecules. FEBS Lett. 1982 Apr 5;140(1):63–66. doi: 10.1016/0014-5793(82)80521-8. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Trapp B. D., McIntyre L. J., Quarles R. H., Sternberger N. H., Webster H. D. Immunocytochemical localization of rat peripheral nervous system myelin proteins: P2 protein is not a component of all peripheral nervous system myelin sheaths. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3552–3556. doi: 10.1073/pnas.76.7.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G. H., Rosen O. M., Rubin C. S. Regulation of fatty acid synthetase concentration and activity during adipocyte differentiation. Studies on 3T3-L1 cells. J Biol Chem. 1980 May 25;255(10):4751–4757. [PubMed] [Google Scholar]
- Wise L. S., Green H. Studies of lipoprotein lipase during the adipose conversion of 3T3 cells. Cell. 1978 Feb;13(2):233–242. doi: 10.1016/0092-8674(78)90192-7. [DOI] [PubMed] [Google Scholar]




