Abstract
Objective
Because few cases of bone metastases of endometrial cancer have been reported, and information is scarce on their incidence, treatment, prognosis, and outcomes, we sought to compile a series of bone metastases of endometrial cancer and to systematically review the medical literature.
Methods
We retrospectively reviewed medical records of patients who had osseous metastases of endometrial cancer treated initially at Mayo Clinic (1984–2001), and of all patients who were referred for treatment of primary bone metastases after primary treatment for endometrial cancer elsewhere.
Results
Of 1632 patients with endometrial cancer, 13 (0.8%) had primary bone dissemination and 6 (0.4%) were referred after initial treatment. Three (15.8%) of these 19 had bone metastases at presentation; in the rest, median time to recurrence was 19.5 months (range, 3–114). The most common sites were the spine and hip. Median survival after metastasis was 12 months (range, 2–267). Median survival after radiotherapy alone vs. multi-modal treatment was 20 months (range, 12–119) vs. 33 months (range, 9–267), respectively (P > .99). Of the 87 caseswe reviewed from the literature, all but 1 (98.9%) had diagnoses based on symptoms. Multiple bone involvement and extraosseous dissemination were associated with poor prognosis. Type II endometrial cancer (i.e., serous or clear-cell histology) was associated with shorter life expectancy after diagnosis of bone metastasis compared to Type I tumors.
Conclusions
The incidence of primary bone metastases of endometrial cancer is <1%. Single bone metastases without extraosseous spread indicate less aggressive disease. Optimal treatment is unclear.
Keywords: Bone, Endometrial neoplasms, Neoplasm metastases, Osseous, Prognosis
Introduction
Endometrial cancer (EC) is the most common malignancy of the female genital tract in the US. Its estimated impact during 2011 was 46,470 newly diagnosed cases and 8120 deaths in the US alone [1].
In most cases, EC is confined at initial diagnosis to the uterus [2]. Nevertheless, nearly 1 in 3 women who die of EC is considered to have localized disease at the time of primary treatment [3]. There are 4 potential routes of dissemination in epithelial corpus cancer: 1) contiguous, 2) hematogenous, 3) lymphatic, and 4) exfoliation followed by intraperitoneal spread [4]. Most hematogenous failures occur in the lung or liver [5].
Bone metastases with EC are infrequent; their real incidence is unknown. Whereas anatomopathologic studies, including those of sub-clinical metastases detected only at autopsy, have an incidence as high as 25% [6], reports of only a few cases of bone metastases have been published [7–51]. In the largest series, Kehoe et al. [52] reported on 21 women with osseous dissemination. However, they made no distinction between bone metastases that were the first site of disease recurrence and those that were subsequent sites, and they provided no information on incidence and factors possibly associated with prognosis.
To estimate the real incidence and evaluate clinical outcomes, we reviewed and analyzed primary bone metastases (discovered either upon EC diagnosis or upon location of the primary site of recurrence) of patients treated at Mayo Clinic. We also conducted a comprehensive review of all available published reports on EC.
Materials and methods
A total of 1632 patients with EC were managed at Mayo Clinic, Rochester, Minnesota, between 1984 and 2001. Staging was defined according to the 1988 staging system of FIGO (Fédération Internationale de Gynécologie et d'Obstétrique [International Federation of Gynecology and Obstetrics]) [53]. Histologic classification was conducted according to that of the World Health Organization [54]. Architectural grading (i.e., the degree of glandular differentiation) was based on FIGO guidelines. Descriptions of tumor characteristics were abstracted from original pathology reports. A pathologist (G.L.K.) retrospectively reviewed all pathology slides (hematoxylin–eosin stain) of primary tumors to confirm original diagnoses (FIGO grade and histologic subtype).
Bone failure consisted of any case of EC metastatic to bone either at presentation with EC or as the primary site of recurrence (alone or in combination with other sites). Bone failure was diagnosed on the basis of clinical, radiographic, surgical, or histologic information in the medical record.
We separately considered patients who received primary treatment for EC elsewhere between 1984 and 2001, and who were referred to Mayo Clinic for treatment of primary bone metastases (as defined above). The referred cases were added to the series of patients who had initial treatment at Mayo Clinic.
All cases were reviewed by a radiologist (J.M.M.) to confirm the diagnosis of bone metastases made at imaging, when pathologic specimens were not available. Bone recurrences were then categorized as either having concomitant hematogenous, lymphatic, peritoneal, or vaginal sites of recurrence, or as consisting of isolated recurrence in 1 or more bones. Bone failures were categorized as having either single or multiple bone localizations. Patients with uterine sarcomas or carcinosarcomas were excluded.
At the discretion of the oncologist, patients with bone failure were treated selectively with radiotherapy or surgery to excise metastases; chemotherapy; hormonal therapy; or a combination.
At follow-up, information was abstracted from the clinical histories of patients. If survival and recurrence were insufficiently detailed, death certificates were obtained and patients and family physicians were contacted by letter or telephone for additional follow-up. Patients were censored if alive (with or without disease) at follow-up or if dead from an unrelated cause. For statistical analysis, we divided patients on the basis of tumor histology, comparing Type I endometrial cancer (defined as endometrioid cancer, endometrioid cancer with squamous differentiation, and adenosquamous cancer) with Type II endometrial cancer (defined as tumors with serous or clear-cell histology). Data on patients with grade 1 and grade 2 lesions were combined for comparison with data on patients with grade 3 lesions.
A systematic literature review was performed by searching the PubMed database for reports published between January 1, 1950, and May 31, 2011, using the terms “bone metast*” and “endometrial cancer”; “bone relapse” and “endometrial cancer”; “osseous dissemination” and “endometrial cancer”; or any combination thereof. We reviewed all publications identified in this search and selected those consisting of clinical case reports (including letters or abstracts) or case series that described patients affected by bone metastases of EC. A manual search of the references in each selected article was performed to identify additional reports of studies not captured by the online search that were potentially relevant for review. Only papers published in English, French, or Italian were considered. Abstracts presented at meetings were reviewed only if also published in indexed journals.
Statistical analysis was performed using the Fisher exact test (to evaluate the association between pairs of categorical variables), the Mann–Whitney U test (to test for differences between groups in the distribution of continuous measures), the Kaplan–Meier product-limit method (to determine survival curves), and the log-rank test (to identify predictors of disease-related survival). Statistically significant difference was defined as P < .05. For analysis, JMP statistical software (version 4.0.4; SAS Institute, Inc.) was used.
Results
Primary bone dissemination developed in 13 (0.8%) of the 1632 patients managed at Mayo Clinic for EC during the study period. Six other patients were referred to Mayo Clinic after receiving initial treatment elsewhere. Therefore, a total of 19 patients were included in the study, with a total of 29 identified sites of osseous metastases (the maximum number of bone metastases identified in a single patient was 4). The overall characteristics of patients are summarized in Table 1, and are described in detail in Table 2.
Table 1.
Overall characteristics of 19 patients with bone metastases of endometrial cancer treated at Mayo Clinic.
| Characteristic | No. (%)a |
|---|---|
| Age, median (range), years | 65 (47–80) |
| Body mass index, median (range) | 31 (17–43) |
| Histology | |
| Endometrioid | 13 (68.4) |
| Nonendometrioid | 6 (31.6) |
| Cancer stage | |
| I | 10 (52.6) |
| II | 1 (5.3) |
| III | 3 (15.8) |
| IV | 5 (26.3) |
| Estrogen and progesterone receptor on primary tumorb | |
| Positive | 10 (52.6) |
| Negative | 2 (10.5) |
| Missing data | 7 (36.8) |
| Diagnosis at presentation of endometrial cancer | 3 (15.8) |
| Time to bone recurrence (if diagnosis not made at presentation), median (range), months | 19.5 (3–114) |
| Involvement of multiple bones | 6 (31.6) |
| Concomitant extraosseous metastases | 9 (47.4) |
| Patients with single bone involvement and no extraosseous spread | 9 (47.4) |
| Overall survival, median (range), months | 12 (2–267) |
| Missing follow-up data | 2 (10.5) |
Values are number (percentage) unless indicated otherwise.
Percentages total < 100% due to rounding.
Table 2.
Individual characteristics of 19 patients with primary bone metastases of endometrial cancer treated at Mayo Clinicaa.
| Pt. no. | Age | Histology, grade, stageb | Symptoms at presentation | Interval to bone met | No. of bone met | Side | Localization | Extraosseous metc | Therapy | Status | Survival after bone met |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 years | ADK, G2, IVB | Pain | 19 months | 1 | R | Sternum (R margin) | No | Surgery, HT | DOD | 60 months |
| 2 | 65 years | ADK, G2, NA | Lack of strength and sensatior | l 8 months | 1 | Median | T5 | No | Surgery, RT, HT | DOD | 9 months |
| 3 | 73 years | ADK, G3, IIB | Pain | 4 months | 2 | R | Ischium, acetabulum | Lung | HT | DOD | 28 months |
| 4 | 71 years | ADK, G3, IC | Fracture | 24 months | 1 | R | Tibia | No | RT, HT | DOD | 25 months |
| 5 | 66 years | Serous, G3, IIIC | Pain, inflammation | 18 months | 2 | Median | T12, sternum | Brain, lung | Bisphosphonates | DOD | 6 months |
| 6 | 52 years | ADK, G3, IVB | Pain | 7 months | 2 | R | Humerus, clavicle | Brain, cervical lymph node | RT, CHT | DOD | 3 months |
| 7 | 71 years | ADK, G3, IC | Pain | 3 months | 1 | Median | Sacrum | Abdomen | RT | DOD | 6 months |
| 8 | 69 years | ADK, G3, IB | Pain | 49 months | 1 | Median | Sacrum | Lung | HT | DOD | 31 months |
| 9 | 62 years | ADK, G3, IIIC | Pain | 14 months | 4 | Bilateral | Skull, T4, T11, sacrum | Para-aortic nodes | RT | DOD | 6 months |
| 10 | 62 years | ADK, G2, IB | Pain, limp | 20 months | 1 | L | Sacrum (sacroiliac joint) | No | RT, HT | DOD | 11 months |
| 11 | 70 years | ADK, G2, IB (adenosquamous) | Pain | 20 months | 4 | L | Clavicle, ribs, T9, L3 | Lung | RT | DOD | 5 months |
| 12 | 59 years | ADK, G1, IC | Pain | 13 months | 1 | Median | T10 | No | RT | NED | 119 months |
| 13 | 64 years | ADK, G2, IVB | Pain | At dx | 1 | R | Hip | No | RT, HT | NED | 267 months |
| 14 | 80 years | ADK, G3, IVB | Pain | Atdx | 1 | L | Sacrum (sacroiliac joint) | Widespread | HT | DOD | 2 months |
| 15 | 70 years | ADK, G3, IC | Pain | 56 months | 1 | R | Femur | No | RT | DOD | 26 months |
| 16 | 60 years | Serous, G3, IB | Pain | 34 months | 2 | L | Tibia, L3 | No | RT | DOD | 14 months |
| 17 | 64 years | Serous, G2, IC | Pain, swelling | 68 months | 1 | R | Calcaneus | No | RT | DOD | 12 months |
| 18 | 73 years | ADK, G3, IC | Pain | 114 months | 1 | R | Femur | Lung | Surgery, RT, HT | DOC | 8 months |
| 19 | 47 years | ADK, G3, IVB (adenosquamous) | Pain | At dx | 1 | L | Hip | No | CHT, RT, HT | NED | 98 months |
Abbreviations: ADK, adenocarcinoma; CHT, chemotherapy; DOC, death by other causes; DOD, death by disease; dx, diagnosis; HT, hormone therapy; L, left; met, metastases; NED, no evidence of disease; Pt., patient; R, right; and RT, radiotherapy.
For this summary table, we used the 1988 FIGO (Federation Internationale de Gynecologie et d'Obstetrique [International Federation of Gynecology and Obstetrics]) staging.
For this summary table, we used the 1988 FIGO (Fédération Internationale de Gynécologie et d'Obstétrique [International Federation of Gynecology and Obstetrics]) staging.
At the time of diagnosis of bone metastasis.
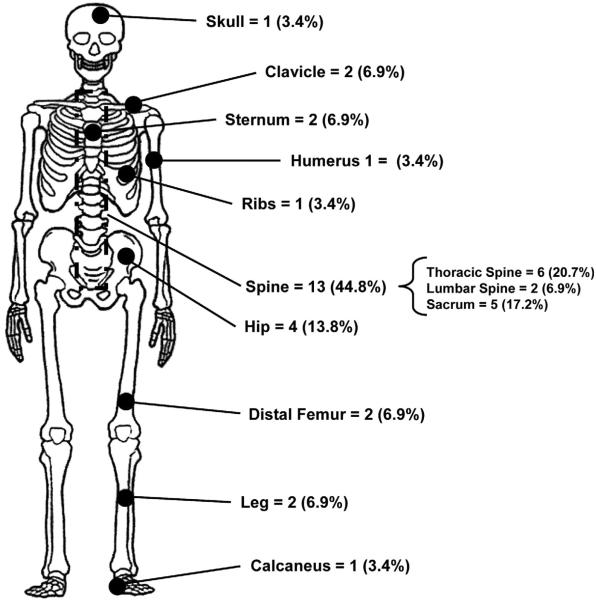
In 3 (15.8%) of the 19 patients, the diagnosis of bone metastases was made upon presentation with EC; 1 of these 3 patients was referred to Mayo Clinic after initial diagnosis of primary EC metastatic to bone. In the remaining 16 (84.2%) patients, the median time to recurrence was 19.5 months (range, 3–114 months). The diagnosis of bone metastasis was made more than 4 years after initial diagnosis of EC in 4 patients, 2 of whom had bone metastasis diagnosed more than 5 years later. The most common sites were the spine (13/29 sites [44.8%]) and the hip (4/29 sites [13.8%]). The sites of osseous metastatic localization are shown in Fig. 1. In 8 (42.1%) of the 19 patients, the metastases were on the right side, in 5 (26.3%) they were on the left, in 5 (26.3%) they were median, and in 1 (5.3%) they were bilateral, with no clear side prevalence.
Fig. 1.
Sites of bone metastases in 19 patients with endometrial cancer. Twenty-nine osseous sites of metastasis were identified in 19 patients. Sites are shown independent of right/left location to avoid misinterpretation.
All patients were symptomatic, and their symptoms warranted further clinical and radiologic assessment to rule out bone metastases. There were no cases of accidental diagnosis; pain at the site of osseous involvement was present in all 19 patients.
The median diameter of osseous lesions was 5 cm (range, 4–8 cm).
In 10 (52.6%) patients, histologic examination of the osseous lesion was consistent with endometrial primary tumor; in 3 (15.8%), cytologic sampling was positive for cells of adenocarcinoma compatible with cancer of the corpus uteri; and in 1 (5.3%), autopsy confirmed the diagnosis of EC metastatic to the bone after suspicion was raised by imaging studies. In the remaining 5 patients, diagnosis was based on radiologic findings. These cases were reviewed and confirmed by the radiologist.
The presence of extraosseous dissemination at diagnosis of bone metastases was detected in 9 (47.4%) patients, whereas multiple bone metastases were discovered in 6 (31.6%). Nine (47.4%) of the 19 patients had a single bone metastasis and no extraosseous spread, 4 (21.1%) had a single bone metastasis and presence of extraosseous dissemination, 1 (5.3%) had multiple (i.e., >2) bone metastases and no extraosseous dissemination, and 5 (26.3%) had both multiple skeletal metastases and extraosseous localizations.
Median survival after bone metastases was 12 months (range, 2–267 months). The presence of a single bone metastasis and no extraosseous dissemination was significantly associated with longer survival (26 vs. 6 months; P = .008) (Table 3). Overall survival was 18 months (range, 1–267 months) vs. 12 months (range, 6–14 months) for Type I vs. Type II endometrial cancer, respectively (P = .70). Of 10 patients without extraosseous spread, 9 had radiotherapy (alone or in combination); among these women, median survival for radiotherapy alone was 20 months (range, 12–119 months) vs. 33 months (range, 9–267 months) for multimodal treatment (P > .99). Surgical resection was attempted in 3 women, 2 of whom had no extraosseous spread. Complete surgical excision was obtained in 1 of these 2, with subsequent hormonal treatment with megestrol; this woman survived 41 months after surgical treatment before dying of recurrent bone disease in the proximity of the osseous excision.
Table 3.
Prognostic factors for survival after diagnosis of bone metastases of endometrial cancer in 19 patients treated at Mayo Clinic.
| Characteristic | Survival, median (range), monthsa,b | P value |
|---|---|---|
| Multiple bone involvement | ||
| Yes (n = 13) | 6 (2–31) | |
| No (n = 6) | 25.5 (9–267) | .006 |
| Extraosseous disease | ||
| Yes (n = 9) | 6 (3–28) | |
| No (n = 10) | 25 (2–267) | .087 |
| Single bone involvement and no extraosseous disease | ||
| Yes (n = 9) | 26 (9–267) | |
| No (n = 10) | 6 (2–31) | .008 |
Values are median (range) unless indicated otherwise.
Estrogen or progesterone receptor status and age at diagnosis were not associated with survival.
Of the 8 (42.1%) patients who survived more than 2 years, all had Type I tumors; in particular, 7 (87.5%) had endometrioid EC, and 1 (12.5%) had adenosquamous histologic findings. In 5 (62.5%) of these 8, EC at initial diagnosis was poorly differentiated. Two (25%) of the 8 had bone metastases at presentation with EC (in both cases, bone metastases were single and without extraosseous spread). They survived 267 and 98 months, respectively. Six (75%) of these 8 patients received hormonal treatment with megestrol, either as the only treatment or in combination with surgery and/or radiotherapy and/or chemotherapy. The 3 women who survived more than 4 years after diagnosis of bone metastasis all received both radiotherapy and hormonal therapy.
Our review of the medical literature identified 46 studies [7–52] describing a total of 68 cases of EC metastatic to bone. Case reports with a maximum of 2 patients per study [7–51] are summarized in Table 4, whereas Table 5 summarizes the 21 cases included in the study by Kehoe et al. [52]. With the addition of our 19 cases, a total of 87 women were affected by osseous dissemination of EC. In 62 patients (43 in the literature and 19 from the present report), bone metastases were the primary site of relapse; in 4 patients, osseous dissemination occurred secondarily, after primary dissemination at another anatomical site; and in 21 patients (all in the same report) [52], bone metastases were not specified as primary or secondary. Of the 87 women whose cases we reviewed, 35 (40.2%) had metastases to the axial skeleton, 37 (42.5%) to the limbs, 6 (6.9%) to the cranium, and the rest to a mixture of these sites. In only 1 (1.1%) case was bone metastasis diagnosed in the absence of symptoms at the site of metastasis (a sacral metastasis was discovered at a routine follow-up computed tomography scan 37 months after primary surgery and diagnosis was confirmed during computed tomography-guided biopsy). Twenty-eight women (32.2%) had diagnosis of bone metastases at initial presentation with EC. They survived longer than patients who received a diagnosis of bone metastases sometime after primary treatment for EC (median duration, 20 vs. 10.5 months; range, 2–267 vs. 1–199 months) (P = .04). This difference was also maintained when analysis was restricted to only those patients from studies describing only primary bone metastases (i.e., excluding the studies by Rocha et al. [48], Sahinler et al. [49], Amiot et al. [50], Oaknin et al. [51], and Kehoe et al. [52]): 24 months (range, 2–267 months) vs. 12 months (range, 1–119 months) (P = .05). Among patients with a diagnosis of bone metastases after primary treatment of EC, the median time between the onset of EC and the occurrence of osseous dissemination was 18 months. When osseous dissemination was diagnosed after primary treatment of EC, time to recurrence did not affect survival.
Table 4.
Characteristics of 47 patients with primary or secondary bone metastases of endometrial cancer reported in case reports.a
| Author, year | Pt. no. | Age | Histology, grade, stageb | Symptoms at presentation | Interval to bone met | Localization | Extraosseous metc | Therapy | Status | Survival after bone met |
|---|---|---|---|---|---|---|---|---|---|---|
| Primary bone metastases | ||||||||||
| Ravault et al. [7] | 1 | 61 years | NA, NA, NA | Pain | 36 months | R tarsus | No | Surgery, RT | NED | 7 months |
| Rouchy et al. [8] | 2 | 61 years | ADK, G1, IVB | NA | At dx | L fibula | No | NA | NA | NA |
| Vanecko et al. [9] | 3 | 67 years | NA, NA, I | Pain, limp | 17 months | R fibula | No | RT | NED | 12 months |
| 4 | 54 years | NA, NA, IVB | Pain | At dx | L fibula | No | RT | DOD | 30 months | |
| Janis and Feldman [10] | 5 | 81 years | ADK, NA, II | Pain | 36 months | L calcaneus | No | RT | NA | NA |
| Beller et al. [11] | 6 | 59 years | ADK, G2, IC | Pain, inflammation | 9 months | L femur | No | RT, CHT, HT | NA | NA |
| Zorzi and Pescatori [12] | 7 | 80 years | ADK, G2, NA | Pain | 10 months | R tarsus | No | Surgery | NA | NA |
| Onuba [13] | 8 | 57 years | ADK, NA, IVB | Pain, fracture | Atdx | R tibia | Lung, kidney | NA | NA | 13 months |
| Litton et al. [14] | 9 | 55 years | ADK, G2, IB | Pain | 24 months | R calcaneus | No | RT | NED | 10 months |
| Maxymiw and Wood [15] | 10 | 63 years | ADK, G3, IVA | Swelling | 4 months | R mandible | No | RT | DOD | 5 months |
| Cooper et al. [16] | 11 | 59 years | ADK, G2, IVB (adenoacanthoma) | Pain, fracture | At dx | R calcaneus | No | RT, CHT, HT | NED | 60 months |
| Nishida et al. [17] | 12 | 61 years | ADK, G1, IIIB | Pain | 2 months | L calcaneus | No | NA | NA | NA |
| Petru et al. [18] | 13 | 61 years | ADK, G1, IVB | Pain, swelling | At dx | L tarsus | No | Surgery, CHT, HT | NED | 10 months |
| Schols et al. [19] | 14 | 66 years | ADK, G3, IA | Pain | 18 months | R humerus | No | RT, HT | NED | 24 months |
| Clarke and Smith [20] | 15 | 55 years | ADK, NA, NA | Pain, swelling | 18 months | R talus, calcaneus | Lung | Surgery, RT | DOD | 36 months |
| Armentano et al. [21] | 16 | 74 years | ADK, NA, IA | Pain, swelling | 144 months | L tibia | No | None | DOD | 1 monthd |
| Malicky et al. [22] | 17 | 44 years | ADK, G2, IVB | Pain | At dx | L femur | No | RT, CHT, HT | NED | 24 months |
| Scott et al. [23] | 18 | 50 years | ADK, G3, IIIC | Pain, mucocele | 3 months | Paranasal sinuses | No | RT, CHT | NA | NA |
| Dosoretz et al. [24] | 19 | 71 years | ADK, G2, IVB | Palpable mass | At dx | L mandible | No | RT, CHT | AWD | 15 months |
| Mustafa et al. [25] | 20 | 45 years | ADK, G2, IA (adenoacanthoma) | Infection | 36 months | Cranium | Lung, pelvic side wall | Surgery, HT | DOD | 6 months |
| Manolitsas et al. [26] | 21 | 76 years | ADK, G3, IVB | Pain | At dx | R calcaneus | No | CHT, RT | DOD | 19 monthse |
| Neto et al. [27] | 22 | 39 years | ADK, G2, IVB | Pain, tumble | At dx | R ischium | No | Surgery, RT | NED | 36 months |
| Ali et al. [28] | 23 | 77 years | ADK, G3, IC | Throbbing, swelling | 24 months | L 4th toe, distal phalanx | Lung | Surgery, HT | AWD | 16 months |
| Arnold et al. [29] | 24 | 63 years | ADK, Gl, IVB | Pain, leg weakness | At dx | 12th thoracic vertebra | No | Surgery, RT, HT | NED | 60 months |
| Dursun et al. [30] | 25 | 51 years | ADK, G3, IIIC | Pain | 1 month | R and L humerus | Lymph nodes | RT | AWD | 6 months |
| Ilvan et al. [31] | 26 | 72 years | Clear cell, NA, IIB | Pain | 14 months | Paranasal sinuses | Lung, liver | None | DOD | 1 month |
| Dursun et al. [32] | 27 | 69 years | Clear cell, G3, IIIC | Pain | 1 month | Widespread | No | CHT, HT | DOD | 1 month |
| Giannakopoulos et al. [33] | 28 | 68 years | ADK, G3, IVB | Pain | At dx | R ischium | No | RT | NED | 48 months |
| Haraguchi et al. [34] | 29 | 87 years | NA, NA, NA | Painful mass | 108 months | Sternum | No | Surgery | NED | 60 months |
| Landoni et al. [35] | 30 | 66 years | ADK, G2, IIIA | Palpable mass | 19 months | Rfoot | Vagina | RT | NA | NA |
| Loizzi et al. [36] | 31 | 73 years | ADK, G3, IVB | Pain | At dx | R tibia | No | CHT | DOD | 9 monthse |
| 32 | 51 years | ADK, G3, IVB | Pain | At dx | Cervical vertebrae | No | CHT | DOD | 2 months | |
| Osanai et al. [37] | 33 | 68 years | ADK, G3, IC | Pain | 22 months | L ischium | No | CHT, bisphosphonates | NED | 36 months |
| Uharcek et al. [38] | 34 | 67 years | ADK, G1, IVB | Pain, erythema, swelling | At dx | R foot | No | Surgery, CHT, HT | NED | 20 months |
| Kaya et al. [39] | 35 | 70 years | ADK, G1, IVB | Pain, swelling | At dx | L tibia | No | RT | NED | 47 months |
| Ishibashi et al. [40] | 36 | 64 years | Carcinosarcoma, G3, IVB | Pain | At dx | R tibia | No | RT | DOD | 6 monthsf |
| Albareda et al. [41] | 37 | 62 years | ADK, G1, IB | None | 37 months | Sacrum | No | Surgery, HT | NED | 26 months |
| Farooq and Chang [42] | 38 | 63 years | ADK, G1, IVB | Painful mass | At dx | Cranium, ribs, vertebrae | No | NA | NA | NA |
| Qin et al. [43] | 39 | 48 years | ADK, G3, IIB | Pain | 22 months | R and L femur | No | Surgery, CHT, RT, HT | NED | 42 months |
| Pakos et al. [44] | 40 | 62 years | ADK, G3, II | Pain | 7 months | R tibia | No | Surgery | NED | 27 months |
| Artioli et al. [45] | 41 | 74 years | ADK, G3, IVB | Pain, infection | At dx | L tibia | No | RT, CHT | NA | NA |
| Chan et al. [46] | 42 | 62 years | NA, NA, NA | Pain | 3 months | Sternum | NA | Surgery | DOD | 18 months |
| Jiang et al. [47] | 43 | 51 years | ADK, G2, IVB | Pain, swelling | At dx | L tibia, calcaneus, tarsus | Lung | Surgery, CHT, HT | NED | 56 months |
| Secondary bone metastases | ||||||||||
| Rocha et al. [48] | 44 | 67 years | NA, NA, NA | Painful mass | 60 months | L mandible | Lung, kidney | Surgery | DOD | 9 months |
| Sahinler et al. [49] | 45 | 67 years | ADK, G3, IC | Pain, infection | 4 months | R and L tibia, R and L | Vagina | RT | DOD | 2 months |
| Amiot et al. [50] | 46 | 86 years | ADK, G3, IIIC | Pain, infection | 18 months | femur, L foot L hallux | Lung, bones (NS) | Surgery | DOD | NA |
| Oaknin et al. [51] | 47 | 69 years | ADK, G1, IA | Pain | 84 months | Spine | Vagina, deltoid | HT, CHT | DOD | 5 months |
Abbreviations: ADK, adenocarcinoma; AWD, alivewith disease; CHT, chemotherapy; DOD, death by disease; dx, diagnosis; HT, hormone therapy; L, left;met,metastases; NA, not available; NED, no evidence of disease; NS, not specified; Pt., patient; R, right; and RT, radiotherapy.
Primary bone metastasis was defined as diagnosis of bone metastasis at the same time as diagnosis of endometrial cancer or as first site of recurrence; secondary bone metastasis was defined as second site of recurrence.
For this summary table, we used the 1988 FIGO (Federation Internationale de Gynecologie et d'Obstetrique [International Federation of Gynecology and Obstetrics]) staging.
At the time of diagnosis of bone metastasis.
This patient refused treatment for primary disease, despite repeated recommendations and warnings by the attending physicians.
No surgical treatment (i.e., hysterectomy plus bilateral salpingo-oophorectomy) was offered to this patient for management of primary disease of the uterus, because of her poor overall general health.
This patient refused hysterectomy plus bilateral salpingo-oophorectomy and chemotherapy but allowed radiotherapy for the bone metastasis. She died of progressive disease.
Table 5.
Characteristics of 21 Patients with primary or secondary bone metastases of endometrial cancer described by Kehoe et al. [52].a
| Pt. no. | Age | Histology, grade, stageb | Symptoms at presentation | Interval to bone met | Localization | Extraosseous metd | Therapy | Status | Survival after bone met |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 61 years | ADK, G1, IIIA | Pain | 44 months | Vertebrae | Yes (NS) | Surgery, RT | Deadd | 12 months |
| 2 | 65 years | ADK, G3, IIIB | Pain | 3 months | Rib, vertebrae | Yes (NS) | CHT | Deadd | 9 months |
| 3 | 55 years | ADK, G3, IVB | Pain | Atdx | Ischium, acetabulum, femur | No | Surgery, RT | Deadd | 10 monthse |
| 4 | 58 years | ADK, G3, IA | Pain | 10 months | L4, L5 | No | RT, Surgery, CHT | AWD | 199 months |
| 5 | 70 years | ADK, G3, IIB | Pain | 10 months | Rib, vertebrae, parietal bone | Yes (NS) | RT | Deadd | 2 months |
| 6 | 65 years | ADK, G1, IIIB | Pain | 7 months | Tibia | Yes (NS) | Surgery, NT | Deadd | 42 months |
| 7 | 47 years | ADK, G2, IVB | Pain | At dx | Vertebrae, femur, acetabulum, humerus | No | RT, CHT | Deadd | 27 months |
| 8 | 55 years | ADK,G1,NA | Pain | 25 months | Pelvis, sacrum, vertebrae, rib | Yes (NS) | CHT | Deadd | 7 months |
| 9 | 60 years | Clear cell, G3, NA | Pain | 12 months | Humerus, clavicle | No | Surgery, CHT, RT | Deadd | 13 months |
| 10 | 71 years | ADK, G2, IVB | Pain | 16 months | L1, L3, L4 | Yes (NS) | None | Deadd | 1 month |
| 11 | 74 years | ADK, G3, IB | Pain | 8 months | Rib, vertebrae | Yes (NS) | RT, CHT | Deadd | 5 months |
| 12 | 62 years | ADK, G3, IVB | Pain | At dx | Vertebrae | Yes (NS) | RT, CHT | Deadd | 16 months |
| 13 | 62 years | ADK, G2, IIIC | Pain | 11 months | Calvaria, femur, spine | Yes (NS) | Surgery | Deadd | 54 months |
| 14 | 60 years | ADK, G3, IVB | Pain | 3 months | Sacroiliac joint | Yes (NS) | CHT, RT | Deadd | 8 months |
| 15 | 52 years | NA, NA, NA | Pain | 148 months | Vertebrae | Yes (NS) | Surgery | Deadd | 7 months |
| 16 | 55 years | ADK, G3, IIIC | Pain | 9 months | Rib | No | RT, surgery | Deadd | 26 months |
| 17 | 32 years | ADK, G3, IVB | Pain | At dx | Pubis, acetabulum | No | RT, CHT | AWD | 5 months |
| 18 | 40 years | ADK, G3, IIIC | Pain | 3 months | Ischium | No | CHT | Deadd | 10 months |
| 19 | 84 years | ADK, G2, IVB | Pain | At dx | Ischium, pubis, acetabulum | No | RT | Deadd | 34 months |
| 20 | 77 years | ADK, G3, IVB | Pain | Atdx | Pubis, sacrum, acetabulum | Yes (NS) | RT, CHT | Deadd | 8 months |
| 21 | 56 years | ADK, G2, IC | Pain | 26 months | Femur | Yes (NS) | Surgery, CHT | AWDe | 12 months |
Abbreviations: ADK, adenocarcinoma; AWD, alive with disease; CHT, chemotherapy; dx, diagnosis; L, left; met, metastases; NA, not available; NS, not specified; Pt., patient; and RT, radiotherapy.
Primary bone metastasis was defined as diagnosis of bone metastasis at the same time as diagnosis of endometrial cancer or as first site of recurrence; secondary bone metastasis was defined as second site of recurrence.
For this summary table, we used the 1988 FIGO (Fédération Internationale de Gynécologie et d'Obstétrique [International Federation of Gynecology and Obstetrics]) staging.
At the time of diagnosis of bone metastasis.
This patient refused hysterectomy plus bilateral salpingo-oophorectomy and chemotherapy but allowed radiotherapy plus surgery for the bone metastasis.
Of the 48 cases of primary bone metastases with available data regarding both tumor histology and survival, 42 had Type I and 6 had Type II histology. Overall survival after diagnosis of bone metastasis was 22 months (range, 1–267 months) among women with Type I EC and 6 months (range, 1–14) among women with Type II EC (P = .02).
Discussion
Bone metastases represent an unusual site of disease dissemination in patients with EC. With the present series, we were able to estimate the exact frequency of this type of event in the clinical setting, for an overall incidence of less than 1%. Bone metastases at presentation with EC are even more uncommon, representing only 0.12% of all patients with EC managed at our institution during the study period.
Although prior reviews have been published [22,25,27,33,41,47], the most updated review [41] captured only 29 cases of EC metastatic to bone. Little information on osseous dissemination of EC is available in the medical literature. An anatomopathologic study published in 1990 that reported data from 305 autopsies of women affected by gynecologic malignancies found 17 cases of bone metastases of EC [6]. All 17 women had concomitant extensive metastases elsewhere; therefore, it is likely that osseous involvement was a marker of disseminated, end-stage disease rather than the first site of relapse of the primary tumor. Fifteen (88%) of the 17 patients had bone metastases to the axial skeleton, either to vertebrae or pelvic bones [6].
A historical study on dissemination of EC noted 6 cases (2.3%) of bone metastases in 266 women [55]. The most common site was the axial skeleton, which was involved in 5 of the 6 (83%) patients. However, it is not clear whether the osseous spread in these 6 patients was the primary site of disease recurrence. Data from our series of 19 patients, despite being limited only to cases of bone metastases as the first localization of recurrent disease, are in agreement with those reported in this and other previous series: in fact, the spine and hip were the most common sites of osseous dissemination in our patients.
More recently, Kehoe et al. [52] published a clinical series in 2010 on bone metastases of EC diagnosed between 1990 and 2007 at Memorial Sloan-Kettering Cancer Center. They reported 21 cases of osseous dissemination of EC, by far the largest series on clinical outcomes until now. The spine was again the most commonly involved site, followed by the pelvic bones. The authors found a 10-month median survival after diagnosis of bone metastasis, which is comparable to the 12 months reported herein. However, Kehoe et al. [52] included cases of later bone recurrence (i.e., bone recurrence not presenting as the first site of EC relapse). Moreover, they did not distinguish between patients initially treated at Memorial Sloan-Kettering Cancer Center and patients secondarily referred there, thus not providing information regarding the exact incidence of this type of metastasis in the general endometrial cancer population. Finally, multiple factors possibly associated with a worse prognosis were not analyzed, apart from the timing of recurrence and the presence of bone dissemination at diagnosis.
Our series illustrates how important it is to emphasize the poor survival of EC patients after diagnosis of bone dissemination (median, 1 year). This is somewhat discouraging if we consider that bone metastases represent a marker of clinically evident widespread metastatic disease in less than 50% of cases. In fact, in 53% of our 19 patients, the skeleton represented the only site of metastasis. However, patients with a single bone metastasis and no evidence of extraosseous disease had a significantly better prognosis than that of patients with more widespread disease.
No routine effort was made during the study period at Mayo Clinic to rule out the presence of bone metastases at the time of primary treatment for EC. Our findings justify this policy, given the low rate of bone metastases either at initial diagnosis or subsequently. Moreover, our literature review underscores the fact that only 1 of 91 cases of bone metastasis was discovered incidentally. Bone metastases are usually symptomatic. In a 1981 report on the routine use of radiographic bone surveys or bone scans during the management of 97 patients with EC [56], 3 cases (3%) of bone metastases were diagnosed; of these, 1 (1%) was found at initial diagnosis. No information was provided on the presence of symptoms accompanying the radiologic findings. However, the authors concluded that routine bone scans and surveys are not justified in patients with EC. Our own data are in agreement with these conclusions.
Both our series and our literature review suggest a better prognosis for patients whose bone metastases are discovered at presentation with EC than with later recurrence (20 vs. 10.5 months; P = .04), particularly when a single bone metastasis is present with no extraosseous dissemination. In fact, 2 of the 3 patients managed at Mayo Clinic who had a diagnosis of osseous spread concomitant with discovery of EC survived more than 7 years. The only patient with a poor prognosis had widespread multiorgan dissemination of metastatic disease; that patient survived only 2 months.
Another finding of our study is the fact that (as might be expected) women with Type II EC who have recurrence to the bone have a lower life expectancy than women with Type I histology.
The optimal elective treatment for bone metastasis of EC is unknown. This uncertainty is probably the result not only of the small number of cases described in the medical literature but also of the different osseous sites involved. Moreover, identifying the extraosseous sites of dissemination is crucial in determining possible therapeutic strategies. To eliminate at least this latter possible bias, we elected to analyze the type of treatment in our study group by focusing on cases without extraosseous spread. Although median survival seemed higher with multimodal therapy in terms of absolute numbers (33 vs. 20 months), statistical significance was not reached and no clear advantage over radiotherapy alone was evident.
Surgery was not related to dramatic improvement in oncologic outcomes, but the limited number of cases in the present series as well as in our literature review does not allow us to draw any definitive conclusions on this issue. Conversely, hormonal therapy with megestrol has been associated with good results, particularly in combination with other treatment modalities. Again, we must emphasize that our data are too scant to support a strong recommendation on the best therapeutic approach for bone metastases of EC.
We must also acknowledge that our literature review is inevitably flawed by publication biases; that is, the published reports likely deal with the most unusual cases or those with the most favorable survival outcomes, thus not providing the real clinical picture of bone metastases of EC. Our series of 19 cases treated at the same institution strengthens the data and provides correction of possible biases.
In conclusion, we suggest that the real incidence of primary bone metastases of EC is low. Markers of better prognosis include the absence of multiple bone metastases and extraosseous spread, and the presence of bone localization at the debut of EC. Further research, including systematic study of progesterone receptor expression, should clarify whether hormonal therapy can have beneficial effects in such patients.
HIGHLIGHTS
Bone metastases have an incidence of almost 1% in endometrial cancer patients.
Diagnosis is almost invariably based on symptoms (mainly pain); routine bone scans during follow-up do not seem justified.
Prognosis appears more favorable when bone metastasis is discovered at diagnosis of endometrial cancer.
Acknowledgments
Role of funding source
The organization providing the grant that partially funded the present article has had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Abbreviations
- EC
endometrial cancer
- FIGO
Fédération Internationale de Gynécologie et d'Obstétrique (International Federation of Gynecology and Obstetrics)
Footnotes
Part of this manuscript was presented as an abstract at the 16th International Meeting of the European Society of Gynaecological Oncology, Belgrade, Serbia, October 11–14, 2009.
This work was partially supported by the Office of Women's Health Research Building Interdisciplinary Careers in Women's Health (BIRCWH award K12 HD065987).
Conflict of interest statement The authors declare that there are no conflicts of interest.
References
- [1].Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011 Jul-Aug;61(4):212–36. doi: 10.3322/caac.20121. Epub 2011 Jun 17. [DOI] [PubMed] [Google Scholar]
- [2].Uccella S, Mariani A. ESGO textbook of gynecologic oncology. Gunes Publishing; 2009. Endometrial cancers; pp. 173–8. [Google Scholar]
- [3].Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. American Cancer Society Cancer statistics, 2004. CA Cancer J Clin. 2004 Jan-Feb;54(1):8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- [4].Mariani A, Dowdy SC, Keeney GL, Long HJ, Lesnick TG, Podratz KC. High-risk endometrial cancer subgroups: candidates for target-based adjuvant therapy. Gynecol Oncol. 2004 Oct;95(1):120–6. doi: 10.1016/j.ygyno.2004.06.042. [DOI] [PubMed] [Google Scholar]
- [5].Mariani A, Webb MJ, Keeney GL, Calori G, Podratz KC. Hematogenous dissemination in corpus cancer. Gynecol Oncol. 2001 Feb;80(2):233–8. doi: 10.1006/gyno.2000.6058. [DOI] [PubMed] [Google Scholar]
- [6].Abdul-Karim FW, Kida M, Wentz WB, Carter JR, Sorensen K, Macfee M, et al. Bone metastasis from gynecologic carcinomas: a clinicopathologic study. Gynecol Oncol. 1990 Nov;39(2):108–14. doi: 10.1016/0090-8258(90)90414-g. [DOI] [PubMed] [Google Scholar]
- [7].Ravault PP, Lejeune E, Bouvier M, Vauzelle JL, Ricard R, Bochu M, Jeanneret J. Isolated metastasis of the tarsal scaphoid bone in the course of cancer of the uterine body. Rev Rhum Mal Osteoartic. 1967 Nov;34(11):650–4. French. PubMed PMID: 5597154. [PubMed] [Google Scholar]
- [8].Rouchy R, Besson J, Grosieux P, Barraya PL. Osseous metastasis revealing endometrial cancer [French] Bull Fed Soc Gynecol Obstet Lang Fr. 1967 Sep-Oct;19(4):352–3. [PubMed] [Google Scholar]
- [9].Vanecko RM, Yao ST, Schmitz RL. Metastasis to the fibula from endometrial carcinoma: report of 2 cases. Obstet Gynecol. 1967 Jun;29(6):803–5. [PubMed] [Google Scholar]
- [10].Janis LR, Feldman EP. Metastatic adenocarcinoma of the calcaneus: case report. J Foot Surg Spring. 1976;15(1):28–32. [PubMed] [Google Scholar]
- [11].Beller U, Beckman EM, Bigelow B, Noumoff J. Early osseous metastasis of stage 1 endometrial carcinoma: report of a case. Gynecol Oncol. 1982 Aug;14(1):141–6. doi: 10.1016/0090-8258(82)90061-0. [DOI] [PubMed] [Google Scholar]
- [12].Zorzi R, Pescatori E. Metastasis of endometrial carcinoma to the tarsus [Italian] Chir Organi Mov. 1982 Jul-Dec;68(4–6):727–30. [PubMed] [Google Scholar]
- [13].Onuba O. Pathological fracture of right tibia, an unusual presentation of endometrial carcinoma: a case report. Injury. 1983 May;14(6):541–5. doi: 10.1016/0020-1383(83)90058-x. [DOI] [PubMed] [Google Scholar]
- [14].Litton GJ, Ward JH, Abbott TM, Williams HJ., Jr Isolated calcaneal metastasis in a patient with endometrial adenocarcinoma. Cancer. 1991 Apr 1;67(7):1979–83. doi: 10.1002/1097-0142(19910401)67:7<1979::aid-cncr2820670726>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [15].Maxymiw WG, Wood RE. Metastatic endometrial carcinoma to the mandible: a case report. J Oral Maxillofac Surg. 1991 Jan;49(1):78–80. doi: 10.1016/0278-2391(91)90271-m. [DOI] [PubMed] [Google Scholar]
- [16].Cooper JK, Wong FL, Swenerton KD. Endometrial adenocarcinoma presenting as an isolated calcaneal metastasis: a rare entity with good prognosis. Cancer. 1994 Jun 1;73(11):2779–81. doi: 10.1002/1097-0142(19940601)73:11<2779::aid-cncr2820731121>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- [17].Nishida Y, Hayata T, Miyakawa I. Metastatic calcaneal adenocarcinoma in a patient with uterine carcinoma. Int J Gynaecol Obstet. 1994 Jun;45(3):287–8. doi: 10.1016/0020-7292(94)90260-7. [DOI] [PubMed] [Google Scholar]
- [18].Petru E, Malleier M, Lax S, Lahousen M, Ehall R, Pickel H, et al. Solitary metastasis in the tarsus preceding the diagnosis of primary endometrial cancer: a case report. Eur J Gynaecol Oncol. 1995;16(5):387–91. [PubMed] [Google Scholar]
- [19].Schols WA, Kock HC, van Etten FH. Recurrent endometrial adenocarcinoma presenting as a solitary humeral metastasis. Gynecol Oncol. 1995 Oct;59(1):148–50. doi: 10.1006/gyno.1995.1282. [DOI] [PubMed] [Google Scholar]
- [20].Clarke SJ, Smith TP. Metastatic endometrial carcinoma of the foot: a case report. J Am Podiatr Med Assoc. 1996 Jul;86(7):331–3. doi: 10.7547/87507315-86-7-331. [DOI] [PubMed] [Google Scholar]
- [21].Armentano G, Bracco PL, Brizio R, Perelli G. Untreated endometrial adenocarcinoma: a case report. Eur J Gynaecol Oncol. 1997;18(2):144–5. [PubMed] [Google Scholar]
- [22].Malicky ES, Kostic KJ, Jacob JH, Allen WC. Endometrial carcinoma presenting with an isolated osseous metastasis: a case report and review of the literature. Eur J Gynaecol Oncol. 1997;18(6):492–4. [PubMed] [Google Scholar]
- [23].Scott A, Raine M, Stansbie JM. Ethmoid metastasis of endometrial carcinoma causing mucocoele of maxillary antrum. J Laryngol Otol. 1998 Mar;112(3):283–5. doi: 10.1017/s0022215100158360. [DOI] [PubMed] [Google Scholar]
- [24].Dosoretz DE, Orr JW, Jr, Salenius SA, Orr PF. Mandibular metastasis in a patient with endometrial cancer. Gynecol Oncol. 1999 Feb;72(2):243–5. doi: 10.1006/gyno.1998.5223. [DOI] [PubMed] [Google Scholar]
- [25].Mustafa MS, Al-Nuaim L, Inayat-Ur-Rahman N. Scalp and cranial bone metastasis of endometrial carcinoma: a case report and literature review. Gynecol Oncol. 2001 Apr;81(1):105–9. doi: 10.1006/gyno.2000.6038. [DOI] [PubMed] [Google Scholar]
- [26].Manolitsas TP, Fowler JM, Gahbauer RA, Gupta N. Pain in the foot: calcaneal metastasis as the presenting feature of endometrial cancer. Obstet Gynecol. 2002 Nov;100(5 Pt 2):1067–9. doi: 10.1016/s0029-7844(02)02015-x. [DOI] [PubMed] [Google Scholar]
- [27].Neto AG, Gupta D, Broaddus R, Malpica A. Endometrial endometrioid adenocarcinoma in a premenopausal woman presenting with metastasis to bon: a case report and review of the literature. Int J Gynecol Pathol. 2002 Jul;21(3):281–4. doi: 10.1097/00004347-200207000-00013. [DOI] [PubMed] [Google Scholar]
- [28].Ali ZA, Wimhurst JA, Ali AA, Tempest ME, Edwards DJ. Endometrial cancer metastasis presenting as a grossly swollen toe. Int J Gynecol Cancer. 2003 Nov-Dec;13(6):909–11. doi: 10.1111/j.1525-1438.2003.13630.x. [DOI] [PubMed] [Google Scholar]
- [29].Arnold J, Charters D, Perrin L. Prolonged survival time following initial presentation with bony metastasis in stage IVb endometrial carcinoma. Aust N Z J Obstet Gynaecol. 2003 Jun;43(3):239–40. doi: 10.1046/j.0004-8666.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- [30].Dursun P, Gultekin M, Basaran M, Aydingoz U, Ayhan A. Bilateral bone metastasis in endometrial adenocarcinoma. Lancet Oncol. 2003 Sep;4(9):547. doi: 10.1016/s1470-2045(03)01193-8. [DOI] [PubMed] [Google Scholar]
- [31].Ilvan S, Akyildiz EU, Calay Z, Celikoyar M, Sahinler I. Endometrial clear cell carcinoma metastatic to the paranasal sinuses: a case report and review of the literature. Gynecol Oncol. 2004 Jul;94(1):232–4. doi: 10.1016/j.ygyno.2004.04.005. [DOI] [PubMed] [Google Scholar]
- [32].Dursun P, Gültekin M, Yüce K, Ayhan A. Skeletal carcinomatosis in endometrial clear cell carcinoma at initial presentation: a case report. Int J Gynecol Cancer. 2006 Mar-Apr;16(2):891–5. doi: 10.1111/j.1525-1438.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- [33].Giannakopoulos CK, Kyriakidou GK, Toufexi GE. Bone metastasis as a presenting feature of endometrial adenocarcinoma: case report and literature review. Eur J Gynaecol Oncol. 2006;27(1):95–7. [PubMed] [Google Scholar]
- [34].Haraguchi S, Hioki M, Hisayoshi T, Yamashita K, Koizumi K, Shimizu K. Resection of sternal metastasis from endometrial carcinoma followed by reconstruction with sandwiched marlex and stainless steel mesh: report of a case. Surg Today. 2006;36(2):184–6. doi: 10.1007/s00595-005-3106-4. [DOI] [PubMed] [Google Scholar]
- [35].Landoni F, Lazzaro G, Lazzari R, Gravante G, Testori A. Endometrial carcinoma bone metastases in unusual sites. Gynecol Oncol. 2006 Aug;102(2):411. doi: 10.1016/j.ygyno.2006.03.013. Epub 2006 May 2. [DOI] [PubMed] [Google Scholar]
- [36].Loizzi V, Cormio G, Cuccovillo A, Fattizzi N, Selvaggi L. Two cases of endometrial cancer diagnosis associated with bone metastasis. Gynecol Obstet Invest. 2006;61(1):49–52. doi: 10.1159/000088530. Epub 2005 Sep 23. [DOI] [PubMed] [Google Scholar]
- [37].Osanai T, Tsuchiya T, Ogino T, Nakahara K. Long-term prevention of skeletal complications by pamidronate in a patient with bone metastasis from endometrial carcinoma: a case report. Gynecol Oncol. 2006 Jan;100(1):195–7. doi: 10.1016/j.ygyno.2005.08.007. Epub 2005 Sep 19. [DOI] [PubMed] [Google Scholar]
- [38].Uharcek P, Mlyncek M, Ravinger J. Endometrial adenocarcinoma presenting with an osseous metastasis. Gynecol Obstet Invest. 2006;61(4):200–2. doi: 10.1159/000091402. Epub 2006 Feb 8. [DOI] [PubMed] [Google Scholar]
- [39].Kaya A, Olmezoglu A, Eren CS, Bayol U, Altay T, Karapinar L, et al. Solitary bone metastasis in the tibia as a presenting sign of endometrial adenocarcinoma: a case report and the review of the literature. Clin Exp Metastasis. 2007;24(2):87–92. doi: 10.1007/s10585-007-9061-2. Epub 2007 Mar 16. [DOI] [PubMed] [Google Scholar]
- [40].Ishibashi M, Fujiwaki R, Nakayama I, Miura H, Sawada K. Endometrial carcinosarcoma presenting as a tibial metastasis. Int J Clin Oncol. 2007 Aug;12(4):305–8. doi: 10.1007/s10147-006-0652-8. Epub 2007 Aug 20. [DOI] [PubMed] [Google Scholar]
- [41].Albareda J, Herrera M, Lopez Salva A, Garcia Donas J, Gonzalez R. Sacral metastasis in a patient with endometrial cancer: case report and review of the literature. Gynecol Oncol. 2008 Dec;111(3):583–8. doi: 10.1016/j.ygyno.2008.04.005. Epub 2008 May 22. [DOI] [PubMed] [Google Scholar]
- [42].Farooq MU, Chang HT. Intracranial and scalp metastasis of endometrial carcinoma. Med Sci Monit. 2008 Sep;14(9):CS87–8. [PubMed] [Google Scholar]
- [43].Qin Y, Peng Z, Gao Y. Bilateral femur metastasis in endometrial adenocarcinoma. Saudi Med J. 2008 May;29(5):766–9. [PubMed] [Google Scholar]
- [44].Pakos EE, Gartzonikas DN, Tsekeris PG, Xenakis TA. Solitary tibial osteolytic lesion. Case Rep Med. 2009;2009:352085. doi: 10.1155/2009/352085. Epub 2009 Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Artioli G, Cassaro M, Pedrini L, Borgato L, Corti L, Cappetta A, et al. Rare presentation of endometrial carcinoma with singular bone metastasis. Eur J Cancer Care (Engl) 2010 Sep;19(5):694–8. doi: 10.1111/j.1365-2354.2008.01045.x. Epub 2009 Jul 29. [DOI] [PubMed] [Google Scholar]
- [46].Chan V, Lau J, Rubens FD, Dennie C, Ruel M. Malignant invasion of sternotomy incision after cardiac operation. Ann Thorac Surg. 2010 Apr;89(4):1295–6. doi: 10.1016/j.athoracsur.2009.09.034. [DOI] [PubMed] [Google Scholar]
- [47].Jiang GQ, Gao YN, Gao M, Zheng H, Yan X, Wang W, et al. Clinicopathological features and treatment of extremity bone metastasis in patients with endometrial carcinoma: a case report and review. Chin Med J (Engl) 2011 Feb;124(4):622–6. [PubMed] [Google Scholar]
- [48].Rocha WC, Curado MP, Vêncio EF, Caixeta WB. Endometrial carcinoma metastatic to the mandible: a case report. J Oral Maxillofac Surg. 2000 Aug;58(8):914–6. doi: 10.1053/joms.2000.8225. [DOI] [PubMed] [Google Scholar]
- [49].Sahinler I, Erkal H, Akyazici E, Atkovar G, Okkan S. Endometrial carcinoma and an unusual presentation of bone metastasis: a case report. Gynecol Oncol. 2001 Jul;82(1):216–8. doi: 10.1006/gyno.2001.6206. [DOI] [PubMed] [Google Scholar]
- [50].Amiot RA, Wilson SE, Reznicek MJ, Webb BS. Endometrial carcinoma metastasis to the distal phalanx of the hallux: a case report. J Foot Ankle Surg. 2005 Nov-Dec;44(6):462–5. doi: 10.1053/j.jfas.2005.07.014. [DOI] [PubMed] [Google Scholar]
- [51].Oaknin A, Barretina MP, Morilla I. Muscle metastasis of low-grade endometrial carcinoma seven years after diagnosis: a case report. Eur J Gynaecol Oncol. 2010;31(1):114–6. [PubMed] [Google Scholar]
- [52].Kehoe SM, Zivanovic O, Ferguson SE, Barakat RR, Soslow RA. Clinicopathologic features of bone metastases and outcomes in patients with primary endometrial cancer. Gynecol Oncol. 2010 May;117(2):229–33. doi: 10.1016/j.ygyno.2010.01.047. Epub 2010 Mar 2. [DOI] [PubMed] [Google Scholar]
- [53].Announcements. FIGO stages Y1988 revision. Gynecol Oncol. 1989;35:125–6. [Google Scholar]
- [54].Scully RE, Bonfiglio TA, Kurman RJ, Silvergerg SG, Wilkinson EJ. Histological typing of female genital tract tumours. 2nd ed. Springer-Verlag; Berlin: 1994. [Google Scholar]
- [55].Finn WF. Time, site, and treatment of recurrences of endometrial carcinoma. Am J Obstet Gynecol. 1950 Oct;60(4):773–82. doi: 10.1016/s0002-9378(16)39102-5. [DOI] [PubMed] [Google Scholar]
- [56].Mettler FA, Jr, Christie JH, Garcia JF, Baldwin MH, Wicks JD, Bartow SA. Radionuclide liver and bone scanning in the evaluation of patients with endometrial carcinoma. Radiology. 1981 Dec;141(3):777–80. doi: 10.1148/radiology.141.3.6272356. [DOI] [PubMed] [Google Scholar]