Abstract
Comorbidity of major depressive disorder (MDD) and cardiovascular disease (CVD) represents the fourth leading cause of morbidity and mortality worldwide, and women have a two times greater risk than men. Thus understanding the pathophysiology has widespread implications for attenuation and prevention of disease burden. We suggest that sex-dependent MDD-CVD comorbidity may result from alterations in fetal programming consequent to the prenatal maternal environments that produce excess glucocorticoids, which then drive sex-dependent developmental alterations of the fetal hypothalamic-pituitary-adrenal (HPA) axis circuitry impacting mood, stress regulation, autonomic nervous system (ANS), and the vasculature in adulthood. Evidence is consistent with the hypothesis that disruptions of pathways associated with gamma aminobutyric acid (GABA) in neuronal and vascular development and growth factors have critical roles in key developmental periods and adult responses to injury in heart and brain. Understanding the potential fetal origins of these sex differences will contribute to development of novel sex-dependent therapeutics.
Keywords: depression, cardiovascular disease, sex differences, hypothalamus, prenatal stress, MDD-CVD comorbidity, fetal hormonal programming
1. Introduction
The co-occurrence (or comorbidity) of major depressive disorder (MDD) and risk for cardiovascular disease (CVD) has a substantial population prevalence of approximately 20% (Kawachi et al., 1994b; Barefoot et al., 1996; Everson et al., 1997; Glassman and Shapiro, 1998; Krishnan et al., 2001; Jones et al., 2003; Scherrer et al., 2003) and is a leading cause of morbidity and mortality worldwide (Murray and Lopez, 1997; Ustun et al., 2004). Further, the comorbidity is significantly higher in women than in men (Naqvi et al., 2005; Moller-Leimkuhler, 2007; Goldstein et al., 2011). MDD alone has a higher prevalence in women (almost 2-fold) (Kessler et al., 1993; Kessler et al., 2003; Kendler et al., 2006), and is an independent risk factor for the development and progression of coronary artery disease (Kawachi et al., 1994a; Kawachi et al., 1994b; Barefoot et al., 1996; Everson et al., 1997; Musselman et al., 1998), even though the risk for CVD alone is higher in men (Lloyd-Jones et al., 2010). Numerous prospective studies demonstrated significantly elevated risks of coronary heart disease, myocardial infarction, or cardiac death among participants with depression (Glassman and Shapiro, 1998; Rozanski et al., 1999; Rutledge et al., 2006a; Rutledge et al., 2006b; Van der Kooy et al., 2007; Vaccarino et al., 2008). Depression predicts first cardiovascular events even among otherwise healthy people (Vaccarino et al., 2008), and particularly among women (Rutledge et al., 2006a). However, the etiologic pathways underlying this comorbidity are unclear, even though it has major public health implications worldwide.
The comorbidity of MDD and CVD, and in particular the association with significant sex differences, may arise in part from hormone-dependent pathogenic processes initiated during fetal development that result in greater risk in women than men. Fetal origins of MDD and CVD may independently result from alterations in the prenatal maternal environment, which drive developmental alterations of the fetal hypothalamic-pituitary-adrenal (HPA) axis circuitry. Several groups have used model animals to study cellular and molecular mechanisms that may relate to human studies of MDD and CVD (McClellan et al., 2010; Goldstein et al., 2011; Holsen et al., 2011; Carbone et al., 2012a; Holsen et al., 2012; Zuloaga et al., 2012b; Weinstock et al., 1992; Henry et al., 1994; Barker, 1995; Arborelius et al., 1999; Seckl, 2001). These independent bodies of work converge on the hypothesis that maternally-driven disruptions of fetal HPA circuitry during development produce shared risk for the adult comorbidity of MDD and CVD, which is significantly higher in females than males.
This review is based on the hypothesis that the key pathways for understanding sex-dependent effects with respect to neuronal and vascular development in HPA circuitry involves the impact of excess maternal glucocorticoids during specific gestational periods on fetal brain development. These mechanisms are shared and influenced by genes and fetal levels of gonadal hormones, growth factors and neurotransmitters such as gamma-aminobutyric acid (GABA). The developmental model is not meant to be an exclusive explanation for sex-dependent comorbidities. However, alternative adult etiologies are reviewed elsewhere (e.g., (Elderon and Whooley, 2013). Brain regions implicated in the stress response circuitry include the paraventricular nucleus in the hypothalamus, central and medial subregions of the amygdala, hippocampus, periaqueductal gray, medial and orbital prefrontal cortices, and anterior cingulate cortex. Many of these brain regions are morphologically or functionally sexually dimorphic (McEwen, 1983; Simerly et al., 1990; Tobet et al., 1993; Filipek et al., 1994; O'Keefe et al., 1995; Giedd et al., 1996; Murphy et al., 1996; Park et al., 1996; Tobet and Hanna, 1997; Gorski, 2000; Goldstein et al., 2001; Chung et al., 2006; Tobet et al., 2009) and implicated in autonomic nervous system (ANS) regulation, the dysregulation of which is a significant risk factor for CVD (Akselrod et al., 1981; Dalack and Roose, 1990; Musselman et al., 1998). Thus, prenatal stress, or an elevated prenatal glucocorticoid model, may produce shared risk for sex differences in MDD-CVD comorbidity by altering the development of common regulatory pathways, such as the ANS, limbic brain areas associated with stress and anxiety-related behaviors, and/or vascular development within brain areas central to HPA control. This review integrates human clinical literature on HPA and HP-gonadal (HPG) abnormalities and brain activity deficits that occur in depression and risk for CVD with developmental and adult preclinical studies, in order to provide convergent evidence for prenatal stress models as key for understanding sex differences in depressive and anxiety-related behaviors, ANS dysregulation, and the vasculature in stress-relevant central nervous system (CNS) regions.
2. Hypothalamo-Pituitary-Adrenal Axis and Stress
2.1 Anatomy of Stress Circuitry
Brain regions implicated in the stress response circuitry all provide inputs through numerous routes to the PVN, which is the final common motor output for the neuroendocrine hypothalamus. These brain regions include central and medial subregions of the amygdala, hippocampus, periaqueductal gray, medial and orbital prefrontal cortices, and anterior cingulate cortex (see Figure 1). Central to the HPA axis are a group of neurons found in the paraventricular nucleus of the hypothalamus that synthesize and secrete corticotropin releasing hormone (CRH). Secretion of CRH from these neurons and co-secretion of arginine vasopressin into the hypothalamo-hypophyseal portal capillaries of the median eminence regulate secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary. In turn, ACTH drives the secretion of cortisol in humans and corticosterone in many other vertebrates from the zona fasciculate of the adrenal cortex. At the same time, preautonomic neurons in the PVN activate the sympathetic nervous system (component of ANS) to stimulate the rapid release of epinephrine from chromaffin cells in the adrenal medulla. This rapid neural response to stress is further augmented by the neuroendocrine system since glucocorticoid release also drives adrenal medullary synthesis of the key enzyme responsible for transforming norepinephrine to epinephrine (phenylethanolamine N-methyltransferase, or PNMT). The combination of glucocorticoids and epinephrine secreted into the bloodstream generates diverse responses throughout the body in addition to the glucocorticoid negative feedback responses at the pituitary and brain levels (see Figure 1).
Figure 1. Shared Brain Circuitry between Stress Response, Mood and HPA Regulation.
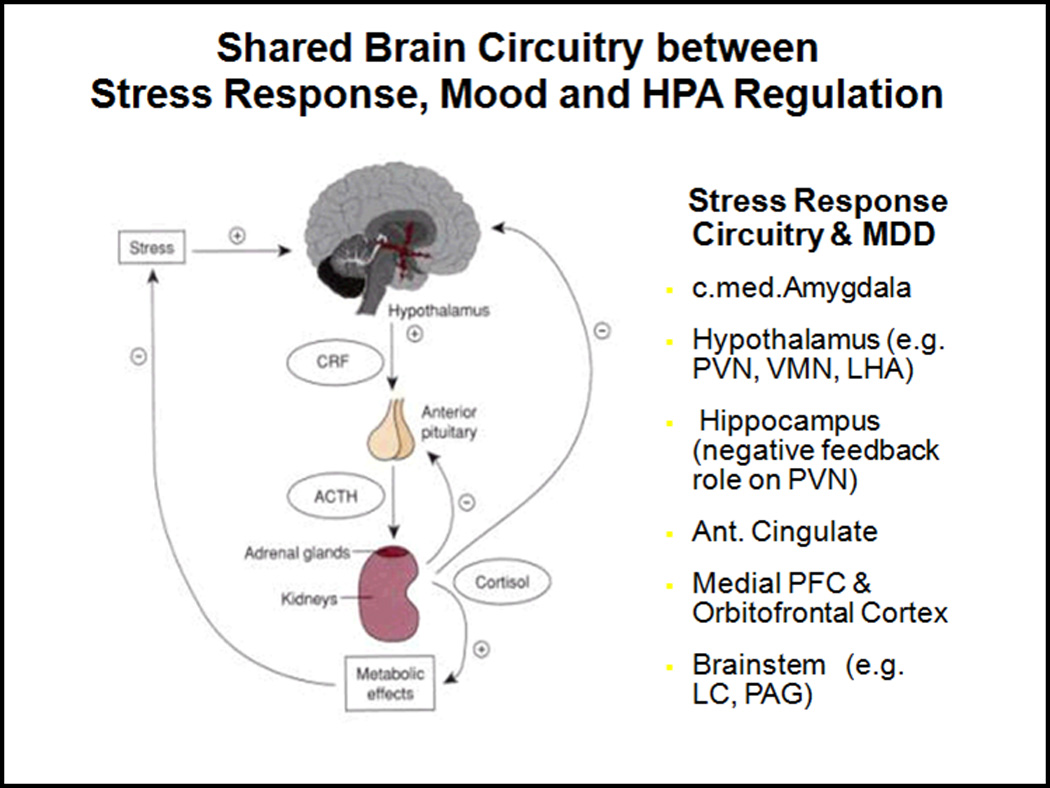
Stress response circuitry in the brain is shared with HPA and HPG regulation and a number of these regions are found to be abnormal in MDD, structurally (operationalized as MRI brain volumes) and functionally (operationalized in studies of fMRI or Positron Emission Tomography or PET).
Among the neuroanatomical components of the HPA axis, the PVN stands out for its unique vascularization. It has been appreciated since the 1930’s that the PVN and its magnocellular companion, supraoptic nuclei, are more highly vascularized than the surrounding brain regions whether in primates or rats (Ambach and Palkovits, 1974; van den Pol AN., 1982). Surprisingly, little is known about the development of PVN vascularity (Menendez and Alvarez-Uria, 1987). However, our recent studies on PVN development demonstrate that the remarkable vascularity of the PVN in mice develops over the first three weeks of postnatal life and can be regulated by neurotransmitter signaling (Frahm et al., 2012). The timing occurs after most forebrain vasculature is established, and thereby identifies a secondary angiogenic period as a potential critical feature for PVN development. The PVN has been thought to be functionally hypo-responsive over the first few postnatal weeks, and several have tried (with varying levels of success) to associate this with the slow emergence of neuronal connections. This new discovery places vascularization as a potential critical developmental variable that may be essential for the normal ‘activation’ of the PVN. The issue can be viewed from at least two perspectives with one being the relationship of neural cells to vasculature and the other being the competency of the vasculature itself (e.g., blood brain barrier function), as illustrated schematically in Figure 2. Investigations will be important to test hypotheses that abnormalities of PVN vascularization are risk factors for HPA dysfunction and contribute to understanding the neural bases for the high comorbidity of depression and cardiovascular disease.
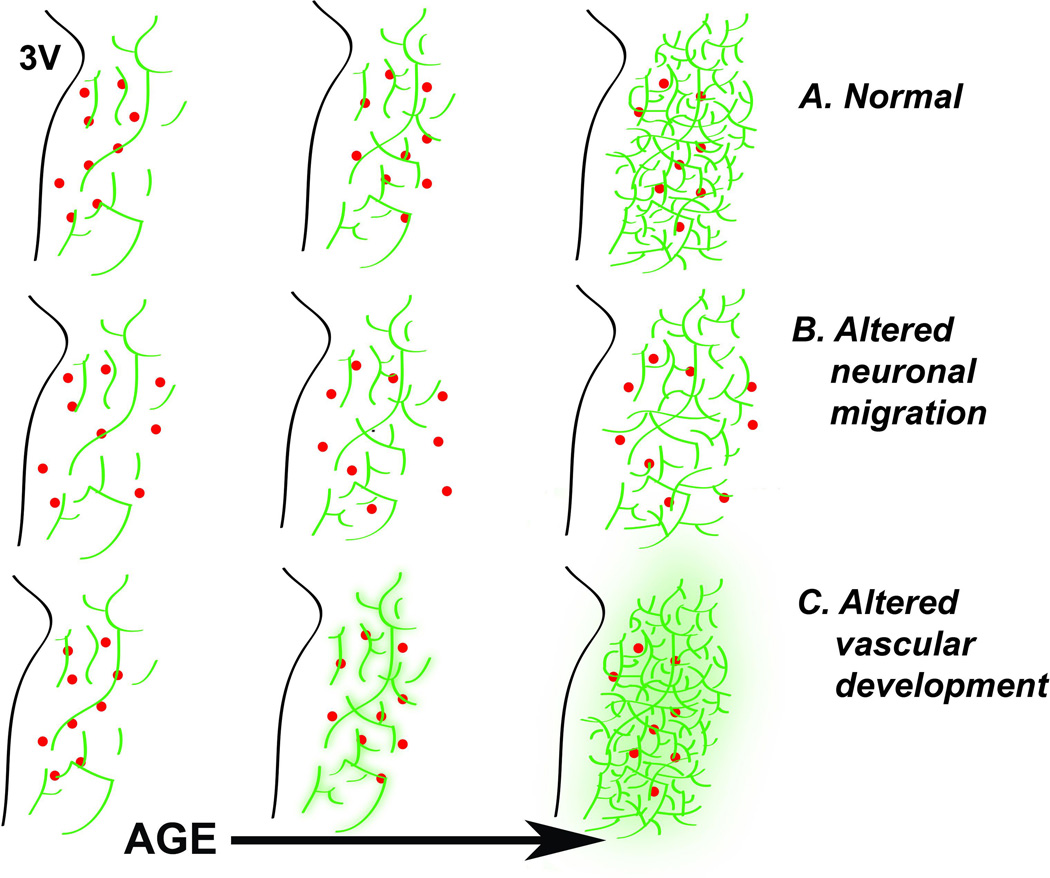
Figure 2. Potential interactions of PVN neuronal and vascular development.
Schematic diagram of coronal section illustrates the development of PVN neurons (red dots) along the proliferative zone of the third ventricle (3V) and their migration laterally with age (A). At later ages there is a major expansion of PVN vasculature (Green lines). The neuronal relationship to vascular elements (e.g., proximity) could be altered to create disease susceptibility (B), or the blood brain barrier function could be compromised leading to disease susceptibility (C).
2.2 Functions of Stress Circuitry
A major role for the HPA axis is to integrate potentially stressful stimuli and respond with a neuroendocrine signal that coordinates homeostatic responses throughout the body. Threatening physical or psychological stimuli or stressors are sensed at many levels of the CNS, including the cerebral cortex, and this information is passed to the hypothalamus after processing in limbic regions (Ferguson et al., 2008; Jankord and Herman, 2008). For example, CRH neurons are directly innervated by neurons of the bed nucleus of the stria terminalis, dorsal medial hypothalamus, preoptic area, and nucleus of the solitary tract. These directly innervating regions receive inputs from the ventral subiculum (glutamatergic), medial prefrontal cortex (glutamatergic), and medial and central amygdala (GABAergic) which communicate with the basolateral amygdala. It is thought that much of the negative feedback on the hypothalamus is mediated by glucocorticoid and mineralocorticoid receptors in the ventral hippocampus (the outflow via ventral subiculum) which relay through inhibitory neurons in areas such as the bed nucleus of the stria terminalis and periventricular PVN. In addition to negative feedback mediation by the hippocampus, glucocorticoid receptors are also present in the amygdala, PVN, bed nucleus stria terminalis, and prefrontal cortex (De Kloet et al., 1998; Myers et al., 2012).
2.3 HPA Axis Dysregulation in MDD
There is a long history of work characterizing the HPA system as central to understanding the development of MDD (Nemeroff et al., 1984; Holsboer et al., 1987; Plotsky et al., 1998; Arborelius et al., 1999; Heim et al., 2002; Parker et al., 2003; Raison and Miller, 2003; Barden, 2004; Swaab et al., 2005; Antonijevic, 2006). Depressive symptoms can occur in the context of either endogenously elevated cortisol (i.e., Cushing syndrome (Sonino et al., 1998) or exogenously administered corticosteroids (Kelly et al., 1980), and patients treated with corticosteroids can develop MDD (Ling et al., 1981)). Human studies demonstrate consistent HPA axis abnormalities associated with MDD, such as elevated cortisol levels in plasma, cerebral spinal fluid (CSF), and 24-hour urine, in addition to high CSF CRH levels, blunted responses to CRH administration, and failure to suppress cortisol secretion after administration of a synthetic glucocorticoid, dexamethasone (Carroll et al., 1976a; Carroll et al., 1976b; Carroll et al., 1981; Jarrett et al., 1983; Nemeroff et al., 1984; Halbreich et al., 1985; Holsboer et al., 1985; Banki et al., 1987; Evans and Nemeroff, 1987; Holsboer et al., 1987; Rubin et al., 1987; Nemeroff et al., 1991; Arborelius et al., 1999; Heim and Nemeroff, 2001; Heim et al., 2001; Newport et al., 2003; Oquendo et al., 2003; Raison and Miller, 2003; Barden, 2004). HPA dysregulation has been related to, among other things, age (Nelson et al., 1984a; Nelson et al., 1984b; Bremmer et al., 2007), depression subtype, hypercortisolemia in atypical depression and normal cortisol levels in melancholia (Brouwer et al., 2005), and single versus recurrent episodes of MDD (Poor et al., 2004).
Several studies have examined the utility of HPA reactivity as an indicator of treatment response in MDD. While elevated CRH levels in CSF have been shown to resolve with antidepressant treatment (Nemeroff et al., 1991; De Bellis et al., 1993; Veith et al., 1993), some studies report an incomplete resolution to normal levels, suggesting that HPA dysregulation may be part of the vulnerability to MDD (or a trait) and not only state-related. Although decreases in elevated pre-treatment cortisol levels have been widely reported in patients following antidepressant treatment (Gibbons and McHugh, 1962; Carroll et al., 1976a; Carroll et al., 1976b), a recent meta-analysis found that cortisol levels (blood, saliva or urine) did not change pre-versus post-treatment in over half of MDD cases (McKay and Zakzanis, 2010). An examination of subject characteristics related to changes in cortisol post-treatment revealed the greatest decreases occurred in those with the melancholic subtype. Time of sample collection, inpatient versus outpatient setting, type of treatment or antidepressant, subject sex, and number of past episodes were not associated with cortisol changes following treatment. However, the length of the current episode was negatively associated with change in cortisol levels (McKay and Zakzanis, 2010). This finding is supported by the hypothesis that the nature of HPA axis dysregulation shifts dramatically from acute (overall hypersecretion of CRH, adrenocorticotropin hormone (ACTH), and cortisol) to chronic (reduced ACTH and hypercortisolemia) phases of MDD (Parker et al., 2003).
Studies have demonstrated more immunoreactive CRH-containing neurons in the human PVN subsequent to hypertension (Goncharuk et al., 2002) as well as for patients with mood disorders (Bao et al., 2005). In the latter case, it was clear that many of the CRH neurons also contained immunoreactive estrogen receptor alpha, providing a potential link for understanding hormone influences in brain regions, such as the PVN, that are associated with MDD and CVD risk. This is not surprising since altered PVN function has been implicated in MDD and CVD in postmortem studies (Mesulam, 1985; Goncharuk et al., 2002; Bao et al., 2005) and, indirectly in vivo human functional imaging studies of sex differences in stress response circuitry in healthy adults (Goldstein et al., 2005; Goldstein et al., 2010), MDD subjects (Holsen et al., 2011; Holsen et al., 2013), and ANS function (Holsen et al., 2012).
It is reasonable to ask whether HPA dysregulation is indicative of enduring trait characteristics associated with MDD or the clinical state that one is in at a given time during an acute episode. The issue of state versus trait (independent of treatment) has not been studied extensively. In remitted MDD patients compared with controls, cortisol levels were reported to be similar (Trestman et al., 1993) or even decreased (Ahrens et al., 2008), although these studies included only small numbers of subjects (n/group ~ 20–30). However, a recent well-powered study examined morning and evening salivary cortisol levels in 308 controls, 579 individuals with remitted MDD, and 701 patients currently in an MDD episode (Vreeburg et al., 2009). Results showed that remitted and current MDD subjects demonstrated significantly higher awakening cortisol levels compared to controls (adjusted for sex), providing evidence that elevated cortisol may be a trait characteristic (Vreeburg et al., 2009). This is consistent with our recent brain imaging study of women with recurrent MDD in remission who were hypercortisolemic compared with healthy women (Holsen et al., 2013). When tracked longitudinally, baseline cortisol and dexamethasone suppression test abnormalities also predicted vulnerability to relapse, necessity of continued medication to sustain remission, and remission rate following hospitalization in MDD (O'Toole et al., 1997; Zobel et al., 1999; Appelhof et al., 2006; Ising et al., 2007).
2.4 Sex differences in HPA Function and MDD
Despite significant advances in understanding the co-occurrence of MDD and HPA axis dysregulation, there is a paucity of data on sex differences in this comorbidity. This is striking even though: 1) there are well-documented sex differences in MDD incidence and prevalence (Kessler, 2003; Kendler et al., 2006); 2) substantial data support sex differences in HPA-HPG axes functioning during stress in healthy populations (Kudielka and Kirschbaum, 2005; Goldstein et al., 2010; Andreano et al., 2011) and in MDD women (Holsen et al., 2011; Holsen et al., 2013); and 3) there are significant interactions of the HPA axis with the HPG axis, which is de facto different between the sexes if based on nothing else than the gonads.
Among the few investigations reporting significant sex differences in HPA axis functioning in MDD, the direction of effects was mixed. Men, but not women, with MDD, demonstrated abnormal plasma ACTH pulsatility (Young et al., 2007b). At baseline, elevated cortisol secretion was documented in depressed men versus depressed women (Bremmer et al., 2007) and non-depressed men (Hinkelmann et al., 2011). However, in contrast, depressed women demonstrated higher 24-hour cortisol secretion versus depressed men (Poor et al., 2004) and non-depressed women (Young and Altemus, 2004). Further, in response to a social stress paradigm, depressed women also exhibited elevated cortisol compared to non-depressed women (Chopra et al., 2009). Conflicting reports on sex differences in cortisol levels may be related to differences in the timing of cortisol assessment (e.g. baseline or under stress) or lack of control for menstrual cycle in women and/or genetic factors.
Recent data suggested an interaction between sex and adrenergic receptor gene polymorphisms in HPA hyperactivity using a dexamethasone/CRH test pre- and post-treatment (Haefner et al., 2008). Specifically, increased ACTH and cortisol responses were seen in males (but not females) homozygous for the alpha(2)-adrenergic receptor (ADRA2A) gene, and females (but not males) homozygous for the beta(2)-adrenergic receptor (ADRB2) gene (Haefner et al., 2008). Collectively, these findings offer initial evidence of sex differences in the role of HPA axis functioning in MDD pathophysiology under different genetic conditions.
By contrast, several reports have found no effect of sex on basal HPA function in MDD (Carroll et al., 1976a; Carroll et al., 1976b; Nelson et al., 1984a; Maes et al., 1987; Dahl et al., 1989; Maes et al., 1989; Maes et al., 1994; Deuschle et al., 1998; Brouwer et al., 2005; Rubin et al., 2006; Vreeburg et al., 2009). However, the majority of these studies did not initially design their studies to investigate sex differences, but rather analyzed the data by sex post hoc. This is problematic since potential confounding (uncontrolled in the initial designs) is typical. For example, the vast majority of studies of MDD oversample women (Maes et al., 1987; Brouwer et al., 2005; Rubin et al., 2006; Young et al., 2007b; Vreeburg et al., 2009; Hinkelmann et al., 2011). Some have matched on sex, whereas some included women using oral contraceptives or estrogen-replacement therapy (Brouwer et al., 2005), which affect plasma levels of cortisol (Kirschbaum et al., 1999). Further, only a few mention “matching for menstrual cycle status” (Maes et al., 1987; Rubin et al., 2006), and those that do generally refer to including similar numbers of women who are pre- or post-menopausal rather than actually controlling for menstrual cycle phase (for example, conducting study visits only within certain phases such as early follicular or late luteal). These methodological confounds present significant challenges to understanding the inconsistencies in the literature on sex differences in human HPA axis dysregulation and MDD comorbidity.
2.5 Postmortem Morphological Changes in HPA in MDD
The importance of HPA axis abnormalities in MDD is underscored by human postmortem studies. It has been reported that there is a 25% decrease in the density of glucocorticoid receptor (GR) mRNA in MDD compared with healthy brains in frontal cortex, dentate gyrus, and subiculum. Such observations showing a down-regulation of GRs suggest that there may be reductions in negative feedback control of the HPA axis, the net result being hypercortisolemia and inability to regulate stress reactivity behaviorally (Webster et al., 2002). This corresponds with the enhanced numbers of arginine vasopressin (AVP) immunoreactive neurons in MDD (von Bardeleben et al., 1989; Muller and Holsboer, 2006). AVP was found to be co-expressed with CRH in some PVN neurons and potentiates the actions of CRH at the anterior pituitary in model animals (Whitnall and Gainer, 1988; Familari et al., 1989). A recent human postmortem study similarly reported increased AVP mRNA in the PVN and supraoptic nucleus in MDD, particularly in brain tissue from patients with melancholic features (Meynen et al., 2006). This is consistent with an increased number of AVP-immunoreactive neurons in PVN (Purba et al., 1996), particularly those co-localizing with increased CRH in PVN in MDD (Raadsheer et al., 1994a; Raadsheer et al., 1994b). It is also consistent with studies of MDD reporting elevated AVP plasma levels (van Londen et al., 1997; van Amelsvoort et al., 2001; de Winter et al., 2003), positive correlations of plasma AVP with cortisol (De Bellis et al., 1994; Inder et al., 1997; de Winter et al., 2003), and increased ACTH and cortisol in MDD and controls after intravenous administration of AVP (Gispen-de Wied et al., 1992), findings that were not due to medication confounds (van Londen et al., 1997; van Amelsvoort et al., 2001; Meynen et al., 2006).
2.6 HPA-HPG axes interactions in MDD
Changes in women’s reproductive system have been consistently related to mood fluctuations and MDD per se (Rabin et al., 1990; Baischer et al., 1995; Rubinow and Schmidt, 1996; Harlow et al., 2003; Payne, 2003; Roca et al., 2003; Spinelli, 2005; Payne et al., 2009). MDD incidence increases with pubertal onset in females (Angold and Costello, 2006), late luteal menstrual cycle phase (Steiner, 1992), chronic use of oral contraceptives (Young et al., 2007a), the postpartum period (Bloch et al., 2000; Brummelte and Galea, 2010), and postmenopause (Graziottin and Serafini, 2009). Population studies have also demonstrated that ovarian dysfunction precedes the onset of MDD (Harlow et al., 2003). In MDD patients, deficits have been found in estradiol (Young et al., 2000), luteinizing hormone and pituitary function (Young et al., 2000; Meller et al., 2001; Daly et al., 2003; Harlow et al., 2003) suggesting a potential causal relationship.
In fact, inhibition of HPG activity occurring in response to stress was linked to HPA hyperactivity (Halbreich and Kahn, 2001). Given that MDD is associated with increased levels of glucocorticoids, women with persistent MDD had two times the risk of earlier perimenopausal transition, and higher FSH and lower estradiol levels, suggesting an early decline in ovarian function (Young et al., 2000; Harlow et al., 2003). Few studies have focused on hormonal deficits in men with MDD, although deficits in androgens have been reported (Baischer et al., 1995; Rubinow and Schmidt, 1996; Schweiger et al., 1999; Seidman et al., 2001; Weiner et al., 2004). Luteinizing hormone pulse frequency and testosterone secretion in males with MDD were also lower (Schweiger et al., 1999), although not consistently (Rubin et al., 1989)
Low levels of estradiol seen in MDD premenopausal women may lead to decreased inhibitory feedback of the HPA axis (by hippocampus) in the presence of increased HPA drive with unopposed progesterone. In fact, in our functional imaging study in MDD women, gonadal hormone abnormalities (lower estradiol) were significantly associated with hippocampal (and amygdalar) brain activity deficits (Holsen et al., 2011). Decreased inhibitory feedback of the HPA axis may in turn account for elevated levels of cortisol in MDD women compared to MDD men or non-depressed women (Young and Altemus, 2004; Holsen et al., 2013). Additionally, in postmortem studies of MDD, CRH-producing neurons in PVN that co-localized with estrogen receptor alpha (ERα) were enhanced in MDD, again suggesting HPA-HPG interactions in MDD (Bao et al., 2005).
In the preclinical literature, sex differences in stress responsiveness for the HPA axis have been known for a long time (Handa et al., 1994a), and sex differences are reported in response to prenatal stress and resilience (Bowman et al., 2004; Richardson et al., 2006; Heim et al., 2009; García-Cáceres et al., 2010). The mechanisms underlying sex differences in the HPA axis can be tied to either organizational or activational responses to steroid hormones. Organizational actions of steroid hormones are those characterized as being permanent changes that occur in response to gonadal steroid hormone exposure during a critical period of development, whereas activational actions are transient effects that occur in response to changes or to sex differences in circulating hormone levels. Both actions are found in the control of the HPA axis. Seale et al (2004, 2005a, b) reported organizational effects of gonadal steroid hormones on the HPA axis where testosterone treatment of neonatal females within 24 hours of birth altered the HPA axis phenotype in adulthood to one resembling that of males. This was characterized by reduced corticosterone pulses, reduced corticosterone responses to stress and lower levels of CRH and arginine vasopressin mRNAs in the PVN. Correspondingly, inhibition of aromatase in neonatal males resulted in higher corticosterone levels at baseline and increased responses to stress in adulthood.
Activational effects of steroid hormones on the HPA axis can also be demonstrated. Sex differences in the localization of PVN peptides or receptors have been seen in adults with females having greater levels of ERα than males in the ventromedial hypothalamic nucleus whereas males had greater levels of ERα in the retrochiasmatic area than females (Scott et al, 2000). Correspondingly, estrogen-dependent effects have been reported for a number of genes, such as the kappa opioid receptors, which increased in the PVN following estradiol treatment (Yukhananov and Handa, 1996). The substrate for such influences was made all the more transparent with the reports of high levels of estrogen receptor beta in the PVN (Hrabovszky et al., 1998; Hrabovszky et al., 2004) and by molecular studies showing the ability of this steroid receptor to affect gene transcription in PVN (Handa et al., 2011). Roles for androgen receptors in the PVN have also been noted (Bingham et al., 2006)..
The possibility that MDD risk may be associated with changes in reproductive function, which can further amplify HPA hyperactivity, is also supported by preclinical studies showing that hormones of the HPA axis can profoundly inhibit reproduction in model animals. The simplest connection between the stress axis and the reproductive axis was suggested by the finding of immunoreactive glucocorticoid receptors in gonadotropin releasing hormone (GnRH) neurons (Ahima and Harlan, 1992). This concept was further bolstered by the discovery of specific promoter elements on the GnRH gene that were sensitive to glucocorticoid receptor activation (Chandran et al., 1996). However, the presence of glucocorticoid receptors in GnRH neurons may be species dependent (e.g., not found in ewes (Dufourny and Skinner, 2002)), and later studies have shifted some emphasis to key interactions at the level of the pituitary (Breen and Karsch, 2006).
Another mechanism by which HPA axis may influence reproductive function is via the peptide corticotrophin-releasing hormone (CRH). CRH is the most upstream hypothalamic factor regulating the HPA response to stress, and CRH also inhibits GnRH and gonadotropin secretion in model animal studies (Nikolarakis et al., 1986; Olster and Ferin, 1987) by disrupting the GnRH pulse generator (Li et al., 2010). More recently, CRH receptor mRNA (CRH-R1) was noted in murine GnRH neurons (Jasoni et al., 2005) and GR and CRH-R1 have been demonstrated in kisspeptin neurons of the anteroventral periventricular nucleus (Takumi et al., 2012), a group of upstream neurons that regulate GnRH function. Nonetheless, other more indirect routes for CRH influences on the HPG axis have been suggested, such as through noradrenergic neurons in the locus coeruleus (Traslaviña and Franci, 2012).
3. Brain circuitry linking MDD, HPA-HPG and ANS
3.1 Rationale
The comorbidity between MDD, HPA-HPG-axis dysregulation and CVD risk is not surprising from a brain circuitry point of view, given that depression is a disorder that involves hypothalamic nuclei (such as PVN and ventromedial nucleus), central medial amygdala, hippocampus, anterior cingulate cortex (ACC), and medial and orbitofrontal cortex (mPFC, OFC) (Dougherty and Rauch, 1997; Mayberg, 1997; Drevets et al., 2002; Sheline et al., 2002; Rauch et al., 2003), regions that are dense in glucocorticoid and sex steroid hormone receptors (MacLusky et al., 1987; Clark et al., 1988; Handa et al., 1994b; Kawata, 1995; Tobet and Hanna, 1997; Donahue et al., 2000; Ostlund et al., 2003) and can relay information that regulates cardiac function through the ANS. The overlap between these circuitries has been historically noted from behavioral and endocrinological findings. However, with the advent of magnetic resonance imaging (MRI) technology, there is a greater focus on the investigation of shared brain circuitry implicated in the regulation of mood, endocrine, and cardiac functioning. This technology allows for hypothesis-driven in vivo exploration of this shared circuitry.
Over the past 5 years, there has been a rapid increase in studies examining the relationship between endogenous and exogenous glucocorticoids and brain activity in stress responsive brain regions using a variety of functional MRI (fMRI) paradigms. These studies typically examine healthy control subjects, generally comprising mixed-gender samples with age ranges between 18–35 years. Amygdala and hippocampal activity in response to stimuli of high negative emotionality was positively associated with pre-versus post-scan (Root et al., 2009) and diurnal rises in salivary cortisol (Cunningham-Bussel et al., 2009). This relationship between hyperactivation in the amygdala and increased diurnal cortisol was supported by evidence indicating that when categorized by level of endogenous cortisol, individuals with high cortisol demonstrated greater amygdala activity than those with low cortisol levels (van Stegeren et al., 2007; van Stegeren et al., 2008). Of interest, the increase in amygdala activation in a high cortisol condition was blocked by administration of a noradrenergic antagonist (van Stegeren et al., 2007; van Stegeren et al., 2008).
Cushing syndrome (CS), associated with chronic hypercortisolemia, also appears to be associated with hyperactivity in arousal regions. Adolescents with CS, compared with age- and gender-matched controls, demonstrated increased activation of the amygdala and hippocampus during successful encoding of emotional faces, despite similar memory performance (Maheu et al., 2008). Further, adults with CS showed hyperactivation in the anterior hippocampus, medial frontal gyrus, ACC, caudate, and superior parietal lobule during identification of emotional facial expressions. Accuracy in CS patients was lower and correlated with brain activity, suggesting these differences could be partially explained by compensatory recruitment of these regions (Langenecker et al., 2012). However, in general, these findings point to a pattern of significantly enhanced activation in the presence of heightened endogenous cortisol levels in healthy controls and Cushing syndrome patients. These relationships between brain activity and HPA response are not surprising given the shared neural circuitry regulating stress and HPA (as well as HPG) responses (see Figure 1).
In cortical stress response circuitry regions, however, somewhat contrasting results emerged with some reporting lower and some reporting higher cortical activity in prefrontal and ACC regions in association with cortisol response to stress (Pruessner et al., 2008; Root et al., 2009). Although several of these studies included sex as a covariate in the analyses, only one focused specifically on sex differences (Wang et al., 2007). Taken together, these findings suggested a complicated picture of endocrine effects on brain activity in subcortical and cortical arousal regions which may be better clarified if studies were specifically designed to investigate sex effects.
Not all subjects can be classified as natural cortisol “responders” to specific stimuli. (Wust et al., 2000a; Wust et al., 2000b; Muehlhan et al., 2011) Thus, one methodological alternative has been to experimentally administer exogenous cortisol (i.e., hydrocortisone). In general, amygdala and hippocampus appeared to be most sensitive to cortisol, demonstrating significant decreases in activation in comparison to placebo (Lovallo et al., 2010). Striking sex differences in the neural response to cortisol (versus placebo) during fear conditioning (circuitry associated with stress response (Lebron-Milad et al., 2012) have been observed, with increased activation in the ACC, OFC, and mPFC in response to the conditioned (versus unconditioned) stimulus in females and decreases in these same regions in males (Stark et al., 2006; Merz et al., 2010).
3.2 HPA-HPG Alters Stress Response Circuitry Activation
Although studies on the HPA hormone-brain relationships occasionally reported controlling for menstrual cycle phase in women (Stark et al., 2006), gonadal hormone relationships with brain activity in functional neuroimaging studies have primarily involved cognitive tasks with fewer reports using emotional tasks as the endpoint. We recently demonstrated that activation in stress response circuitry regions was modulated across menstrual cycle in healthy women in response to stressful images, with greater activation in anterior hypothalamus, amygdala, hippocampus, ACC, and OFC during the early follicular phase compared with late follicular/midcycle (Goldstein et al., 2005). Further, in imaging studies of healthy women, hyperactivity of the amygdala and hippocampus was found during late luteal compared with early follicular menstrual cycle phase (Andreano and Cahill, 2010). Importantly, estradiol levels were negatively associated with amygdala activation (Andreano and Cahill, 2010). A direct comparison between males and females showed a consistency with these patterns, with greater hyperactivity in men than women in a number of subcortical and cortical stress response regions (especially the latter), particularly when women were scanned during their late follicular/midcycle compared to when the men were compared to the same woman scanned during the early follicular menstrual cycle phase (Goldstein et al., 2010). The results suggested that gonadal hormones modulated subcortical arousal by prefrontal circuitry in the healthy brain (Goldstein et al., 2005; Goldstein et al., 2010).
Inhibitory responses to negative (versus neutral) emotional stimuli targets during the luteal phase (versus follicular) were associated with greater activation in the medial OFC (Protopopescu et al., 2005), ACC, dorsolateral prefrontal cortex, and putamen (Amin et al., 2006), although not consistently (Dreher et al., 2007; Rupp et al., 2009a). Discrepancies may have been related to differences in menstrual phase definition, with follicular phase defined as days 4–8 (Dreher et al., 2007), 8–12 (Protopopescu et al., 2005), or 10–12 after the start of menstruation (Rupp et al., 2009a), and luteal phase defined as 19–23 days following the start of menstruation (Rupp et al., 2009b), 6–10 days post luteinizing hormone surge (Dreher et al., 2007), or 1–5 days before menses onset (Protopopescu et al., 2005). Although healthy control women in these samples had regular cycles, this variation in phase definition across studies could have had significant effects on interpreting the changing circulating estradiol and progesterone levels, leading to substantial differences reported in these studies in brain activity across the menstrual cycle.
Experimental studies of effects of exogenous gonadal hormone regulation of neural responses to emotional stimuli demonstrated that compared with placebo, progesterone administration during the early follicular phase was related to increased amygdala reactivity to emotional face processing, increased amygdala-dorsal ACC connectivity, and decreased amygdala-fusiform gyrus connectivity (van Wingen et al., 2008b). In contrast, testosterone administration increased hippocampus and inferior temporal gyrus activation during memory formation and retrieval of male faces in middle-aged women (van Wingen et al., 2008a). Compared with placebo, testosterone increased amygdala responsiveness to levels equivalent to those observed in young women (van Eijndhoven et al., 2009) and reduced functional connectivity between the amygdala and OFC (van Wingen et al., 2010). Thus, administration of exogenous gonadal hormones exerted significant influence on amygdala responsiveness in general and coupling between the amygdala and other limbic and cortical regions during evaluation of emotionally salient cues.
A few studies recently demonstrated compelling evidence of links between HPA-HPG hormone dysregulation and brain activity deficits in MDD. Cortisol administration to currently depressed women resulted in increased hippocampal activation during encoding of neutral (versus negative or positive) words in comparison to healthy control women, a trend not observed during placebo and not in men (Abercrombie et al., 2011). Further, premenopausal women with recurrent MDD displayed hypoactivity in a number of regions involved in the stress response circuitry that were significantly associated with gonadal hormone deficits (Holsen et al., 2011), i.e., decreased estradiol and increased progesterone levels in MDD women during late follicular/midcycle phase of the menstrual cycle. Finally, hypoactivation to positive stimuli in the nucleus accumbens and hyperactivations in the amygdala and lateral OFC in response to negative stimuli during the luteal phase (versus late follicular) were reported in women with premenstrual dysphoric disorder compared with healthy controls (Protopopescu et al., 2008). Taken together, findings from these studies indicated complex interaction between HPA (cortisol) and HPG (progesterone, estradiol) dysregulation and brain activation during emotional processing (respectively) in women with mood disorders, providing support for mechanisms implicating neuroendocrine systems associated with sex differences in depression.
3.3 Mood, Endocrine and ANS Share Brain Circuitries
Historically, emotions and visceral function have been intimately associated, the latter of which is controlled by the ANS, coordinated to a large extent by the hypothalamus (Papez, 1995). Subsequently, a more complex network has been implicated including some highly sexually dimorphic brain regions of interest, such as amygdala (Zola-Morgan et al., 1991; Kluver and Bucy, 1997), hippocampus, cingulate cortex (Mac, 1949; Papez, 1995), medial PFC, and periacqueductal gray (Price and Drevets, 2010). In fact, MDD has been characterized by some authors as maladaptive stress-induced neuroplastic alterations in the medial prefrontal cortico-amygdalo/hippocampo-hypothalamo-brainstem circuits (Krishnan and Nestler, 2008; Murray et al., 2011). Vagal dyscontrol of the solitary nucleus and motor nucleus of the vagus in the brainstem, which are innervated by preautonomic neurons of the PVN, can affect heart and cardiovascular function (Swaab et al., 2003); (see Figure 3).
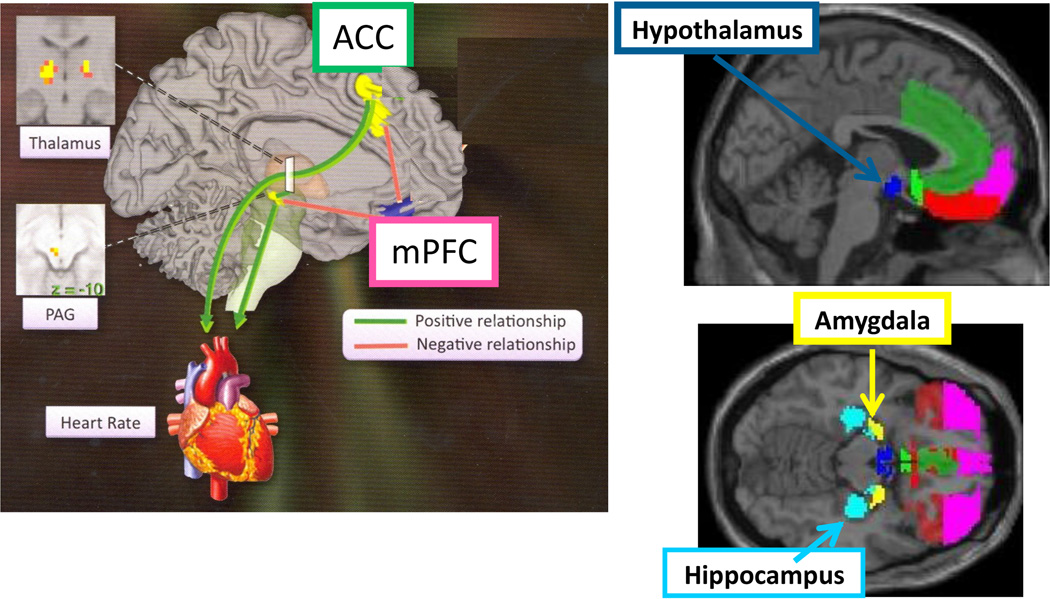
Figure 3. Brain Regions Shared between Mood, Stress, and ANS.
Brain regions implicated in CNS regulation of stress, mood and anxiety disorders are also involved in the CNS control of the heart (through ANS). Left image and upper right is parasagittal and lower right is horizontal. Shared sex-dependent development of this neural circuitry provides a rationale for the fetal programming of sex differences in the comorbidity of MDD and CVD. (Image adapted from Lane and Wager, NeuroImage 2009, volume 47, issue 3)
Linking ANS activity to various metabolic abnormalities and MDD has gained popularity in the brain imaging literature (Licht et al., 2008; Dao et al., 2010; Henry et al., 2010). The time interval between two R-waves during the recording of heart rate (HR) by electrocardiogram is referred to as the R-R interval. Fourier or autoregressive analysis of the cyclical oscillations in the R-R interval (or R-R variability; RRV) produces power spectra, portions of which reflect different autonomic influences on heart rate and blood pressure. Sympathetic activity causes a slow increase in HR and is thus reflected in the low-frequency (LF) range of 0.04 to 0.15 Hz. Parasympathetic or vagal activity causes a rapid decrease in HR and is associated with the relatively high-frequency (HF) range of 0.15 to 0.40 Hz (Malik et al., 1996). The spectral components of the RR-intervals have been defined as heart-rate variability (HRV) or RRV, and reflect sympathovagal balance (Malik et al., 1996).
Research suggests that increased sympathetic and decreased parasympathetic activity (cardiac regulation from the CNS through the vagal nerve) in response to stress may represent a unique window to visualize underlying biological mechanisms involved in MDD and its comorbidity with CVD. Decreased RRV is associated with the incidence of cardiac events and coronary disease (Tsuji et al., 1996; Liao et al., 1997), atherosclerosis (Huikuri et al., 1999), mortality in men (Dekker et al., 1997), and MDD (Gorman and Sloan, 2000; Nahshoni et al., 2004; van der Kooy et al., 2006; Licht et al., 2008; Lane and Wager, 2009); (Licht et al., 2010), and with the comorbidity of MDD and CVD risk (particularly in women), demonstrated in our recent study (Goldstein et al., 2011). Specifically, MDD has been associated with parasympathetic cardiac dysregulation (Yeragani et al., 1991; Gorman and Sloan, 2000; Nahshoni et al., 2004; van der Kooy et al., 2006; Holsen et al., 2012), operationalized primarily as the high frequency component of the R-R interval variability.
Recent studies have investigated the abnormal brain circuitry correlates of ANS dysregulation, although primarily in healthy participants. The investigations on heart rate reactivity and RRV in association with emotional tasks (Lane et al., 2001; Simpson et al., 2001; Kuniecki et al., 2003; Critchley et al., 2005; O'Connor et al., 2007; Ahs et al., 2009; Lutz et al., 2009; Mujica-Parodi et al., 2009; Wager et al., 2009a; Wager et al., 2009b) reported activation of CNS regions implicated in ANS control, including hypothalamic nuclei, brainstem regions, and amygdala, and medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and insula. This circuitry was activated regardless of sample size or whether assessed using pulse oximetry or an electrocardiogram.
Reviewing the literature in healthy populations, Lane and Wager (Lane and Wager, 2009) defined a medial-prefrontal-brainstem network implicated in ANS control in tandem with endocrine, emotion, and behavior (see Figure 3). In an elegant series of studies by Wager and colleagues (Wager et al., 2009a; Wager et al., 2009b), two main pathways mediated brain-heart rate (HR) relationships in response to stress: increases in the pregenual ACC [mediated by connections with periaqueductal gray (PAG)] and decreases in ventromedial PFC (vmPFC)/medial OFC (mOFC). Ventromedial PFC/mOFC was particularly important in mediating anxiety and HR associations (Wager et al., 2009a). We extended this work to investigate brain CNS-ANS associations in stress response circuitry in women with recurrent MDD, demonstrating that hypoactivations of stress response circuitry in anterior hypothalamus, hippocampus, ACC, and OFC were significantly associated with a loss of parasympathetic cardiac regulation in premenopausal MDD women (Holsen et al., 2012), controlled for menstrual status and potential medication confounds.
Findings that implicated limbic brain regions in ANS regulation were not surprising given that they are critical in the regulation of mood. Experimental evidence for the fact that limbic regions, such as hippocampus and amygdala, impact changes in autonomic tone date as far back as 1956 (Anand and Dua, 1956; Schwaber et al., 1982). These regions also affect autonomic regulation through their projections to the anterior hypothalamus and the control of the HPA and HPG circuitries. Further, paralimbic regions, such as the orbitofrontal cortex and anterior cingulate gyrus, also regulate autonomic tone, demonstrated in human studies (Mesulam and Mufson, 1982) as well as stimulation studies in monkeys (Kaada et al., 1949; Hoffman and Rasmussen, 1953). Previous work demonstrated that menstrual status significantly affected RRV (Dart et al., 2002; McKinley et al., 2009), and most studies have not controlled for menstrual status in female participants. Sex differences in RRV (Zhang, 2007) were reported in one study, and thus we are currently investigating sex differences in shared brain circuitries regulating mood and cardiac dysregulation. Increased sympathetic activity and decreased vagal activity have been proposed as biomarkers of the severity of depression (Kemp et al., 2010) and thus understanding this comorbidity and associated sex differences will be key to understanding the nature of MDD and associated risk for CVD.
3.4 Sex differences in shared mood, endocrine and ANS circuitries
Circuitry shared by mood, endocrine and ANS regulation includes the most highly sexually dimorphic regions in the brain (see Figure 4). Thus, we have argued that an understanding of the disruption of this circuitry during fetal development may provide critical clues to understanding the fetal programming of sex differences in their comorbidity. On the human level, in vivo imaging and postmortem studies demonstrated sex differences in these regions at the gross brain volume level in brain imaging studies or nuclei level in postmortem work. In women, relative to cerebrum size, findings demonstrated greater relative volumes of hippocampus (Filipek et al., 1994; Giedd et al., 1996; Murphy et al., 1996; Goldstein et al., 2001), ACC (Paus et al., 1996; Goldstein et al., 2001), and OFC (Goldstein et al., 2001). In men, there are greater volumes (relative to cerebrum size) of the amygdala (Giedd et al., 1996; Goldstein et al., 2001), hypothalamus (Swaab and Fliers, 1985; Allen et al., 1989; Goldstein et al., 2001), and paracingulate gyrus (Paus et al., 1996; Goldstein et al., 2001). Thus, women tend to have relatively larger volumes of hippocampus, OFC and ACC, whereas men have relatively larger amygdala and hypothalamic volumes (see Figure 4). Recent findings offer additional evidence that even structural brain volumes in women may show some variation at different points in the menstrual cycle, as demonstrated with hippocampal gray matter volume increased and dorsal basal ganglia gray matter volume decreased during follicular compared with luteal phase (Protopopescu et al., 2008). Further, estradiol, progesterone, and testosterone levels in young adults were associated with 13%, 13%, and 2% of the variation in superior parietal gyrus, medial temporal pole, and inferior frontal gyrus gray matter volumes, respectively (Licht et al., 2010), suggesting significant activational associations between gonadal hormone levels and neuroanatomic variation in humans.
Figure 4. Stress Response Circuitry in the Brain is Highly Sexually Dimorphic.
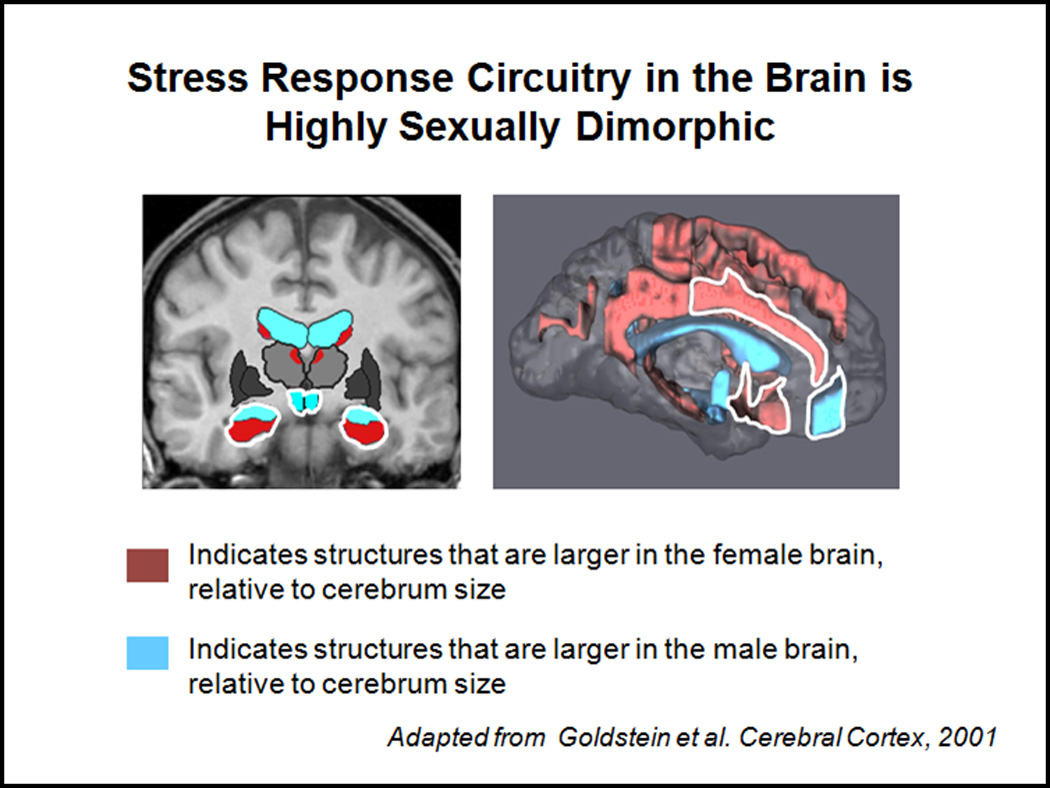
Brain regions implicated in the stress response circuitry are some of the most highly sexually dimorphic brain regions in the brain (e.g., hypothalamic nuclei, central medial amygdala, hippocampus, mPFC, ACC). Figure 3 highlights the brain regions in MR images and shows which regions are larger in volume (cm3) in the female brain (in red), relative to cerebrum size, and which are larger in the male brain, relative to cerebrum size (in blue). (Adapted from Goldstein, et al., 2001, Cerebral Cortex)
4. Towards Determination of Mechanisms
Together, studies on HPA, HPG, stress behavior, and ANS circuitries suggest a shared biologic substrate underlying comorbidity of MDD and CVD that has developmental origins and is sex-dependent. A growing body of data is consistent with a prenatal stress model that involves excess maternal glucocorticoids impacting sex-dependent development of fetal stress circuitry implicating disruptions in GABA and growth factors with consequences for mood, endocrine function, ANS and vascular abnormalities (as illustrated in Figure 5).
Figure 5. Mechanisms Associated with Sex-Dependent Fetal Stress Circuitry Development Implicating Comorbidity of MDD and CVD in Adulthood.
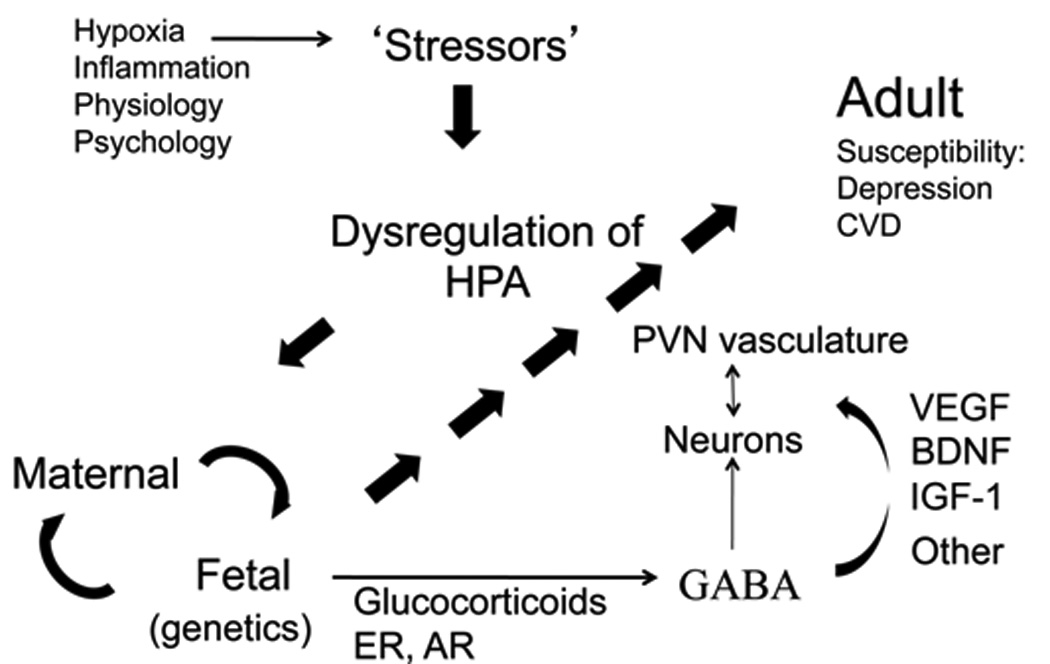
Schematic depiction of the interactions among stressors of the mother leading to dysregulation of her HPA axis that then influences maternal-fetal interactions in a sex-dependent developmental manner. In the fetal compartment, glucocorticoids and sex steroid influences (e.g., estrogen and androgen receptors (ER, AR)) impact GABA and growth factors, which alter neuronal and vascular cellular components.
4.1 Impact of Sex Differences in Brain Development
Sex differences in brain development are driven by synergies of hormone influences and selective gene transcription. Although recent work has identified direct genetic contributions to sexual differentiation of the brain (De Vries et al., 2002; Arnold, 2011; Majdic and Tobet, 2011), the primary driver of this process has always been noted at the level of gonadal hormone regulation. This is initiated when the testes begin to secrete testosterone at the beginning of second trimester, at which time both androgens and estradiol (which increases by the conversion of testosterone to estradiol by the enzyme aromatase) masculinize the male brain. This has been demonstrated in animal studies (McEwen, 1983; Tobet et al., 1985; Simerly et al., 1990; Tobet et al., 1993; Handa et al., 1994b; O'Keefe et al., 1995; Park et al., 1996; Gorski, 2000; Chung et al., 2006). Findings in humans indirectly suggest that this too may, in part, contribute to understanding sexual dimorphisms in adulthood (Goldstein et al., 2001), although the role of estradiol in humans is less clear as neither aromatase (Grumbach and Auchus, 1999; Maffei et al., 2004) or estrogen receptor (Smith et al., 1994) deficient individuals are lacking in sexual interest. In animals, nuclei of the corticomedial amygdala, PVN, ventromedial hypothalamic nucleus, hippocampus, OFC and ACC express high concentrations of gonadal and/or adrenal hormone receptors compared with other brain regions (Handa et al., 1994b; Pacak et al., 1995; Koob, 1999; Solum and Handa, 2002; Tobet, 2002; Ostlund et al., 2003; Lund et al., 2004a; Lund et al., 2004b; Suzuki and Handa, 2004; Lund et al., 2006). These brain regions have been implicated in regulating HPA and ANS functions and are abnormal in MDD patients. The hypotheses in this review are, in part, based on the premise, supported by work on sex differences in another disorder with fetal origins (schizophrenia) (Goldstein et al., 2002), that normal sexual dimorphisms during fetal development go awry in brain regions associated with mood, HPA and ANS functions, and that mechanisms involved in understanding normal sexual dimorphisms, such as the roles of gonadal and adrenal hormones (in association with genes) (Handa et al., 1994c; Majdic and Tobet, 2011) will contribute to understanding sex differences in risk for comorbid MDD and CVD risk in adulthood. Work with model animals has been a primary driver of this notion.
4.2 Changes in GABA Implicated in MDD
There is a long history of study on the role of GABA in brain sexual differentiation (e.g., (Davis et al., 1996; McCarthy et al., 2002; Tobet et al., 2009). Sex-dependent disruption of the development of sexually dimorphic brain regions implicated in MDD may involve GABA signaling pathways (Gao and Bao, 2011; Stratton et al., 2011; Tobet et al., 2013). GABA has been implicated in the development of MDD in ex vivo and in vivo studies, particularly its role in understanding the efficacy of antidepressant medications. MDD patients have consistently shown reduced plasma, cerebrosprinal fluid (CSF), and cortical levels of GABA as compared to healthy controls (Chang et al., 2003; Pilc and Nowak, 2005; Kalueff and Nutt, 2007; Sanacora and Saricicek, 2007), findings that have been in part replicated in model animal studies (Alcaro et al., 2010). The majority of GABA studies in MDD have focused on its therapeutic role, including interactions with serotonin and neuronal systems targeted by selective serotonin reuptake inhibitors (SSRIs) (Taylor et al., 2003), although GABAergic pharmaceuticals in and of themselves have shown low efficacy in MDD patients. Polymorphisms in GABAergic genes have been identified in MDD patients (Liu et al., 2007) as has altered levels of GABA in first-degree unaffected relatives of MDD patients (Petty, 1994). Postmortem findings demonstrated that mRNA levels of GABA receptors in frontopolar cortex were lower in MDD suicide cases than controls (Merali et al., 2004; Choudary et al., 2005). Sex differences in GABA in MDD cases have also been reported previously (Sanacora et al., 1999), although few have focused on this issue and designed studies to avoid confounding the associations among sex, hormones, medications, and GABA. In fact, earlier studies reported that GABA levels decreased in the mid-follicular menstrual cycle stage among healthy women (Halbreich et al., 1996), although this was not replicated at the level of cortical GABA levels and gonadal hormones (Epperson et al., 2006).
There have been a number of preclinical studies indicating a role for GABA using model animals (reviewed (Cryan and Slattery, 2010; Luscher et al., 2010)). Although animal findings based on the forced swim test (a model of depressive-like behaviors) did not identify significant associations with GABAA, alpha 1, or GABAA, alpha 3 (Verkuyl et al., 2004), knockout mice for GABAA gamma2 subunits may provide a substantially better model (Shen et al., 2010; Shen et al., 2012). Further, examining the metabotropic GABAB receptors, knockout mice for GABAB R1 subunit displayed antidepressant-like activity and GABA dysfunction (Cryan and Kaupmann, 2005). Given the pivotal role of the PVN in HPA axis outflow, it is perhaps not surprising that GABA may play role(s) in prenatal stress models, not only for PVN neuronal development but also PVN vasculature, suggesting potential links between MDD and CVD risk. As we recently demonstrated, PVN development is regulated by glucocorticoids and GABA, with females being particularly vulnerable to GABA disruption in PVN neuronal (McClellan et al., 2010; Stratton et al., 2011) and vascular development (Frahm et al., 2012).
It is important to note that there are other neurotransmitter connections that can be drawn across multiple disorders (Gao and Bao, 2011; Blier and El Mansari, 2013). As reviewed above, there are substantial reasons for focusing on GABA, but these are not meant to be exclusive of other mechanisms, some of which are reviewed further below.
4.3 Glucocorticoid Excess during Development Programs Adult Function
The hypothesis that glucocorticoid excess leads to the propensity for adult disorders has been tested in a number of studies over the last two decades (Reynolds, 2012). This work received a great deal of attention when Barker and colleagues in the 1980’s proposed a fetal programming model of CVD (Barker, 2012). Preclinical studies using a number of different model animals demonstrated the impact of prenatal stress on a host of HPA-related outcomes, including hypothalamic and hippocampal structure and function (Takahashi et al., 1992; Matsumoto and Arai, 1997; Weinstock, 1997), with lasting effects on the HPA axis by programming a “hyperactive” system vulnerable to ANS deficits, hyperglycemia and hypertension (Weinstock et al., 1992; Henry et al., 1994; Barker, 1995; Arborelius et al., 1999; Seckl, 2001). Evidence from model animal and human studies suggested that a variety of prenatal stressors altered fetal cardiac function and parasympathetic regulation of the heart (Manning, 1995; Schifrin, 1995); (Prechtl, 1984; Lee et al., 1998). Inflammation (which has been associated with prenatal stress models) has been postulated as one pathway linking autonomic dysregulation to atherosclerosis, through what Tracey and colleagues called the vagal anti-inflammatory reflex (Tracey, 2002).
Exactly how glucocorticoid excess in development programs elevations in blood pressure in adulthood is still under investigation (Edwards et al., 1996). In observational studies of humans though, higher blood pressures were observed among adolescents exposed to corticosteroids antenatally (Doyle et al., 2000). Explanations have included, for example, long term changes in glucocorticoid receptors (Levitt et al., 1996) or reduction in endothelial nitric oxide (NO) production (Wallerath et al., 1999). Further, hypotheses focused on placental compromise are critical in the pathway between maternal excess glucocorticoids and fetal response and are currently under investigation (O'Regan et al., 2001; Howerton et al., 2013).
CRH is produced in large quantities by the healthy placenta in late pregnancy and contributes to the initiation of parturition (Frim et al., 1988; Giles et al., 1996). Placental 11-beta-hydroxysteroid dehydrogenase (11-beta-HSD, type 2 isoform) is produced to protect the fetus from overexposure to glucocorticoids. Investigators have proposed that the generalized placental response when compromised is to produce increased quantities of CRH. In preclinical studies, even a reduction of protein intake during rat pregnancy decreased 11-beta-HSD activity (Edwards et al., 1993; Edwards et al., 1996; Langley-Evans et al., 1996a; Langley-Evans et al., 1996b; Churchill et al., 1997; Langley-Evans, 1997; Seckl, 2001). Thus, conditions producing a maternal prenatal stress response may result in placental compromise, which may be related to studies in humans demonstrating a significant association between reduction in placental 11-beta-HSD, type 2 isoform and MDD in adulthood (Poor et al., 2004).
In fact, pregnancy conditions associated with placental compromise and fetal vascular adverse outcomes were related to disproportionate elevations in circulating CRH levels, including fetal growth restriction (Goland et al., 1993; Petraglia et al., 1995; Giles et al., 1996), inflammation in general (Petraglia et al., 1995), preeclampsia and other hypertensive states in pregnancy (Warren et al., 1995), and preterm delivery (Wolfe et al., 1988; Warren et al., 1992), pregnancy complications that have been associated with adult risk for MDD and CVD. Chronic villitis and uteroplacental ischemia evoked enhanced placental CRH release. Furthermore, vascular compromise, assessed by umbilical artery velocimetry, was associated with increased CRH concentrations in the fetal compartment (Giles et al., 1996). Unlike in the hypothalamus, placental CRH and cortisol participate in a feedforward loop (Robinson et al., 1988; Karalis et al., 1996; Majzoub et al., 1999). Thus, when placental compromise elevated CRH, it had the potential to increase fetal adrenal activity and hence, fetal exposure to glucocorticoids and vascular compromise.
There is recent evidence that vascular compromise and cardiac dysregulation are sex-dependent. For example, human studies of the impact of low birth weight on young adult ANS and baroreceptor control of cardiac function demonstrated that females compared with males showed greater cardiac dysregulation and reduced baroreflex sensitivity in response to stress (Jones et al., 2007). Sex differences in ANS function were reported in several studies (Evans et al., 2001; Snieder et al., 2007), with an emphasis on women’s reliance on parasympathetic ANS control and the impact of the menstrual cycle on RRV (Dart et al., 2002; McKinley et al., 2009). These studies, although few in number, provide initial evidence of the importance of designing studies to test the sex-dependency of the fetal programming of vascular and cardiac outcomes, which is rarely considered at the human or preclinical level.
A number of preclinical studies using model animals have underscored this point. Administration of dexamethasone to pregnant ewes resulted in elevated blood pressure later in life (Dodic et al., 1999) (Dodic et al., 2006) In the rat model, such administration resulted in modified hepatic receptor expression and glucose intolerance in the adult offspring (Nyrienda et al., 1998). Similarly, prenatal exposure to dexamethasone in rat models demonstrated reductions in growth and alterations in hepatic triglyceride levels (Drake et al., 2010). When this model was explicitly investigated by sex, these alterations were more severe in females, accompanied by increased hepatic steatosis when placed on a high fat diet (Carbone et al., 2012a). Sex-dependent outcomes (i.e., more in females) were characterized by deficits in growth hormone releasing hormone mRNA within the arcuate nucleus and circulating insulin-like growth factor-1(IGF-1) (Carbone et al., 2012a). Further, in a different study, sex-dependent effects were found in rodents exposed to prenatal dexamethasone, which resulted in lower resting state blood pressure in adulthood. However, they were more susceptible to a hypertensive phenotype even after a very mild stressors (O'Regan et al., 2008), a susceptibility that was more prevalent in females (O'Regan et al., 2004).
Independent of the rise of fetal programming models of CVD in the mid-1980’s, psychiatry since Freud has had a much longer history of an etiologic focus on early developmental factors (including fetal brain development) that impact adult psychiatric outcomes. Population-level studies have demonstrated fetal risk factors for adult onset MDD (van Os et al., 1997; Watson et al., 1999; Brown et al., 2000), for which a final common pathway involving maternal-fetal HPA circuitry activation was proposed (Nemeroff et al., 1984; Holsboer et al., 1987; Arborelius et al., 1999; Heim et al., 2002; Parker et al., 2003; Raison and Miller, 2003; Barden, 2004; Owen et al., 2005). Further, at the human population level, there was a sex-dependent (females higher) risk of the comorbidity of MDD and CVD when exposed to preeclampsia or conditions producing fetal growth restriction (Goldstein et al., 2011).
Preclinical studies have demonstrated sex-dependent effects of prenatal stress on behavioral development for a long time, e.g., (Joffe, 1965). The influence of sex steroids in the process came to the forefront with experiments that more selectively addressed sex behavior (Ward, 1972) and then progressed to other behaviors (Meisel et al., 1979). A selective tie to sex steroids was connected to direct changes in the androgen-metabolizing enzyme, aromatase, in the brains of prenatally stressed rats (Weisz et al., 1982). Between the early studies where stress was administered only late in pregnancy and more recent studies where stress has been examined as a function of different trimesters of pregnancy (Goel and Bale, 2009), it is increasingly clear that the effects of sex steroids, and mechanistic pathways to understand these effects differ across prenatal stages.
Along with considering that different stages of pregnancy evoke differential susceptibilities is also the issue of defining just what can be considered as “models” of prenatal stress. Studies range from restraint stress with light and heat (Ward, 1972) to chronic variable stress (Mueller and Bale, 2006) that involved a combination of mild stressors that were chosen based on their lack of causing pain or impacting maternal energy balance. On the other hand, one aspect of maternal stress is an increase in plasma glucocorticoids. In this sense, a substantial number of studies examined the influence of excess fetal glucocorticoid exposure (see above and also (Harris and Seckl, 2011; Carbone et al., 2012b). Thus, given independent evidence for the impact of excess maternal glucocorticoid exposure in fetal development on cardiac and depression outcomes, some of which are sex-dependent, we argued here that this is one mechanism in the pathway for understanding the fetal programming of sex-dependent comorbidity of these outcomes (Musselman et al., 1998; Seckl, 2001; Grippo and Johnson, 2009; Goldstein et al., 2011; Tobet et al., 2013).
4.4 Growth Factors, MDD and CVD
Growth factors (brain-derived nerve growth factor (BDNF), vascular endothelial growth factor (VEGF), and IGF-1) are expressed by cells in the brain and heart and are critical for normal development and in response to injury. We argued that maternal glucocorticoid elevations during pregnancy can affect the sex-dependent development of HPA regions through mechanisms related to the disruption of these growth factors, whose actions are shared by the brain and vasculature (Tobet et al., 2013). The neurotrophin, BDNF, has been found in the heart, and the angiogenic factor, VEGF, has been found in the brain. These growth factors are critical for the proper development of the brain and heart, but they also have significant roles in the maintenance of adult blood vessels and normal response to injury such as with inflammation.
BDNF and its receptor TrkB have critical neurotrophic roles in brain development and functioning, in particular, in PVN and hippocampus, and implicated in the etiology of MDD (Schumacher et al., 2005; Martinowich et al., 2007; Pittenger and Duman, 2007). Serum BDNF is decreased in unmedicated MDD patients relative to healthy controls, an effect that is reversed following antidepressant treatment (Shimizu et al., 2003). Postmortem studies demonstrated that hippocampal BDNF and trkB expression were decreased in unmedicated and increased in medicated MDD patients (Chen et al., 2001), an effect linked to BDNF’s role in adult neurogenesis (Duman and Monteggia, 2006). Preclinical studies demonstrated that stress decreases (Smith et al., 1995) while antidepressant treatment increases (Nibuya et al., 1995; Russo-Neustadt et al., 2000) expression of BDNF mRNA and protein. Reciprocally, direct hippocampal infusions of BDNF protein produced antidepressant effects (Siuciak et al., 1997; Shirayama et al., 2002). Conditional gene knock-out models and genetic association studies further implicated BDNF variants in depressive behaviors (Monteggia et al., 2007) and MDD (Schumacher et al., 2005). A common polymorphism of the BDNF gene, val66met, is characterized by reduced levels of BDNF and has been linked to impaired hippocampal and prefrontal cortical function in humans (Egan et al., 2003), regions important in the regulation of the stress response. Imaging genetics studies have also found evidence of dysregulated stress circuitry activity in met carriers, including greater amygdala and hippocampal activity during a stress response task (Montag et al., 2008; Schofield et al., 2009; Lau et al., 2010). Thus, a substantial amount of research in MDD has focused on the importance of the role of BDNF, although there is a lack of focus on the sex dependent impact of BDNF.
Growth factors in general often have effects on cell survival, and in preclinical studies, it has been shown that their expression is regulated by hormones, such as estradiol and glucocorticoids (Carbone and Handa, 2012; Gray et al., 2012; Jeanneteau and Chao, 2012; Suri and Vaidya, 2012). Further, it has been shown that prolonged glucocorticoid levels enhance neuronal cell death (Reagan and McEwen, 1997), perhaps through morphological changes in hippocampus (Conrad et al., 2007) that may make those neurons more susceptible to neurotoxic challenges (Landfield et al., 1978; Uno et al., 1989; Conrad et al., 2007). More relevant to the programming of fetal brain, increased apoptotic cell death has also been described in the amygdala and other limbic structures after glucocorticoid treatment in developing rats (Zuloaga et al., 2011; Zuloaga et al., 2012a; Zuloaga et al., 2012c).
In fact, maternal treatment with DEX caused hypomethylation of the BDNF promoter in females only in PVN and hippocampus (Carbone and Handa, 2012). This raises the possibility that epigenetic changes (i.e. functional changes to the genome that are not through changes in nucleotide sequence) may be involved in the actions of steroid hormones. Indeed, epigenetic effects on the developing nervous system that result in sex differences in adult brain function have now been reported (McCarthy et al., 2009; Qureshi and Mehler, 2010). However, the absence of GABAB signaling resulted in lower BDNF levels in PVN of mice (McClellan et al., 2010).
Although BDNF has been implicated in MDD etiology (as discussed above) (Nibuya et al., 1995; Duman, 2004), less is known about the role of this neurotrophin in non-neuronal cells, such as endothelial cells, and in vessel survival and stabilization following vascular injury (Kermani and Hempstead, 2007). Endothelial cells in heart arteries and capillaries, regulators of vascular homeostasis, express BDNF and trkB during development, are particularly high in adulthood (Kermani et al., 2005), and are increased following vascular injury (Kraemer, 2002). These studies suggest an important BDNF role in vascular repair in addition to its role in neuron maintenance and synaptogenesis (Lafuente et al., 2012).
VEGF is a major angiogenic protein that also acts in development and in response to injury in adulthood. In fact, VEGF is found in high density in PVN (Alonso et al., 2005), one of the most highly vascularized brain regions (Finley, 1937; Menendez and Alvarez-Uria, 1987; Frahm et al., 2012), and in hippocampus (Newton et al., 2003). It has also been found to mediate the effect of BDNF on antidepressant treatment in MDD in the hippocampus (Cao et al., 2004; Warner-Schmidt and Duman, 2007), suggesting a neurogenic role for VEGF in the adult hippocampus in MDD. It will be important to test the hypothesis that disruption of maternal gestational glucocorticoids work in part through dysregulation of growth factors in the heart and brain, particularly in PVN and hippocampus, areas important for HPA axis regulation. In fact, DEX administration into adult PVN suppressed VEGF (Alonso et al., 2005), suggesting that effects of glucocorticoids in PVN suppress growth factors.
There is a great deal of work on the role of IGF-1 in the development of CVD, given its relationship to growth hormone (GH) and insulin. Studies have supported a U-shaped relationship in that lower fetal IGF-1 in mice impaired cardiac tissue development, but excess IGF-1 led to excessive growth (LeRoith, 2010). In clinical studies, IGF-1 was inversely proportional to high sensitivity C-reactive protein, a critical inflammatory CVD risk indicator (Sesmilo et al., 2001; Lawson et al., 2007). These studies further reported that estradiol reduced IGF-1 and promoted GH resistance (Heald et al., 2005; Lawson et al., 2007), suggesting potential sex differences in the regulation of IGF-1. In this regard, Carbone and Handa (Carbone et al., 2012a) also demonstrated that, in rats, fetal exposure to synthetic glucocorticoids reduced circulating IGF-1 levels and arcuate nucleus Growth Hormone Releasing Hormone mRNA levels in adulthood, an effect that was only seen in females.
Animal models of chronic stress in IGF-1 treated mice (Duman et al., 2009) and IGF-1 KO mice had a significant impact on reducing immobility, underscoring its antidepressant-like properties (Hoshaw et al., 2005; Malberg et al., 2007; Duman et al., 2009). In healthy populations, IGF-1 decreased with age (Deuschle et al., 1997; Franz et al., 1999; Unden et al., 2002; Weber-Hamann et al., 2009) and was positively associated with decreased levels of depressive symptoms (Unden et al., 2002). However, in MDD, increased IGF-1 was associated with hypercortisolemia (Weber-Hamann et al., 2009); (Deuschle et al., 1997; Franz et al., 1999) but dependent on the woman’s menstrual cycle phase (Franz et al., 1999). Thus the role of IGF-1 in MDD is still unclear and in part inconsistent. This may be due to the fact that most of the animal studies included only male mice, and clinical studies of MDD have been primarily female.
4.5 Impact of Developmental Rates on Sex Differences in Comorbidity
Several brain characteristics develop at different rates in males and females (Tobet and Hanna, 1997), and these rates of development can depend on the perinatal gonadal steroid milieu. There are a number of molecules that have shown sex-dependent differences in the developing hypothalamus, including ERα and ERβ and GABAB receptor R1 subunit (Wolfe et al., 2005), islet-1 (Davis et al., 2004), calbindin and nNOS (Edelmann et al., 2007), all of which may appear sex-dependent only transiently during development. Studies are needed to assess the rate at which the PVN and other key stress circuitry brain regions, such as the hippocampus and amygdala, develop in males versus females, the role of gonadal steroid hormones and their receptors, and sex-dependent genetic factors. These studies will determine whether changing the developmental rate may be a critical factor for determining the developmental susceptibility of the PVN and/or other HPA regions to stress-induced alterations leading to selective sex-dependent vulnerability for MDD and/or CVD in adulthood.
5. Conclusions
The comorbidity of major depressive disorder (MDD) and cardiovascular disease (CVD) will be the number one cause of disability worldwide in 2020 and thus a major public health problem that will necessitate new therapeutic initiatives. Unfortunately, there are few scientists in either psychiatry or cardiology that focus their investigations on understanding the higher rate (almost twice the risk) in women than men. The fetal programming of MDD and CVD has been proposed independently of each other, and both fields have proposed prenatal stress models. This review argues that the comorbidity of MDD and CVD, and in particular the significant sex differences, arises in part from hormone-dependent pathogenic processes initiated during fetal development that result in greater risk in women than men. Specifically, the fetal origins of MDD and CVD result from alterations in the prenatal maternal environment that result in her producing excess glucocorticoids, which then drive sex-dependent developmental alterations of the fetal HPA axis circuitry with implications for mood and stress regulation, ANS function, and the vasculature into adulthood. These alterations include disruptions of pathways associated with GABA in neuronal and vascular development and growth factors (such as BDNF, VEGF, and IGF-1) that have critical roles in development and adult response to injury in the heart and the brain. Further, developmental timing of these disruptions is key, given that the impact of some of these molecules on neuronal and vascular development only occur in sex-dependent ways at particular periods in fetal development. Thus, further investigations of the sex-dependent development of these key brain regions, such as the PVN, the role of gonadal steroid hormones and their receptors, and sex-dependent genetic effects will be critical for an understanding of the origins of sex differences in the comorbidity of depression and cardiovascular disease and the potential development of novel sex-dependent therapeutics.
Highlights.
Fetal origins of depression-CVD comorbidity are sex-dependent and produce higher risk in women.
Prenatal stress models explain shared sex-dependent effects on adult mood, stress, ANS, and vasculature.
Disruptions of developmental pathways associated with glucocorticoids, GABA, growth factors, gonadal steroids and genes are key.
The sex-dependent development of the hypothalamic paraventricular nucleus is critical.
Developmental timing is essential for understanding sex-dependent effects.
Acknowledgements
The ideas in this review emanated from research on a translational (human to animal) program project that was supported by the Office for Research on Women’s Health (ORWH) and National Institute of Mental Health (ORWH-NIMH SCOR P50 MH082679; Goldstein, Tobet, Handa, PIs; http://mddscor.bwh.harvard.edu) and NIMH-NHLBI RO1 MH074679 (Goldstein, P.I.) We are very grateful for the funding support and our incredible research teams at Brigham and Women’s Hospital (Clinical Neuroscience Laboratory for Sex Differences in the Brain (http://cnl-sd.bwh.harvard.edu), Colorado State University, and University of Arizona College of Medicine. Special thanks to Laura Holsen, Ph.D. for help with some of the clinical and brain imaging literature on MDD, and Krystal Frahm, Ph.D. for her work on Figure 2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest The authors declare that they have no conflict of interest.
References
- Abercrombie HC, Jahn AL, Davidson RJ, Kern S, Kirschbaum C, Halverson J. Cortisol's effects on hippocampal activation in depressed patients are related to alterations in memory formation. Journal of Psychiatric Research. 2011;45:15–23. doi: 10.1016/j.jpsychires.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Harlan R. Glucocorticoid receptors in LHRH neurons. Neuroendocrinology. 1992;56:845–850. doi: 10.1159/000126315. [DOI] [PubMed] [Google Scholar]
- Ahrens T, Deuschle M, Krumm B, van der Pompe G, den Boer JA, Lederbogen F. Pituitary-adrenal and sympathetic nervous system responses to stress in women remitted from recurrent major depression. Psychosomatic Medicine. 2008;70:461–467. doi: 10.1097/PSY.0b013e31816b1aaa. [DOI] [PubMed] [Google Scholar]
- Ahs F, Pissiota A, Michelgard A, Frans O, Furmark T, Appel L, et al. Disentangling the web of fear: amygdala reactivity and functional connectivity in spider and snake phobia. Psychiatry Research. 2009;172:103–108. doi: 10.1016/j.pscychresns.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Barger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- Alcaro A, Panksepp J, Witczak J, Hayes DJ, Northoff G. Is subcortical-cortical midline activity in depression mediated by glutamate and GABA? A cross-species translational approach. Neuroscience and Biobehavioral Reviews. 2010;34:592–605. doi: 10.1016/j.neubiorev.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. Journal of Neuroscience. 1989;9:497–506. doi: 10.1523/JNEUROSCI.09-02-00497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G, Galibert E, Duvoid-Guillou A, Vincent A. Hyperosmotic stimulus induces reversible angiogenesis within the hypothalamic magnocellular nuclei of the adult rat: a potential role for neuronal vascular endothelial growth factor. BMC Neurosci. 2005;6:20. doi: 10.1186/1471-2202-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambach G, Palkovits M. Blood supply of the rat hypothalamus. II. Nucleus paraventricularis. Acta Morphologica Academiae Scientiarum Hungaricae. 1974;22:311. [PubMed] [Google Scholar]
- Amin Z, Epperson CN, Constable RT, Canli T. Effects of estrogen variation on neural correlates of emotional response inhibition. NeuroImage. 2006;32:457–464. doi: 10.1016/j.neuroimage.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Anand BK, Dua S. Electrical stimulation of the limbic system of brain (visceral brain) in the waking animals. Indian Journal of Medical Research. 1956;44:107–119. [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage. 2010;53:1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Waisman J, Donley L, Cahill L. Effects of breast cancer treatment on the hormonal and cognitive consequences of acute stress. Psycho-Oncology. 2011 doi: 10.1002/pon.2006. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ. Puberty and depression. Child and Adolescent Psychiatric Clinics of North America. 2006;15:919–937. doi: 10.1016/j.chc.2006.05.013. ix. [DOI] [PubMed] [Google Scholar]
- Antonijevic IA. Depressive disorders -- is it time to endorse different pathophysiologies? Psychoneuroendocrinology. 2006;31:1–15. doi: 10.1016/j.psyneuen.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Appelhof BC, Huyser J, Verweij M, Brouwer JP, van Dyck R, Fliers E, et al. Glucocorticoids and relapse of major depression (dexamethasone/corticotropin-releasing hormone test in relation to relapse of major depression) Biol Psychiatry. 2006;59:696–701. doi: 10.1016/j.biopsych.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Arnold AP. The end of gonad-centric sex determination in mammals. Trends in Genetics. 2011;28:55–61. doi: 10.1016/j.tig.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baischer W, Koinig G, Hartmann B, Huber J, Langer G. Hypothalamic-pituitary-gonadal axis in depressed premenopausal women: elevated blood testosterone concentrations compared to normal controls. Psychoneuroendocrinology. 1995;20:553–559. doi: 10.1016/0306-4530(94)00081-k. [DOI] [PubMed] [Google Scholar]
- Banki CM, Bissette G, Arato M, O'Connor L, Nemeroff CB. CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry. 1987;144:873–877. doi: 10.1176/ajp.144.7.873. [DOI] [PubMed] [Google Scholar]
- Bao AM, Hestiantoro A, Van Someren EJ, Swaab DF, Zhou JN. Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128:1301–1313. doi: 10.1093/brain/awh448. [DOI] [PubMed] [Google Scholar]
- Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psychiatry Neurosci. 2004;29:185–193. [PMC free article] [PubMed] [Google Scholar]
- Barefoot JC, Helms MJ, Mark DB, Blumenthal JA, Califf RM, Haney TL, et al. Depression and long-term mortality risk in patients with coronary artery disease. American Journal of Cardiology. 1996;78:613–617. doi: 10.1016/s0002-9149(96)00380-3. [DOI] [PubMed] [Google Scholar]
- Barker D. Developmental origins of chronic disease: The Richard Doll lecture. Public Health. 2012;126:185–189. doi: 10.1016/j.puhe.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Intrauterine programming of adult disease. Mol Med Today. 1995;1:418–423. doi: 10.1016/s1357-4310(95)90793-9. [DOI] [PubMed] [Google Scholar]
- Bingham B, Williamson M, Viau V. Androgen and estrogen receptor-β distribution within spinal-projecting and neurosecretory neurons in the paraventricular nucleus of the male rat. The Journal of Comparative Neurology. 2006;499:911–923. doi: 10.1002/cne.21151. [DOI] [PubMed] [Google Scholar]
- Blier P, El Mansari M. Serotonin and beyond: therapeutics for major depression. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368:1615. doi: 10.1098/rstb.2012.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. American Journal of Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Bowman RE, MacLusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 2004;145:3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- Breen KM, Karsch FJ. New insights regarding glucocorticoids, stress and gonadotropin suppression. Frontiers in Neuroendocrinology. 2006;27:233–245. doi: 10.1016/j.yfrne.2006.03.335. [DOI] [PubMed] [Google Scholar]
- Bremmer MA, Deeg DJ, Beekman AT, Penninx BW, Lips P, Hoogendijk WJ. Major depression in late life is associated with both hypo- and hypercortisolemia. Biol Psychiatry. 2007;62:479–486. doi: 10.1016/j.biopsych.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Brouwer JP, Appelhof BC, Hoogendijk WJ, Huyser J, Endert E, Zuketto C, et al. Thyroid and adrenal axis in major depression: a controlled study in outpatients. Eur J Endocrinol. 2005;152:185–191. doi: 10.1530/eje.1.01828. [DOI] [PubMed] [Google Scholar]
- Brown AS, van Os J, Driessens C, Hoek HW, Susser ES. Further evidence of relation between prenatal famine and major affective disorder. American Journal of Psychiatry. 2000;157:190–195. doi: 10.1176/appi.ajp.157.2.190. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:766–776. doi: 10.1016/j.pnpbp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nature Genetics. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Handa RJ. Sex and Stress Hormone Influences on the Expression and Activity of Brain-Derived Neurotrophic Factor. Neuroscience. 2012;239:295–303. doi: 10.1016/j.neuroscience.2012.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone DL, Zuloaga DG, Hiroi R, Foradori CD, Legare ME, Handa RJ. Prenatal Dexamethasone Exposure Potentiates Diet-Induced Hepatosteatosis and Decreases Plasma IGF-I in a Sex-Specific Fashion. Endocrinology. 2012a;153:295–306. doi: 10.1210/en.2011-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone DL, Zuloaga DG, Lacagnina AF, Handa RJ. A unique population of prepro-thyrotropin releasing hormone expressing neurons in the lateral hypothalamus that are activated by leptin and altered by prenatal glucocorticoid exposure. Brain Research. 2012b;1477:19–26. doi: 10.1016/j.brainres.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, Davies BM, Mendels J, Sugerman AA. Urinary free cortisol excretion in depression. Psychol Med. 1976a;6:43–50. doi: 10.1017/s0033291700007480. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, Mendels J. Cerebrospinal fluid and plasma free cortisol concentrations in depression. Psychol Med. 1976b;6:235–244. doi: 10.1017/s0033291700013775. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Feinberg M, Greden JF, Tarika J, Albala AA, Haskett RF, et al. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch Gen Psychiatry. 1981;38:15–22. doi: 10.1001/archpsyc.1981.01780260017001. [DOI] [PubMed] [Google Scholar]
- Chandran UR, Attardi B, Friedman R, Zheng Z-w, Roberts JL, DeFranco DB. Glucocorticoid repression of the mouse gonadotropin-releasing hormone gene is mediated by promoter elements that are recognized by heteromeric complexes containing glucocorticoid receptor. Journal of Biological Chemistry. 1996;271:20412–20420. doi: 10.1074/jbc.271.34.20412. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak CC, Ernst T. Magnetic resonance spectroscopy studies of GABA in neuropsychiatric disorders. Journal of Clinical Psychiatry. 2003;64(Suppl 3):7–14. [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang J-F, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biological Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Chopra KK, Ravindran A, Kennedy SH, Mackenzie B, Matthews S, Anisman H, et al. Sex differences in hormonal responses to a social stressor in chronic major depression. Psychoneuroendocrinology. 2009;34:1235–1241. doi: 10.1016/j.psyneuen.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WC, Pak TR, Weiser MJ, Hinds LR, Andersen ME, Handa RJ. Progestin receptor expression in the developing rat brain depends upon activation of estrogen receptor alpha and not estrogen receptor beta. Brain Research. 2006;1082:50–60. doi: 10.1016/j.brainres.2006.01.109. [DOI] [PubMed] [Google Scholar]
- Churchill D, Perry IJ, Beevers DG. Ambulatory blood pressure in pregnancy and fetal growth. Lancet. 1997;349:7–10. doi: 10.1016/s0140-6736(96)06297-6. [DOI] [PubMed] [Google Scholar]
- Clark AS, MacLusky NJ, Goldman-Rakic PS. Androgen binding and metabolism in the cerebral cortex of the developing rhesus monkey. Endocrinology. 1988;123:932–940. doi: 10.1210/endo-123-2-932. [DOI] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, et al. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. The Journal of Neuroscience. 2007;27:8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Rotshtein P, Nagai Y, O'Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. NeuroImage. 2005;24:751–762. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kaupmann K. Don't worry 'B' happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26:36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Slattery DA. GABA Receptors and Depression: Current Status. Advances in Pharmacology. 2010;58:427–451. doi: 10.1016/S1054-3589(10)58016-5. [DOI] [PubMed] [Google Scholar]
- Cunningham-Bussel AC, Root JC, Butler T, Tuescher O, Pan H, Epstein J, et al. Diurnal cortisol amplitude and fronto-limbic activity in response to stressful stimuli. Psychoneuroendocrinology. 2009;34:694–704. doi: 10.1016/j.psyneuen.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R, Puig-Antich J, Ryan N, Nelson B, Novacenko H, Twomey J, et al. Cortisol secretion in adolescents with major depressive disorder. Acta Psychiatr Scand. 1989;80:18–26. doi: 10.1111/j.1600-0447.1989.tb01295.x. [DOI] [PubMed] [Google Scholar]
- Dalack GW, Roose SP. Perspectives on the relationship between cardiovascular disease and affective disorder. Journal of Clinical Psychiatry. 1990;51(Suppl):4–9. [PubMed] [Google Scholar]
- Daly RC, Danaceau MA, Rubinow DR, Schmidt PJ. Concordant restoration of ovarian function and mood in perimenopausal depression. Am J Psychiatry. 2003;160:1842–1846. doi: 10.1176/appi.ajp.160.10.1842. [DOI] [PubMed] [Google Scholar]
- Dao TK, Youssef NA, Gopaldas RR, Chu D, Bakaeen F, Wear E, et al. Autonomic cardiovascular dysregulation as a potential mechanism underlying depression and coronary artery bypass grafting surgery outcomes. J Cardiothorac Surg. 2010;5:36. doi: 10.1186/1749-8090-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart AM, Du XJ, Kingwell BA. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovascular Research. 2002;53:678–687. doi: 10.1016/s0008-6363(01)00508-9. [DOI] [PubMed] [Google Scholar]
- Davis AM, Grattan DR, Selmanoff M, Mccarthy MM. Sex differences in glutamic acid decarboxylase mRNA in neonatal rat brain: implications for sexual differentiation. Hormones and Behavior. 1996;30:538–552. doi: 10.1006/hbeh.1996.0057. [DOI] [PubMed] [Google Scholar]
- Davis AM, Seney ML, Walker HJ, Tobet SA. Differential colocalization of Islet-1 and estrogen receptor α in the murine preoptic area and hypothalamus during development. Endocrinology. 2004;145:360–366. doi: 10.1210/en.2003-0996. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Chrousos GP, Dorn LD, Burke L, Helmers K, Kling MA, et al. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. J Clin Endocrinol Metab. 1994;78:249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Gold PW, Geracioti TD, Jr, Listwak SJ, Kling MA. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry. 1993;150:656–657. doi: 10.1176/ajp.150.4.656. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Winter RF, van Hemert AM, DeRijk RH, Zwinderman KH, Frankhuijzen-Sierevogel AC, Wiegant VM, et al. Anxious-retarded depression: relation with plasma vasopressin and cortisol. Neuropsychopharmacology. 2003;28:140–147. doi: 10.1038/sj.npp.1300002. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen Study. American Journal of Epidemiology. 1997;145:899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Blum WF, Strasburger CJ, Schweiger U, Weber B, Körner A, et al. Insulin-like growth factor-I (IGF-I) plasma concentrations are increased in depressed patients. Psychoneuroendocrinology. 1997;22:493–503. doi: 10.1016/s0306-4530(97)00046-2. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Weber B, Colla M, Depner M, Heuser I. Effects of major depression, aging and gender upon calculated diurnal free plasma cortisol concentrations: a re-evaluation study. Stress. 1998;2:281–287. doi: 10.3109/10253899809167292. [DOI] [PubMed] [Google Scholar]
- Dodic M, McAlinden A, Jefferies A, Wintour E, Cock M, May C, et al. Differential effects of prenatal exposure to dexamethasone or cortisol on circulatory control mechanisms mediated by angiotensin II in the central nervous system of adult sheep. The Journal of physiology. 2006;571:651–660. doi: 10.1113/jphysiol.2005.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodic M, Wintour EM, Whitworth JA, Coghlan JP. Effect of steroid hormones on blood pressure. Clin Exp Pharmacol Physiol. 1999;26:550–552. doi: 10.1046/j.1440-1681.1999.03076.x. [DOI] [PubMed] [Google Scholar]
- Donahue JE, Stopa EG, Chorsky RL, King JC, Schipper HM, Tobet SA, et al. Cells containing immunoreactive estrogen receptor-alpha in the human basal forebrain. Brain Research. 2000;856:142–151. doi: 10.1016/s0006-8993(99)02413-0. [DOI] [PubMed] [Google Scholar]
- Dougherty D, Rauch SL. Neuroimaging and neurobiological models of depression. Harv Rev Psychiatry. 1997;5:138–159. doi: 10.3109/10673229709000299. [DOI] [PubMed] [Google Scholar]
- Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Lond) 2000;98:137–142. [PubMed] [Google Scholar]
- Drake AJ, Raubenheimer PJ, Kerrigan D, McInnes KJ, Seckl JR, Walker BR. Prenatal dexamethasone programs expression of genes in liver and adipose tissue and increased hepatic lipid accumulation but not obesity on a high-fat diet. Endocrinology. 2010;151:1581–1587. doi: 10.1210/en.2009-1088. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Dufourny L, Skinner DC. Type II glucocorticoid receptors in the ovine hypothalamus: distribution, influence of estrogen and absence of co-localization with GnRH. Brain Research. 2002;946:79–86. doi: 10.1016/s0006-8993(02)02829-9. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Terwilliger R, Russell DS, Newton SS, Duman RS. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behavioural Brain Research. 2009;198:366–371. doi: 10.1016/j.bbr.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Edelmann M, Wolfe C, Scordalakes EM, Rissman EF, Tobet S. Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Developmental neurobiology. 2007;67:1371–1381. doi: 10.1002/dneu.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. Dysfunction of placental glucocorticoid barrier: link between fetal environment and adult hypertension? Lancet. 1993;341:355–357. doi: 10.1016/0140-6736(93)90148-a. [DOI] [PubMed] [Google Scholar]
- Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. 11 beta-Hydroxysteroid dehydrogenases: key enzymes in determining tissue-specific glucocorticoid effects. Steroids. 1996;61:263–269. doi: 10.1016/0039-128x(96)00033-5. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Elderon L, Whooley MA. Depression and cardiovascular disease. Progress in Cardiovascular Diseases. 2013;55:511–523. doi: 10.1016/j.pcad.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krystal JH, et al. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology (Berl) 2006;186:425–433. doi: 10.1007/s00213-006-0313-7. [DOI] [PubMed] [Google Scholar]
- Evans DL, Nemeroff CB. The clinical use of the dexamethasone suppression test in DSM-III affective disorders: correlation with the severe depressive subtypes of melancholia and psychosis. J Psychiatr Res. 1987;21:185–194. doi: 10.1016/0022-3956(87)90018-5. [DOI] [PubMed] [Google Scholar]
- Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, et al. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. Journal of Applied Physiology. 2001;91:2611–2618. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- Everson SA, Kaplan GA, Goldberg DE, Salonen R, Salonen JT. Hopelessness and 4-year progression of carotid atherosclerosis; the Kuopio Ischemic Heart Disease Risk Factor Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17:1490–1495. doi: 10.1161/01.atv.17.8.1490. [DOI] [PubMed] [Google Scholar]
- Familari M, Smith A, Smith R, Funder J. Arginine vasopressin is a much more potent stimulus to ACTH release from ovine anterior pituitary cells than ovine corticotropin-releasing factor. Neuroendocrinology. 1989;50:152–157. doi: 10.1159/000125214. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus-a potential target for integrative treatment of autonomic dysfunction. Expert Opinion on Therapeutic Targets. 2008;12:717–727. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: An MRI-based morphometric analysis. Cerebral Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Finley KH. The capillary bed of the paraventricular and supraoptic nuclei of the hypothalamus. Research Publications - Association for Research in Nervous and Mental Disease. 1937;18:94–109. [Google Scholar]
- Frahm KA, Schow MJ, Tobet SA. The Vasculature within the Paraventricular Nucleus of the Hypothalamus in Mice Varies as a Function of Development, Subnuclear Location, and GABA Signaling. Hormone and metabolic research. 2012;44:619–624. doi: 10.1055/s-0032-1304624. [DOI] [PubMed] [Google Scholar]
- Franz B, Buysse DJ, Cherry CR, Gray NS, Grochocinski VJ, Frank E, et al. Insulin-like growth factor 1 and growth hormone binding protein in depression: a preliminary communication. Journal of Psychiatric Research. 1999;33:121–127. doi: 10.1016/s0022-3956(98)00066-1. [DOI] [PubMed] [Google Scholar]
- Frim DM, Emanuel RL, Robinson BG, Smas CM, Adler GK, Majzoub JA. Characterization and gestational regulation of corticotropin-releasing hormone messenger RNA in human placenta. J Clin Invest. 1988;82:287–292. doi: 10.1172/JCI113585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S-F, Bao A-M. Corticotropin-releasing hormone, glutamate, and γ-aminobutyric acid in depression. The Neuroscientist. 2011;17:124–144. doi: 10.1177/1073858410361780. [DOI] [PubMed] [Google Scholar]
- García-Cáceres C, Lagunas N, Calmarza-Font I, Azcoitia I, Diz-Chaves Y, García-Segura LM, et al. Gender differences in the long-term effects of chronic prenatal stress on the HPA axis and hypothalamic structure in rats. Psychoneuroendocrinology. 2010;35:1525–1535. doi: 10.1016/j.psyneuen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Gibbons JL, McHugh PR. Plasma cortisol in depressive illness. J Psychiatr Res. 1962;1:162–171. doi: 10.1016/0022-3956(62)90006-7. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Giles WB, McLean M, Davies JJ, Smith R. Abnormal umbilical artery Doppler waveforms and cord blood corticotropin-releasing hormone. Obstet Gynecol. 1996;87:107–111. doi: 10.1016/0029-7844(95)00338-x. [DOI] [PubMed] [Google Scholar]
- Gispen-de Wied CC, Westenberg HG, Koppeschaar HP, Thijssen JH, van Ree JM. Stimulation of the pituitary-adrenal axis with a low dose [Arg8]-vasopressin in depressed patients and healthy subjects. Eur Neuropsychopharmacol. 1992;2:411–419. doi: 10.1016/0924-977x(92)90003-q. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Shapiro PA. Depression and the course of coronary artery disease. Am J Psychiatry. 1998;155:4–11. doi: 10.1176/ajp.155.1.4. [DOI] [PubMed] [Google Scholar]
- Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. Journal of Neuroendocrinology. 2009;21:415–420. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goland RS, Jozak S, Warren WB, Conwell IM, Stark RI, Tropper PJ. Elevated levels of umbilical cord plasma corticotropin-releasing hormone in growth-retarded fetuses. J Clin Endocrinol Metab. 1993;77:1174–1179. doi: 10.1210/jcem.77.5.8077309. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Cherkerzian S, Buka SL, Fitzmaurice G, Hornig M, Gillman M, et al. Sex-Specific Impact of Maternal-Fetal Risk Factors on Depression and Cardiovascular Risk 40 Years Later. Journal of Developmental Origins of Health and Disease. 2011;6:353–364. doi: 10.1017/S2040174411000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. The Journal of Neuroscience. 2010;30:431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. Journal of Neuroscience. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, O'Brien LM, Horton NJ, Kennedy DN, Makris N, et al. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Archives of General Psychiatry. 2002;59:154–164. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- Goncharuk VD, Van Heerikhuize J, Swaab DF, Buijs RM. Paraventricular nucleus of the human hypothalamus in primary hypertension: activation of corticotropin-releasing hormone neurons. J Comp Neurol. 2002;443:321–331. doi: 10.1002/cne.10124. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Sloan RP. Heart rate variability in depressive and anxiety disorders. American Heart Journal. 2000;140:77–83. doi: 10.1067/mhj.2000.109981. [DOI] [PubMed] [Google Scholar]
- Gorski RA. Sexual differentiation of the nervous system. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. New York: McGraw-Hill Health Professions Division; 2000. pp. 1131–1146. [Google Scholar]
- Gray JD, Milner TA, McEwen BS. Dynamic Plasticity: The role of glucocorticoids, BDNF and other trophic factors. Neuroscience. 2012;239:214–227. doi: 10.1016/j.neuroscience.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziottin A, Serafini A. Depression and the menopause: why antidepressants are not enough? Menopause Int. 2009;15:76–81. doi: 10.1258/mi.2009.009021. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12:1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. Journal of Clinical Endocrinology and Metabolism. 1999;84:4677–4694. doi: 10.1210/jcem.84.12.6290. [DOI] [PubMed] [Google Scholar]
- Haefner S, Baghai TC, Schule C, Eser D, Spraul M, Zill P, et al. Impact of gene-gender effects of adrenergic polymorphisms on hypothalamic-pituitary-adrenal axis activity in depressed patients. Neuropsychobiology. 2008;58:154–162. doi: 10.1159/000182891. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Asnis GM, Shindledecker R, Zumoff B, Nathan RS. Cortisol secretion in endogenous depression. I. Basal plasma levels. Arch Gen Psychiatry. 1985;42:904–908. doi: 10.1001/archpsyc.1985.01790320076010. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Kahn LS. Role of estrogen in the aetiology and treatment of mood disorders. CNS Drugs. 2001;15:797–817. doi: 10.2165/00023210-200115100-00005. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Petty F, Yonkers K, Kramer GL, Rush AJ, Bibi KW. Low plasma gamma-aminobutyric acid levels during the late luteal phase of women with premenstrual dysphoric disorder. The American Journal of Psychiatry. 1996;153:718–720. doi: 10.1176/ajp.153.5.718. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Hormones and Behavior. 1994a;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Hormones and Behavior. 1994b;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiology and Behavior. 1994c;55:117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Sharma D, Uht R. A role for the androgen metabolite, 5alpha androstane 3beta, 17beta Diol (3β-Diol) in the regulation of the hypothalamo-pituitary–adrenal axis. Frontiers in Endocrinology. 2011;2:65. doi: 10.3389/fendo.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow BL, Wise LA, Otto MW, Soares CN, Cohen LS. Depression and its influence on reproductive endocrine and menstrual cycle markers associated with perimenopause: the Harvard Study of Moods and Cycles. Arch Gen Psychiatry. 2003;60:29–36. doi: 10.1001/archpsyc.60.1.29. [DOI] [PubMed] [Google Scholar]
- Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Hormones and Behavior. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Heald A, Kaushal K, Anderson S, Redpath M, Durrington PN, Selby PL, et al. Effects of hormone replacement therapy on insulin-like growth factor (IGF)-I, IGF-II and IGF binding protein (IGFBP)-1 to IGFBP-4: implications for cardiovascular risk. Gynecological Endocrinology. 2005;20:176–182. doi: 10.1080/09513590400027406. [DOI] [PubMed] [Google Scholar]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, et al. Effect of childhood trauma on adult depression and neuroendocrine function: sex-specific moderation by CRH receptor 1 gene. Frontiers in behavioral neuroscience. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Paulus MP, Geyer MA, Perry W. Heart rate variability in bipolar mania and schizophrenia. Journal of Psychiatric Research. 2010;44:168–176. doi: 10.1016/j.jpsychires.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Botzenhardt J, Muhtz C, Agorastos A, Wiedemann K, Kellner M, et al. Sex differences of salivary cortisol secretion in patients with major depression. Stress. 2011;15:105–109. doi: 10.3109/10253890.2011.582200. [DOI] [PubMed] [Google Scholar]
- Hoffman BL, Rasmussen T. Stimulation studies of insular cortex of Macaca mulatta. Journal of Neurophysiology. 1953;16:343–351. doi: 10.1152/jn.1953.16.4.343. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Gerken A, Stalla GK, Muller OA. ACTH, cortisol, and corticosterone output after ovine corticotropin-releasing factor challenge during depression and after recovery. Biol Psychiatry. 1985;20:276–286. doi: 10.1016/0006-3223(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Gerken A, Stalla GK, Muller OA. Blunted aldosterone and ACTH release after human CRH administration in depressed patients. Am J Psychiatry. 1987;144:229–231. doi: 10.1176/ajp.144.2.229. [DOI] [PubMed] [Google Scholar]
- Holsen L, Lancaster K, Klibanksi A, Whitfield-Gabrieli S, Cherkerzian S, Buka S, et al. HPA-Axis Hormone Modulation of Stress Response Circuitry Activity in Women with Remitted Major Depression. 2013 doi: 10.1016/j.neuroscience.2013.07.042. (under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Lee JH, Spaeth SB, Ogden LA, Klibanski A, Whitfield-Gabrieli S, et al. Brain hypoactivation, autonomic nervous system dysregulation, and gonadal hormones in depression: A preliminary study. Neuroscience Letters. 2012;514:57–61. doi: 10.1016/j.neulet.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Spaeth SB, Lee JH, Ogden LA, Klibanski A, Whitfield-Gabrieli S, et al. Stress response circuitry hypoactivation related to hormonal dysfunction in women with major depression. Journal of Affective Disorders. 2011;1–3:379–387. doi: 10.1016/j.jad.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Research. 2005;1037:204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Howerton CL, Morgan CP, Fischer DB, Bale TL. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proceedings of the National Academy of Sciences. 2013;110:5169–5174. doi: 10.1073/pnas.1300065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Kalló I, Hajszán T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-β messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinology. 1998;139:2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Kalló I, Steinhauser A, Merchenthaler I, Coen CW, Petersen SL, et al. Estrogen receptor-β in oxytocin and vasopressin neurons of the rat and human hypothalamus: Immunocytochemical and in situ hybridization studies. The Journal of Comparative Neurology. 2004;473:315–333. doi: 10.1002/cne.20127. [DOI] [PubMed] [Google Scholar]
- Huikuri HV, Jokinen V, Syvanne M, Nieminen MS, Airaksinen KE, Ikaheimo MJ, et al. Heart rate variability and progression of coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:1979–1985. doi: 10.1161/01.atv.19.8.1979. [DOI] [PubMed] [Google Scholar]
- Inder WJ, Donald RA, Prickett TC, Frampton CM, Sullivan PF, Mulder RT, et al. Arginine vasopressin is associated with hypercortisolemia and suicide attempts in depression. Biol Psychiatry. 1997;42:744–747. doi: 10.1016/s0006-3223(97)00301-6. [DOI] [PubMed] [Google Scholar]
- Ising M, Horstmann S, Kloiber S, Lucae S, Binder EB, Kern N, et al. Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression - a potential biomarker? Biol Psychiatry. 2007;62:47–54. doi: 10.1016/j.biopsych.2006.07.039. [DOI] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of Hypothalamo-Pituitary-Adrenocortical function during acute and chronic stress. Annals of the New York Academy of Sciences. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett DB, Coble PA, Kupfer DJ. Reduced cortisol latency in depressive illness. Arch Gen Psychiatry. 1983;40:506–511. doi: 10.1001/archpsyc.1983.01790050032004. [DOI] [PubMed] [Google Scholar]
- Jasoni CL, Todman MG, Han S-K, Herbison AE. Expression of mRNAs encoding receptors that mediate stress signals in gonadotropin-releasing hormone neurons of the mouse. Neuroendocrinology. 2005;82:320–328. doi: 10.1159/000093155. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Chao MV. Are BDNF and glucocorticoid activities calibrated? Neuroscience. 2012;239:173–195. doi: 10.1016/j.neuroscience.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe J. Genotype and prenatal and premating stress interact to affect adult behavior in rats. Science. 1965;150(3705):1844–1845. doi: 10.1126/science.150.3705.1844. [DOI] [PubMed] [Google Scholar]
- Jones A, Beda A, Ward AM, Osmond C, Phillips DI, Moore VM, et al. Size at birth and autonomic function during psychological stress. Hypertension. 2007;49:548–555. doi: 10.1161/01.HYP.0000257196.13485.9b. [DOI] [PubMed] [Google Scholar]
- Jones DJ, Bromberger JT, Sutton-Tyrrell K, Matthews KA. Lifetime history of depression and carotid atherosclerosis in middle-aged women. Arch Gen Psychiatry. 2003;60:153–160. doi: 10.1001/archpsyc.60.2.153. [DOI] [PubMed] [Google Scholar]
- Kaada BR, Pribram KH, Epstein JA. Respiratory and vascular responses in monkeys from temporal pole, insula, orbital surface and cingulate gyrus; a preliminary report. Journal of Neurophysiology. 1949;12:347–356. doi: 10.1152/jn.1949.12.5.347. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depression and Anxiety. 2007;24:495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- Karalis K, Goodwin G, Majzoub JA. Cortisol blockade of progesterone: a possible molecular mechanism involved in the initiation of human labor. Nat Med. 1996;2:556–560. doi: 10.1038/nm0596-556. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Colditz GA, Ascherio A, Rimm EB, Giovannucci E, Stampfer MJ, et al. Prospective study of phobic anxiety and risk of coronary heart disease in men. Circulation. 1994a;89:1992–1997. doi: 10.1161/01.cir.89.5.1992. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Symptoms of anxiety and risk of coronary heart disease. The Normative Aging Study. Circulation. 1994b;90:2225–2229. doi: 10.1161/01.cir.90.5.2225. [DOI] [PubMed] [Google Scholar]
- Kawata M. Roles of steroid hormones and their receptors in structural organization in the nervous system. Neuroscience Research. 1995;24:1–46. doi: 10.1016/0168-0102(96)81278-8. [DOI] [PubMed] [Google Scholar]
- Kelly WF, Checkley SA, Bender DA. Cushing's syndrome, tryptophan and depression. Br J Psychiatry. 1980;136:125–132. doi: 10.1192/bjp.136.2.125. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biological Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends in Cardiovascular Medicine. 2007;17:140–143. doi: 10.1016/j.tcm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermani P, Rafii D, Jin DK, Whitlock P, Schaffer W, Chiang A, et al. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. Journal of Clinical Investigation. 2005;115:653–663. doi: 10.1172/JCI200522655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. Journal of Affective Disorders. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. Journal of Affective Disorders. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. 1939. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9:606–620. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Kraemer R. Reduced apoptosis and increased lesion development in the flow-restricted carotid artery of p75(NTR)-null mutant mice. Circulation Research. 2002;91:494–500. doi: 10.1161/01.res.0000035245.83233.2a. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Doraiswamy PM, Clary CM. Clinical and treatment response characteristics of late-life depression associated with vascular disease: a pooled analysis of two multicenter trials with sertraline. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2001;25:347–361. doi: 10.1016/s0278-5846(00)00168-8. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological Psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kuniecki M, Urbanik A, Sobiecka B, Kozub J, Binder M. Central control of heart rate changes during visual affective processing as revealed by fMRI. Acta Neurobiol Exp (Wars) 2003;63:39–48. doi: 10.55782/ane-2003-1453. [DOI] [PubMed] [Google Scholar]
- Lafuente JV, Ortuzar N, Bengoetxea H, Bulnes S, Argandona EG. Vascular endothelial growth factor and other angioglioneurins: key molecules in brain development and restoration. New Perspectives of Central Nervous System Injury and Neuroprotection. 2012;102:317–346. doi: 10.1016/B978-0-12-386986-9.00012-0. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Waymire J, Lynch G. Hippocampal aging and adrenocorticoids: quantitative correlations. Science. 1978;202(4372):1098–1102. doi: 10.1126/science.715460. [DOI] [PubMed] [Google Scholar]
- Lane R, Reiman E, Ahern GT, Thayer J. Activity in medial prefrontal cortex correlates with vagal component of heart rate variability during emotion. Brain and Cognition. 2001;47:97–100. [Google Scholar]
- Lane RD, Wager TD. The new field of Brain-Body Medicine: What have we learned and where are we headed? NeuroImage. 2009;47:1135–1140. doi: 10.1016/j.neuroimage.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Weisenbach SL, Giordani B, Briceno EM, Guidotti Breting LM, Schallmo MP, et al. Impact of chronic hypercortisolemia on affective processing. Neuropharmacology. 2012;62:217–225. doi: 10.1016/j.neuropharm.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley-Evans SC. Intrauterine programming of hypertension by glucocorticoids. Life Sci. 1997;60:1213–1221. doi: 10.1016/s0024-3205(96)00611-x. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Phillips GJ, Benediktsson R, Gardner DS, Edwards CR, Jackson AA, et al. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 1996a;17:169–172. doi: 10.1016/s0143-4004(96)80010-5. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci (Lond) 1996b;91:607–615. doi: 10.1042/cs0910607. [DOI] [PubMed] [Google Scholar]
- Lau JY, Goldman D, Buzas B, Hodgkinson C, Leibenluft E, Nelson E, et al. BDNF gene polymorphism (Val66Met) predicts amygdala and anterior hippocampus responses to emotional faces in anxious and depressed adolescents. NeuroImage. 2010;53:952–961. doi: 10.1016/j.neuroimage.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Miller KK, Mathur VA, Misra M, Meenaghan E, Herzog DB, et al. Hormonal and nutritional effects on cardiovascular risk markers in young women. Journal of Clinical Endocrinology and Metabolism. 2007;92:3089–3094. doi: 10.1210/jc.2007-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron-Milad K, Abbs B, Milad MR, Linman C, Rougemount-Bücking A, Zeidan MA, et al. Sex differences in the neurobiology of fear conditioning and extinction: a preliminary fMRI study of shared sex differences with stress-arousal circuitry. Biology of Mood and Anxiety Disorders. 2012;2(1):1–10. doi: 10.1186/2045-5380-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Park KS, Hwang JH, Park MI, Yum MK. Chaotic and periodic heart rate dynamics in uncomplicated intrauterine growth restricted fetuses. Early Human Development. 1998;53:121–128. doi: 10.1016/s0378-3782(98)00046-2. [DOI] [PubMed] [Google Scholar]
- LeRoith D. IGF-I: panacea or poison? Journal of Clinical Endocrinology and Metabolism. 2010;95:4549–4551. doi: 10.1210/jc.2010-1737. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- Li X, Knox A, O'Byrne K. Corticotrophin-releasing factor and stress-induced inhibition of the gonadotrophin-releasing hormone pulse generator in the female. Brain Research. 2010;1364:153–163. doi: 10.1016/j.brainres.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, et al. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. American Journal of Epidemiology. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- Licht CM, de Geus EJ, Zitman FG, Hoogendijk WJ, van Dyck R, Penninx BW. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA) Archives of General Psychiatry. 2008;65:1358–1367. doi: 10.1001/archpsyc.65.12.1358. [DOI] [PubMed] [Google Scholar]
- Licht CM, Vreeburg SA, van Reedt Dortland AK, Giltay EJ, Hoogendijk WJ, DeRijk RH, et al. Increased sympathetic and decreased parasympathetic activity rather than changes in hypothalamic-pituitary-adrenal axis activity is associated with metabolic abnormalities. Journal of Clinical Endocrinology and Metabolism. 2010;95:2458–2466. doi: 10.1210/jc.2009-2801. [DOI] [PubMed] [Google Scholar]
- Ling MH, Perry PJ, Tsuang MT. Side effects of corticosteroid therapy. Psychiatric aspects. Arch Gen Psychiatry. 1981;38:471–477. doi: 10.1001/archpsyc.1981.01780290105011. [DOI] [PubMed] [Google Scholar]
- Liu GX, Cai GQ, Cai YQ, Sheng ZJ, Jiang J, Mei Z, et al. Reduced anxiety and depression-like behaviors in mice lacking GABA transporter subtype 1. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:1531–1539. doi: 10.1038/sj.npp.1301281. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Robinson JL, Glahn DC, Fox PT. Acute effects of hydrocortisone on the human brain: an fMRI study. Psychoneuroendocrinology. 2010;35:15–20. doi: 10.1016/j.psyneuen.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2004a;16:272–278. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neurosci Lett. 2004b;365:43–47. doi: 10.1016/j.neulet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Molecular Psychiatry. 2010;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Greischar LL, Perlman DM, Davidson RJ. BOLD signal in insula is differentially related to cardiac function during compassion meditation in experts vs. novices. Neuroimage. 2009;47:1038–1046. doi: 10.1016/j.neuroimage.2009.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac LP. Psychosomatic disease and the visceral brain; recent developments bearing on the Papez theory of emotion. Psychosomatic Medicine. 1949;11:338–353. doi: 10.1097/00006842-194911000-00003. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Clark AS, Naftolin F, Goldman-Rakic PS. Estrogen formation in the mammalian brain: possible role of aromatase in sexual differentiation of the hippocampus and neocortex. Steroids. 1987;50:459–474. doi: 10.1016/0039-128x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Maes M, Calabrese J, Meltzer HY. The relevance of the in- versus outpatient status for studies on HPA-axis in depression: spontaneous hypercortisolism is a feature of major depressed inpatients and not of major depression per se. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:503–517. doi: 10.1016/0278-5846(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Maes M, De Ruyter M, Claes R, Bosma G, Suy E. The cortisol responses to 5-hydroxytryptophan, orally, in depressive inpatients. J Affect Disord. 1987;13:23–30. doi: 10.1016/0165-0327(87)90070-x. [DOI] [PubMed] [Google Scholar]
- Maes M, De Ruyter M, Suy E. Use of the dexamethasone suppression test in an inpatient setting: a replication and new findings. Psychoneuroendocrinology. 1989;14:231–239. doi: 10.1016/0306-4530(89)90021-8. [DOI] [PubMed] [Google Scholar]
- Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M, et al. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. Journal of Clinical Endocrinology and Metabolism. 2004;89:61–70. doi: 10.1210/jc.2003-030313. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Mazzone L, Merke DP, Keil MF, Stratakis CA, Pine DS, et al. Altered amygdala and hippocampus function in adolescents with hypercortisolemia: a functional magnetic resonance imaging study of Cushing syndrome. Development and Psychopathology. 2008;20:1177–1189. doi: 10.1017/S0954579408000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdic G, Tobet S. Cooperation of sex chromosomal genes and endocrine influences for hypothalamic sexual differentiation. Frontiers in Neuroendocrinology. 2011;32:137–145. doi: 10.1016/j.yfrne.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majzoub JA, McGregor JA, Lockwood CJ, Smith R, Taggart MS, Schulkin J. A central theory of preterm and term labor: putative role for corticotropinreleasing hormone. Am J Obstet Gynecol. 1999;180:S232–S241. doi: 10.1016/s0002-9378(99)70707-6. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Platt B, Rizzo SJS, Ring RH, Lucki I, Schechter LE, et al. Increasing the levels of insulin-like growth factor-I by an IGF binding protein inhibitor produces anxiolytic and antidepressant-like effects. Neuropsychopharmacology. 2007;32:2360–2368. doi: 10.1038/sj.npp.1301358. [DOI] [PubMed] [Google Scholar]
- Malik M, Camm A Cardiology, M.o.t.T.F.o.t.E.S.o. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Manning FA. Dynamic ultrasound-based fetal assessment: the fetal biophysical profile score. Clinical Obstetrics and Gynecology. 1995;38:26–44. doi: 10.1097/00003081-199503000-00006. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nature Neuroscience. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Sexual differentiation of neuronal circuitry in the neuroendocrine hypothalamus. Biomedical Review. 1997;7:5–15. [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, et al. The epigenetics of sex differences in the brain. The Journal of Neuroscience. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Perrot-Sinal TS. Getting excited about GABA and sex differences in the brain. Trends in Neurosciences. 2002;25:307–312. doi: 10.1016/s0166-2236(02)02182-3. [DOI] [PubMed] [Google Scholar]
- McClellan KM, Stratton MS, Tobet SA. Roles for gamma-aminobutyric acid in the development of the paraventricular nucleus of the hypothalamus. The Journal of Comparative Neurology. 2010;518:2710–2728. doi: 10.1002/cne.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Gonadal steroid influences on brain development and sexual differentiation. In: Greep R, editor. Reproductive Physiology IV. Baltimore: University Park; 1983. pp. 99–145. [PubMed] [Google Scholar]
- McKay MS, Zakzanis KK. The impact of treatment on HPA axis activity in unipolar major depression. J Psychiatr Res. 2010;44:183–192. doi: 10.1016/j.jpsychires.2009.07.012. [DOI] [PubMed] [Google Scholar]
- McKinley PS, King AR, Shapiro PA, Slavov I, Fang Y, Chen IS, et al. The impact of menstrual cycle phase on cardiac autonomic regulation. Psychophysiology. 2009;46:904–911. doi: 10.1111/j.1469-8986.2009.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RL, Dohanich GP, Ward IL. Effects of prenatal stress on avoidance acquisition, open-field performance and lordotic behavior in male rats. Physiology & Behavior. 1979;22:527–530. doi: 10.1016/0031-9384(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Meller WH, Grambsch PL, Bingham C, Tagatz GE. Hypothalamic pituitary gonadal axis dysregulation in depressed women. Psychoneuroendocrinology. 2001;26:253–259. doi: 10.1016/s0306-4530(00)00050-0. [DOI] [PubMed] [Google Scholar]
- Menendez A, Alvarez-Uria M. The development of vascularization in the postnatal rat paraventricular nucleus: a morphometric analysis. Journal fur Hirnforschung. 1987;28:325–329. [PubMed] [Google Scholar]
- Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24:1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, et al. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology. 2010;35:33–46. doi: 10.1016/j.psyneuen.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Patterns in Behavioral Neuroanatomy: Association Areas, The Limbic System, and Hemispheric Specialization. In: Mesulam MM, editor. Principles of Behavioral Neurology. Philadelphia: F.A. Davis Company; 1985. pp. 1–58. [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical ouput and comments on function. Journal of Comparative Neurology. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Meynen G, Unmehopa UA, Heerikhuize JJ, Hofman MA, Swaab DF, Hoogendijk WJ. Increased Arginine Vasopressin mRNA Expression in the Human Hypothalamus in Depression: A Preliminary Report. Biol Psychiatry. 2006;60(8):892–895. doi: 10.1016/j.biopsych.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Moller-Leimkuhler AM. Gender differences in cardiovascular disease and comorbid depression. Dialogues in Clinical Neuroscience. 2007;9:71–83. doi: 10.31887/DCNS.2007.9.1/ammoeller. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C, Reuter M, Newport B, Elger C, Weber B. The BDNF Val66Met polymorphism affects amygdala activity in response to emotional stimuli: evidence from a genetic imaging study. NeuroImage. 2008;42:1554–1559. doi: 10.1016/j.neuroimage.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Muehlhan M, Lueken U, Wittchen HU, Kirschbaum C. The scanner as a stressor: evidence from subjective and neuroendocrine stress parameters in the time course of a functional magnetic resonance imaging session. International journal of psychophysiology: official journal of the International Organization of Psychophysiology. 2011;79:118–126. doi: 10.1016/j.ijpsycho.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiology & Behavior. 2006;88:605–614. doi: 10.1016/j.physbeh.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Mujica-Parodi LR, Korgaonkar M, Ravindranath B, Greenberg T, Tomasi D, Wagshul M, et al. Limbic dysregulation is associated with lowered heart rate variability and increased trait anxiety in healthy adults. Human Brain Mapping. 2009;30:47–58. doi: 10.1002/hbm.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MB, Holsboer F. Mice with mutations in the HPA-system as models for symptoms of depression. Biol Psychiatry. 2006;59:1104–1115. doi: 10.1016/j.biopsych.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Murphy DG, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, et al. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53:585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- Murray EA, Wise SP, Drevets WC. Localization of dysfunction in major depressive disorder: prefrontal cortex and amygdala. Biological Psychiatry. 2011;69:e43–e54. doi: 10.1016/j.biopsych.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Archives of General Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Herman JP. Neural Regulation of the Stress Response: The Many Faces of Feedback. Cellular and Molecular Neurobiology. 2012;32(5):683–694. doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahshoni E, Aizenberg D, Sigler M, Strasberg B, Zalsman G, Imbar S, et al. Heart rate variability increases in elderly depressed patients who respond to electroconvulsive therapy. Journal of Psychosomatic Research. 2004;56:89–94. doi: 10.1016/S0022-3999(03)00037-0. [DOI] [PubMed] [Google Scholar]
- Naqvi TZ, Naqvi SS, Merz CN. Gender differences in the link between depression and cardiovascular disease. Psychosomatic Medicine. 2005;67(Suppl 1):S15–S18. doi: 10.1097/01.psy.0000164013.55453.05. [DOI] [PubMed] [Google Scholar]
- Nelson WH, Khan A, Orr WW, Jr, Tamragouri RN. The dexamethasone suppression test: interaction of diagnosis, sex, age in psychiatric inpatients. Biol Psychiatry. 1984a;19:1293–1304. [PubMed] [Google Scholar]
- Nelson WH, Orr WW, Jr, Shane SR, Stevenson JM. Hypothalamic-pituitary-adrenal axis activity and age in major depression. J Clin Psychiatry. 1984b;45:120–121. [PubMed] [Google Scholar]
- Nemeroff CB, Bissette G, Akil H, Fink M. Neuropeptide concentrations in the cerebrospinal fluid of depressed patients treated with electroconvulsive therapy. Corticotrophin-releasing factor, beta-endorphin and somatostatin. Br J Psychiatry. 1991;158:59–63. doi: 10.1192/bjp.158.1.59. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Heim C, Owens MJ, Ritchie JC, Ramsey CH, Bonsall R, et al. Cerebrospinal fluid corticotropin-releasing factor (CRF) and vasopressin concentrations predict pituitary response in the CRF stimulation test: a multiple regression analysis. Neuropsychopharmacology. 2003;28:569–576. doi: 10.1038/sj.npp.1300071. [DOI] [PubMed] [Google Scholar]
- Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, et al. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. Journal of Neuroscience. 2003;23:10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. Journal of Neuroscience. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolarakis KE, Almeida OF, Herz A. Corticotropin-releasing factor (CRF) inhibits gonadotropin-releasing hormone (GnRH) release from superfused rat hypothalami in vitro. Brain Res. 1986;377:388–390. doi: 10.1016/0006-8993(86)90887-5. [DOI] [PubMed] [Google Scholar]
- Nyrienda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glococorticoid exposure in late gestation permanently programs rat hepatic phosphoenolypyruvate carboxykinase and glcocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101:2174–2181. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MF, Gundel H, McRae K, Lane RD. Baseline vagal tone predicts BOLD response during elicitation of grief. Neuropsychopharmacology. 2007;32:2184–2189. doi: 10.1038/sj.npp.1301342. [DOI] [PubMed] [Google Scholar]
- O'Keefe JA, Li Y, Burgess LH, Handa RJ. Estrogen receptor mRNA alterations in the developing rat hippocampus. Brain Research. Molecular Brain Research. 1995;30:115–124. doi: 10.1016/0169-328x(94)00284-l. [DOI] [PubMed] [Google Scholar]
- O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab. 2004;287:E863–E870. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Prenatal dexamethasone 'programmes' hypotension, but stress-induced hypertension in adult offspring. Journal of Endocrinology. 2008;196:343–352. doi: 10.1677/JOE-07-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Regan D, Welberg LL, Holmes MC, Seckl JR. Seminars in Neonatology. Elsevier; 2001. Glucocorticoid programming of pituitary–adrenal function: mechanisms and physiological consequences; pp. 319–329. [DOI] [PubMed] [Google Scholar]
- O'Toole SM, Sekula LK, Rubin RT. Pituitary-adrenal cortical axis measures as predictors of sustained remission in major depression. Biol Psychiatry. 1997;42:85–89. doi: 10.1016/S0006-3223(96)00293-4. [DOI] [PubMed] [Google Scholar]
- Olster DH, Ferin M. Corticotropin-releasing hormone inhibits gonadotropin secretion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab. 1987;65:262–267. doi: 10.1210/jcem-65-2-262. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Echavarria G, Galfalvy HC, Grunebaum MF, Burke A, Barrera A, et al. Lower cortisol levels in depressed patients with comorbid post-traumatic stress disorder. Neuropsychopharmacology. 2003;28:591–598. doi: 10.1038/sj.npp.1300050. [DOI] [PubMed] [Google Scholar]
- Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Kopin IJ, Goldstein DS. Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: In vivo microdialysis studies. Front Neuroendocrinol. 1995;16:89–150. doi: 10.1006/frne.1995.1004. [DOI] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. 1937. Journal of Neuropsychiatry and Clinical Neurosciences. 1995;7:103–112. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- Park J-J, Baum MJ, Paredes RG, Tobet SA. Neurogenesis and cell migration into the sexually dimorphic preoptic area/anterior hypothalamus of the fetal ferret. Journal of Neurobiology. 1996;30:315–328. doi: 10.1002/(SICI)1097-4695(199607)30:3<315::AID-NEU1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Paus T, Otaky N, Caramanos Z, MacDonald D, Zijdenbos A, D'Avirro D, et al. In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior-rostral sulci: Hemispheric asymmetries, gender differences and probability maps. Journal of Comparative Neurology. 1996;376:664–673. doi: 10.1002/(SICI)1096-9861(19961223)376:4<664::AID-CNE12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Payne JL. The role of estrogen in mood disorders in women. Int Rev Psychiatry. 2003;15:280–290. doi: 10.1080/0954026031000136893. [DOI] [PubMed] [Google Scholar]
- Payne JL, Palmer JT, Joffe H. A reproductive subtype of depression: conceptualizing models and moving toward etiology. Harvard Review of Psychiatry. 2009;17:72–86. doi: 10.1080/10673220902899706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia F, Aguzzoli L, Florio P, Baumann P, Genazzani AD, Di Carlo C, et al. Maternal plasma and placental immunoreactive corticotrophin-releasing factor concentrations in infection-associated term and pre-term delivery. Placenta. 1995;16:157–164. doi: 10.1016/0143-4004(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Petty F. Plasma concentrations of gamma-aminobutyric acid (GABA) and mood disorders: a blood test for manic depressive disease? Clinical Chemistry. 1994;40:296–302. [PubMed] [Google Scholar]
- Pilc A, Nowak G. GABAergic hypotheses of anxiety and depression: focus on GABA-B receptors. Drugs of today. 2005;41:755–766. doi: 10.1358/dot.2005.41.11.904728. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2007;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Poor V, Juricskay S, Gati A, Osvath P, Tenyi T. Urinary steroid metabolites and 11beta-hydroxysteroid dehydrogenase activity in patients with unipolar recurrent major depression. J Affect Disord. 2004;81:55–59. doi: 10.1016/S0165-0327(03)00199-X. [DOI] [PubMed] [Google Scholar]
- Prechtl HF. Continuity and change in early neural development. In: Prechtl HF, editor. Continuity of Neural Functions from Prenatal to Postnatal Life. Philadelphia: Lippincott; 1984. pp. 1–15. [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Butler T, Pan H, Altemus A, Polanescsky M, McEwen B, et al. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18:985–988. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci U S A. 2005;102:16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, et al. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. 6 Genetic and epigenetic underpinnings of sex differences in the brain and in neurological and psychiatric disease susceptibility. Progress in Brain Research. 2010;186:77. doi: 10.1016/B978-0-444-53630-3.00006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994a;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Oorschot DE, Verwer RW, Tilders FJ, Swaab DF. Age-related increase in the total number of corticotropin-releasing hormone neurons in the human paraventricular nucleus in controls and Alzheimer's disease: comparison of the disector with an unfolding method. Journal of Comparative Neurology. 1994b;339:447–457. doi: 10.1002/cne.903390311. [DOI] [PubMed] [Google Scholar]
- Rabin DS, Schmidt PJ, Campbell G, Gold PW, Jensvold M, Rubinow DR, et al. Hypothalamic-pituitary-adrenal function in patients with the premenstrual syndrome. Journal of Clinical Endocrinology and Metabolism. 1990;71:1158–1162. doi: 10.1210/jcem-71-5-1158. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Reynolds RM. Glucocorticoid excess and the developmental origins of disease: Two decades of testing the hypothesis. Psychoneuroendocrinology. 2012;38(1):1–11. doi: 10.1016/j.psyneuen.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- Robinson BG, Emanuel RL, Frim DM, Majzoub JA. Glucocorticoid stimulates expression of corticotropin-releasing hormone gene in human placenta. Proc Natl Acad Sci U S A. 1988;85:5244–5248. doi: 10.1073/pnas.85.14.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, et al. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. J Clin Endocrinol Metab. 2003;88:3057–3063. doi: 10.1210/jc.2002-021570. [DOI] [PubMed] [Google Scholar]
- Root JC, Tuescher O, Cunningham-Bussel A, Pan H, Epstein J, Altemus M, et al. Frontolimbic function and cortisol reactivity in response to emotional stimuli. Neuroreport. 2009;20:429–434. doi: 10.1097/WNR.0b013e328326a031. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Miller TH, Rhodes ME, Czambel RK. Adrenal cortical responses to low- and high-dose ACTH(1–24) administration in major depressives vs. matched controls. Psychiatry Res. 2006;143:43–50. doi: 10.1016/j.psychres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Poland RE, Lesser IM. Neuroendocrine aspects of primary endogenous depression VIII. Pituitary-gonadal axis activity in male patients and matched control subjects. Psychoneuroendocrinology. 1989;14:217–229. doi: 10.1016/0306-4530(89)90020-6. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Poland RE, Lesser IM, Winston RA, Blodgett AL. Neuroendocrine aspects of primary endogenous depression. I. Cortisol secretory dynamics in patients and matched controls. Arch Gen Psychiatry. 1987;44:328–336. doi: 10.1001/archpsyc.1987.01800160032006. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. Androgens, brain, and behavior. American Journal of Psychiatry. 1996;153:974–984. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- Rupp HA, James TW, Ketterson ED, Sengelaub DR, Janssen E, Heiman JR. Neural activation in the orbitofrontal cortex in response to male faces increases during the follicular phase. Hormones and Behavior. 2009a;56:66–72. doi: 10.1016/j.yhbeh.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp HA, James TW, Ketterson ED, Sengelaub DR, Janssen E, Heiman JR. Neural Activation in Women in Response to Masculinized Male Faces: Mediation by Hormones and Psychosexual Factors. Evolution and human behavior : official journal of the Human Behavior and Evolution Society. 2009b;30:1–10. doi: 10.1016/j.evolhumbehav.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo-Neustadt A, Beard R, Huang Y, Cotman C. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Rutledge T, Reis SE, Olson MB, Owens J, Kelsey SF, Pepine CJ, et al. Depression symptom severity and reported treatment history in the prediction of cardiac risk in women with suspected myocardial ischemia: The NHLBI-sponsored WISE study. Archives of General Psychiatry. 2006a;63:874–880. doi: 10.1001/archpsyc.63.8.874. [DOI] [PubMed] [Google Scholar]
- Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. Journal of the American College of Cardiology. 2006b;48:1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Archives of General Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Saricicek A. GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action. CNS Neurol Disord Drug Targets. 2007;6:127–140. doi: 10.2174/187152707780363294. [DOI] [PubMed] [Google Scholar]
- Scherrer JF, Xian H, Bucholz KK, Eisen SA, Lyons MJ, Goldberg J, et al. A twin study of depression symptoms, hypertension, and heart disease in middle-aged men. Psychosom Med. 2003;65:548–557. doi: 10.1097/01.psy.0000077507.29863.cb. [DOI] [PubMed] [Google Scholar]
- Schifrin BS. Antenatal fetal assessment: overview and implications for neurologic injury and routine testing. Clinical Obstetrics and Gynecology. 1995;38:132–141. doi: 10.1097/00003081-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Schofield PR, Williams LM, Paul RH, Gatt JM, Brown K, Luty A, et al. Disturbances in selective information processing associated with the BDNF Val66Met polymorphism: evidence from cognition, the P300 and fronto-hippocampal systems. Biological Psychology. 2009;80:176–188. doi: 10.1016/j.biopsycho.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Jamra RA, Becker T, Ohlraun S, Klopp N, Binder EB, et al. Evidence for a relationship between genetic variants at the brain-derived neurotrophic factor (BDNF) locus and major depression. Biol Psychiatry. 2005;58:307–314. doi: 10.1016/j.biopsych.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Schwaber JS, Kapp BS, Higgins GA, Rapp PR. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. Journal of Neuroscience. 1982;2:1424–1438. doi: 10.1523/JNEUROSCI.02-10-01424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger U, Deuschle M, Weber B, Korner A, Lammers CH, Schmider J, et al. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med. 1999;61:292–296. doi: 10.1097/00006842-199905000-00007. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoid programming of the fetus; adult phenotypes and molecular mechanisms. Molecular and Cellular Endocrinology. 2001;185:61–71. doi: 10.1016/s0303-7207(01)00633-5. [DOI] [PubMed] [Google Scholar]
- Seidman SN, Araujo AB, Roose SP, McKinlay JB. Testosterone level, androgen receptor polymorphism, and depressive symptoms in middle-aged men. Biol Psychiatry. 2001;50:371–376. doi: 10.1016/s0006-3223(01)01148-9. [DOI] [PubMed] [Google Scholar]
- Sesmilo G, Miller KK, Hayden D, Klibanski A. Inflammatory cardiovascular risk markers in women with hypopituitarism. Journal of Clinical Endocrinology and Metabolism. 2001;86:5774–5781. doi: 10.1210/jcem.86.12.8087. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Mittler BL, Mintun MA. The hippocampus and depression. Eur Psychiatry. 2002;17(Suppl 3):300–305. doi: 10.1016/s0924-9338(02)00655-7. [DOI] [PubMed] [Google Scholar]
- Shen Q, Fuchs T, Sahir N, Luscher B. GABAergic Control of Critical Developmental Periods for Anxiety-and Depression-Related Behavior in Mice. PLoS ONE. 2012;7:e47441. doi: 10.1371/journal.pone.0047441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, Luscher B. γ-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biological Psychiatry. 2010;68:512–520. doi: 10.1016/j.biopsych.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biological Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. Journal of Comparative Neurology. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacology Biochemistry and Behavior. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. New England Journal of Medicine. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. The Journal of Neuroscience. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snieder H, van Doornen LJ, Boomsma DI, Thayer JF. Sex differences and heritability of two indices of heart rate dynamics: a twin study. Twin Res Hum Genet. 2007;10:364–372. doi: 10.1375/twin.10.2.364. [DOI] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. Journal of Neuroscience. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonino N, Fava GA, Raffi AR, Boscaro M, Fallo F. Clinical correlates of major depression in Cushing's disease. Psychopathology. 1998;31:302–306. doi: 10.1159/000029054. [DOI] [PubMed] [Google Scholar]
- Spinelli MG. Neuroendocrine effects on mood. Rev Endocr Metab Disord. 2005;6:109–115. doi: 10.1007/s11154-005-6723-8. [DOI] [PubMed] [Google Scholar]
- Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmermann M, Kirsch P, et al. Influence of the stress hormone cortisol on fear conditioning in humans: evidence for sex differences in the response of the prefrontal cortex. NeuroImage. 2006;32:1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Steiner M. Female-specific mood disorders. Clinical Obstetrics and Gynecology. 1992;35:599–611. doi: 10.1097/00003081-199209000-00020. [DOI] [PubMed] [Google Scholar]
- Stratton MS, Budefeld T, Majdic G, Tobet S. Embryonic GABA-B receptor blockade alters adult hypothalamic structure and anxiety- and depression-like behaviors in mice [abstract]. Society of Neuroscience 41st Annual Meeting; Washington, D.C.. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri D, Vaidya VA. Glucocorticoid regulation of BDNF: Relevance to hippocampal structural and functional plasticity. Neuroscience. 2012;239:196–213. doi: 10.1016/j.neuroscience.2012.08.065. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Handa RJ. Regulation of estrogen receptor-beta expression in the female rat hypothalamus: Differential effects of dexamethasone and estradiol. Endocrinology. 2004;145:3658–3670. doi: 10.1210/en.2003-1688. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Chung WC, Kruijver FP, Hofman MA, Hestiantoro A. Sex differences in the hypothalamus in the different stages of human life. Neurobiol Aging. 2003;24(Suppl 1):S1–S16. doi: 10.1016/s0197-4580(03)00059-9. discussion S17-9. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E. A sexually dimorphic nucleus in the human brain. Science. 1985;228:1112–1115. doi: 10.1126/science.3992248. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Sudo M, Minokoshi Y, Shimazu T. Effects of ventromedial hypothalamic stimulation on glucose transport system in rat tissues. Am J Physiol. 1992;263:R1228–R1234. doi: 10.1152/ajpregu.1992.263.6.R1228. [DOI] [PubMed] [Google Scholar]
- Takumi K, Iijima N, Higo S, Ozawa H. Immunohistochemical analysis of the colocalization of corticotropin-releasing hormone receptor and glucocorticoid receptor in kisspeptin neurons in the hypothalamus of female rats. Neuroscience Letters. 2012;531(1):40–45. doi: 10.1016/j.neulet.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18:650–659. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Tobet S, Handa R, Goldstein JM. Sex-dependent Pathophysiology as Predictors of Comorbidity of Major Depressive Disorder and Cardiovascular Disease. Pflügers Archiv - European Journal of Physiology. 2013;465(5):585–594. doi: 10.1007/s00424-013-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobet S, Knoll JG, Hartshorn C, Aurand E, Stratton M, Kumar P, et al. Brain sex differences and hormone influences: a moving experience? Journal of Neuroendocrinology. 2009;21:387–392. doi: 10.1111/j.1365-2826.2009.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobet SA. Genes controlling hypothalamic development and sexual differentiation. European Journal of Neuroscience. 2002;16:373–376. doi: 10.1046/j.1460-9568.2002.02105.x. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Basham ME, Baum MJ. Estrogen receptor immunoreactive neurons in the fetal ferret forebrain. Brain Research. Developmental Brain Research. 1993;72:167–180. doi: 10.1016/0165-3806(93)90182-a. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Baum MJ, Tang HB, Shim JH, Canick JA. Aromatase activity in the perinatal rat forebrain: Effect of age, sex and intrauterine position. Developmental Brain Research. 1985;23:171–178. doi: 10.1016/0165-3806(85)90038-0. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Hanna IK. Ontogeny of sex differences in the mammalian hypothalamus and preoptic area. Cellular and Molecular Neurobiology. 1997;17:565–601. doi: 10.1023/A:1022529918810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Traslaviña GAA, Franci CR. Divergent Roles of the CRH Receptors in the Control of Gonadotropin Secretion Induced by Acute Restraint Stress at Proestrus. Endocrinology. 2012;153:4838–4848. doi: 10.1210/en.2012-1333. [DOI] [PubMed] [Google Scholar]
- Trestman RL, Coccaro EF, Mitropoulou V, Gabriel SM, Horvath T, Siever LJ. The cortisol response to clonidine in acute and remitted depressed men. Biol Psychiatry. 1993;34:373–379. doi: 10.1016/0006-3223(93)90181-c. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- Unden A-L, Elofsson S, Knox S, Lewitt MS, Brismar K. IGF-I in a normal population: relation to psychosocial factors. Clinical Endocrinology. 2002;57:793–803. doi: 10.1046/j.1365-2265.2002.01671.x. [DOI] [PubMed] [Google Scholar]
- Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. The Journal of Neuroscience. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustun TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. British Journal of Psychiatry. 2004;184:386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, McClure C, Johnson BD, Sheps DS, Bittner V, Rutledge T, et al. Depression, the metabolic syndrome and cardiovascular risk. Psychosomatic Medicine. 2008;70:40–48. doi: 10.1097/PSY.0b013e31815c1b85. [DOI] [PubMed] [Google Scholar]
- van Amelsvoort TAMJ, Abel KM, Robertson DMR, Daly E, Critchley H, Whitehead M, et al. Prolactin response to d-fenfluramine in postmenopausal women on and off ERT: comparison with young women. Psychoneuroendocrinology. 2001;26:493–502. doi: 10.1016/s0306-4530(01)00008-7. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. The magnocellular and parvocellular paraventricular nucleus of rat: intrinsic organization. Journal of Comparative Neurology. 1982;206:317–345. doi: 10.1002/cne.902060402. [DOI] [PubMed] [Google Scholar]
- Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. International Journal of Geriatric Psychiatry. 2007;22:613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- van der Kooy KG, van Hout HP, van Marwijk HW, de Haan M, Stehouwer CD, Beekman AT. Differences in heart rate variability between depressed and non-depressed elderly. International Journal of Geriatric Psychiatry. 2006;21:147–150. doi: 10.1002/gps.1439. [DOI] [PubMed] [Google Scholar]
- van Eijndhoven P, van Wingen G, van Oijen K, Rijpkema M, Goraj B, Jan Verkes R, et al. Amygdala volume marks the acute state in the early course of depression. Biol Psychiatry. 2009;65:812–818. doi: 10.1016/j.biopsych.2008.10.027. [DOI] [PubMed] [Google Scholar]
- van Londen L, Goekoop JG, van Kempen GM, Frankhuijzen-Sierevogel AC, Wiegant VM, van der Velde EA, et al. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology. 1997;17:284–292. doi: 10.1016/S0893-133X(97)00054-7. [DOI] [PubMed] [Google Scholar]
- van Os J, Jones P, Lewis G, Wadsworth M, Murray R. Developmental precursors of affective illness in a general population birth cohort. Arch Gen Psychiatry. 1997;54:625–631. doi: 10.1001/archpsyc.1997.01830190049005. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Everaerd W, Rombouts SA. Interaction of endogenous cortisol and noradrenaline in the human amygdala. Progress in Brain Research. 2008;167:263–268. doi: 10.1016/S0079-6123(07)67020-4. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, Rombouts SA. Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiology of Learning and Memory. 2007;87:57–66. doi: 10.1016/j.nlm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- van Wingen G, Mattern C, Verkes RJ, Buitelaar J, Fernandez G. Testosterone biases automatic memory processes in women towards potential mates. NeuroImage. 2008a;43:114–120. doi: 10.1016/j.neuroimage.2008.07.002. [DOI] [PubMed] [Google Scholar]
- van Wingen G, Mattern C, Verkes RJ, Buitelaar J, Fernandez G. Testosterone reduces amygdala-orbitofrontal cortex coupling. Psychoneuroendocrinology. 2010;35:105–113. doi: 10.1016/j.psyneuen.2009.09.007. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar JK, et al. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. 2008b;13:325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- Veith RC, Lewis N, Langohr JI, Murburg MM, Ashleigh EA, Castillo S, et al. Effect of desipramine on cerebrospinal fluid concentrations of corticotropin-releasing factor in human subjects. Psychiatry Res. 1993;46:1–8. doi: 10.1016/0165-1781(93)90002-x. [DOI] [PubMed] [Google Scholar]
- Verkuyl JM, Hemby SE, Joels M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur J Neurosci. 2004;20:1665–1673. doi: 10.1111/j.1460-9568.2004.03568.x. [DOI] [PubMed] [Google Scholar]
- von Bardeleben U, Holsboer F, Gerken A, Benkert O. Mood elevating effect of fluoxetine in a diagnostically homogeneous inpatient population with major depressive disorder. Int Clin Psychopharmacol. 1989;4(Suppl 1):31–35. [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66:617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009a;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009b;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallerath T, Witte K, Schafer SC, Schwarz PM, Prellwitz W, Wohlfart P, et al. Down-regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoid-mediated hypertension. Proc Natl Acad Sci U S A. 1999;96:13357–13362. doi: 10.1073/pnas.96.23.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, et al. Gender difference in neural response to psychological stress. Social Cognitive and Affective Neuroscience. 2007;2:227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward IL. Prenatal stress feminizes and demasculinizes the behavior of males. Science. 1972 doi: 10.1126/science.175.4017.82. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren WB, Gurewitsch ED, Goland RS. Corticotropin-releasing hormone and pituitary-adrenal hormones in pregnancies complicated by chronic hypertension. Am J Obstet Gynecol. 1995;172:661–666. doi: 10.1016/0002-9378(95)90589-8. [DOI] [PubMed] [Google Scholar]
- Warren WB, Patrick SL, Goland RS. Elevated maternal plasma corticotropin-releasing hormone levels in pregnancies complicated by preterm labor. Am J Obstet Gynecol. 1992;166:1198–1204. doi: 10.1016/s0002-9378(11)90606-1. discussion 1204-7. [DOI] [PubMed] [Google Scholar]
- Watson JB, Mednick SA, Huttunen M, Wang X. Prenatal teratogens and the development of adult mental illness. Development and Psychopathology. 1999;11:457–466. doi: 10.1017/s0954579499002151. [DOI] [PubMed] [Google Scholar]
- Weber-Hamann B, Blum WF, Kratzsch J, Gilles M, Heuser I, Deuschle M. Insulin-like growth factor-I (IGF-I) serum concentrations in depressed patients: relationship to saliva cortisol and changes during antidepressant treatment. Pharmacopsychiatry. 2009;42:23–28. doi: 10.1055/s-0028-1085442. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, O'Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol Psychiatry. 2002;7:985–994. 924. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- Weiner CL, Primeau M, Ehrmann DA. Androgens and mood dysfunction in women: comparison of women with polycystic ovarian syndrome to healthy controls. Psychosom Med. 2004;66:356–362. doi: 10.1097/01.psy.0000127871.46309.fe. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Does prenatal stress impair coping and regulation of hypothalamic-pituitary-adrenal axis? Neurosci Biobehav Rev. 1997;21:1–10. doi: 10.1016/s0149-7634(96)00014-0. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992;595:195–200. doi: 10.1016/0006-8993(92)91049-k. [DOI] [PubMed] [Google Scholar]
- Weisz J, Brown B, Ward I. Maternal stress decreases steroid aromatase activity in brains of male and female rat fetuses. Neuroendocrinology. 1982;35:374–379. doi: 10.1159/000123410. [DOI] [PubMed] [Google Scholar]
- Whitnall M, Gainer H. Major pro-vasopressin-expressing and pro-vasopressin-deficient subpopulations of corticotropin-releasing hormone neurons in normal rats. Neuroendocrinology. 1988;47:176–180. doi: 10.1159/000124910. [DOI] [PubMed] [Google Scholar]
- Wolfe CA, Van Doren M, Walker HJ, Seney ML, McClellan KM, Tobet SA. Sex differences in the location of immunochemically defined cell populations in the mouse preoptic area/anterior hypothalamus. Brain Res Dev Brain Res. 2005;157:34–41. doi: 10.1016/j.devbrainres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Wolfe CD, Patel SP, Linton EA, Campbell EA, Anderson J, Dornhorst A, et al. Plasma corticotrophin-releasing factor (CRF) in abnormal pregnancy. Br J Obstet Gynaecol. 1988;95:1003–1006. doi: 10.1111/j.1471-0528.1988.tb06504.x. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000a;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response - normal values and confounds. Noise and Health. 2000b;2:79–88. [PubMed] [Google Scholar]
- Yeragani VK, Pohl R, Balon R, Ramesh C, Glitz D, Jung I, et al. Heart rate variability in patients with major depression. Psychiatry Research. 1991;37:35–46. doi: 10.1016/0165-1781(91)90104-w. [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M. Puberty, ovarian steroids, and stress. Ann N Y Acad Sci. 2004;1021:124–133. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]
- Young EA, Kornstein SG, Harvey AT, Wisniewski SR, Barkin J, Fava M, et al. Influences of hormone-based contraception on depressive symptoms in premenopausal women with major depression. Psychoneuroendocrinology. 2007a;32:843–853. doi: 10.1016/j.psyneuen.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Midgley AR, Carlson NE, Brown MB. Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Archives of General Psychiatry. 2000;57:1157–1162. doi: 10.1001/archpsyc.57.12.1157. [DOI] [PubMed] [Google Scholar]
- Young EA, Ribeiro SC, Ye W. Sex differences in ACTH pulsatility following metyrapone blockade in patients with major depression. Psychoneuroendocrinology. 2007b;32:503–507. doi: 10.1016/j.psyneuen.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukhananov RY, Handa RJ. Alterations in kappa opioid receptor mRNA levels in the paraventricular nucleus of the hypothalamus by stress and sex steroids. Neuroreport Oxford. 1996;7:1690–1694. doi: 10.1097/00001756-199607080-00033. [DOI] [PubMed] [Google Scholar]
- Zhang J. Effect of age and sex on heart rate variability in healthy subjects. Journal of Manipulative and Physiological Therapeutics. 2007;30:374–379. doi: 10.1016/j.jmpt.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Yassouridis A, Frieboes RM, Holsboer F. Prediction of medium-term outcome by cortisol response to the combined dexamethasone-CRH test in patients with remitted depression. Am J Psychiatry. 1999;156:949–951. doi: 10.1176/ajp.156.6.949. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Alvarez-Royo P, Clower RP. Independence of memory functions and emotional behavior: separate contributions of the hippocampal formation and the amygdala. Hippocampus. 1991;1:207–220. doi: 10.1002/hipo.450010208. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Carbone DL, Handa RJ. Prenatal dexamethasone selectively decreases calretinin expression in the adult female lateral amygdala. Neuroscience Letters. 2012a;521:109–114. doi: 10.1016/j.neulet.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Carbone DL, Hiroi R, Chong DL, Handa RJ. Dexamethasone induces apoptosis in the developing rat amygdala in an age, region, and sex specific manner. Neuroscience. 2011;199:535–547. doi: 10.1016/j.neuroscience.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Carbone DL, Quihuis A, Hiroi R, Chong DL, Handa RJ. Perinatal dexamethasone-induced alterations in apoptosis within the hippocampus and paraventricular nucleus of the hypothalamus are influenced by age and sex. Journal of Neuroscience Research. 2012b;90:1403–1412. doi: 10.1002/jnr.23026. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Carbone DL, Quihuis A, Hiroi R, Chong DL, Handa RJ. Perinatal dexamethasone-induced alterations in apoptosis within the hippocampus and paraventricular nucleus of the hypothalamus are influenced by age and sex. Journal of Neuroscience Research. 2012c;90:1403–1412. doi: 10.1002/jnr.23026. [DOI] [PubMed] [Google Scholar]