Abstract
BACKGROUND
Radiation therapy can result in osteoradionecrosis (ORN) and mucosal ulceration predisposing to infection.
METHODS
Fourteen patients presenting with infectious sequelae related to mandibular ORN were retrospectively reviewed.
RESULTS
In most patients, infection followed diagnosis of ORN but in 4 patients ORN was not diagnosed until after the time of infection and imaging. An early imaging finding of ORN was lingual cortical defects near the last molar. Pain followed by erythema, purulent drainage and sub-periosteal abscess by imaging were the most common signs of infection. In most patients conservative management eventually failed and segmental mandibulectomies were required.
CONCLUSIONS
Soft tissue infection with characteristic bone findings such as subperiosteal abscess and cortical bone erosions helps to distinguish infected ORN from recurrent tumor or sterile ORN. In patients previously treated with radiation who present with infection, pain or an avid PET scan with bone involvement, the mandible should be scrutinized.
Introduction
Although radiation treatment is a mainstay of therapy for head and neck squamous cell carcinoma (SCC), it is not without potential complications. One of the more common acute complications is mucosal ulceration, which can be detected radiographically1–3. The loss of the mucosal barrier provides a route for oral microbes to enter a normally sterile area such as the masticator space and the mandible.
The mandible is a common site for osteoradionecrosis (ORN) when high dose radiation (>50–60Gy) is given, due to its tenuous blood supply4–6. Mandibular ORN can be a debilitating disease secondary to pain and bone loss which can result in pathologic fractures 7. Many factors have been implicated in the pathogenesis of ORN including the amount of radiation to affect the bone, trauma (such as dental extraction), oral hygiene, and preexisting conditions which may delay healing, such as diabetes mellitus7. There is a wide range of incidence of mandibular ORN related to radiation (2–22%) with superimposed infection in the minority of ORN patients8. ORN is the most common cause of mandibular osteomyelitis9.
Previous reports10–13 have described characteristic imaging findings of ORN and emphasized that the soft tissue enhancement adjacent to the bone should not be confused with recurrent tumor. We are continuing this work with the following retrospective case series of 14 patients who developed infectious complications related to mandibular ORN. There have been few case reports on the imaging of infection superimposed on mandibular ORN to date14. Several helpful signs for differentiating infected from sterile ORN will be discussed. Additionally, this series gives evidence for the debate of how infection relates to mandibular ORN, which may relate to prognosis.
Methods and Materials
Fourteen patients were identified with imaging evidence and subsequent documentation of infection with concomitant diagnosis of mandibular ORN based on a search of the teaching files of a senior neuroradiologist (L.G.). The reporting period was from 2000 to 2012, given the availability of imaging in the electronic medical record. The medical records were retrospectively reviewed for relevant clinical and imaging data. Computer tomography (CT) was available in all patients and was the main means of assessment. Six patients also had positron emission tomography (PET) available. All patients presented with infectious sequelae related to mandibular ORN. Twelve of fourteen patients (86%) had T2-T4 SCC tumor involving the oral tongue and/or base of tongue (Table 1). The remaining patients had a T4 SCC centered in the palatine tonsil (Figure 1, Patient 7) or metachronous bilateral parotid tumors (Patient 13). Most patients were radiated with 60–70 Gy to the primary tumor site (Figure 2a). Three patients were re-irradiated and likely received larger total doses. The average estimated dose to the affected area of the mandible was ~40–68 Gy for the patients with available treatment plans.
Table 1.
Demographic and diagnostic information for the 14 patients with infections related to osteoradionecrosis of the mandible. Demographics” gives the age in years and gender of patient, location” is the site of the primary squamous cell carcinoma. Radiation” is the total dose of radiation that the patient received. Time to ORN” is the number of months from finishing radiation to the first signs of ORN by imaging or clinically. Time to infection” is the number of months from the first signs (imaging or clinical) of ORN to the development of infection. Known diagnosis ORN/ ID” is for if the patient’s diagnosis of ORN or infection was known clinically at the time of imaging which confirmed or revealed the diagnosis. Presenting symptom” is the first complaints that the patient had at presentation with infection. Treatment” is the means of therapy given to the patient. Abbreviation guide: M= male, F= female, T= primary tumor stage, BOT= base of tongue, OT= oral tongue, Gy= Grey (unit of radiation), n/a= not available, redo= re-irradiated, mo= months, ORN= osteoradionecrosis of the mandible, Bacteria= bacterial infection, Fungal= fungal infection, Y= yes, N= no, Abx = antibiotics, I&D= incision and drainage, Seq = sequestrectomy, Sx = surgery (some form of segmental mandibulectomy), HBO= hyperbaric oxygen.
| Patient Number | Demographics | Location | Radiation (Gy) | Time to ORN (mo) | Time to Infection (mo) | Type of Infection | Known Diagnosis ORN/ID | Presenting Symptom | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 M | T2 BOT | 70 | 24 | 0.75 | Bacteria, Fungal | Y/Y | Pain, drainage, rubor | Abx, I&D |
| 2 | 66 M | T2 BOT | n/a | 5 | 8 | Fungal | Y/Y | Pain, rubor | Abx, Seq |
| 3 | 70 M | T3 BOT | n/a | 17 | 0 | n/a | Y/Y | Trismus, fistula | Abx, Seq, Sx |
| 4 | 61 F | T2 OT | 70 | 7 | 10 | Bacteria/ Fungal | Y/Y | Pain, swelling | Abx, Sx |
| 5 | 68 M | T4 BOT | 72 | 60 | 12 | n/a | Y/Y | Worse trismus, swelling | Abx, Sx |
| 6 | 54 M | T2BOT | 70 | 16 | 2 | Bacteria/ Fungal | Y/Y | Swelling, drainage | Abx, extraction, Sx |
| 7 | 59 M | T4 tonsil | n/a | 27 | 45 | Bacteria | N/Y | Trismus, rubor | Abx, HBO, I&D, Sx |
| 8 | 50 M | T2 BOT | n/a | 18 | 3 | n/a | Y/N | Trismus, pain | Abx, HBO, Sx |
| 9 | 70 M | Tonsil, OT, T4 BOT | n/a (redo) | 168 (1st); 5 (2nd) | 12 | Bacteria | Y/N | Trismus, pain | Abx, Sx |
| 10 | 69 M | T2 OT | >60 (redo) | 60 (1st); 5 (2nd) | 8 | n/a | Y/Y | Trismus, pain | Abx, Sx |
| 11 | 67 M | T2 OT | 70 | 13 | 37 | Bacteria | N/N | Pain, drainage | Abx, HBO, Sx |
| 12 | 74 F | BOT | n/a | 60 | 0 | Bacteria | N/N | Trismus, pain, weight loss | Abx |
| 13 | 82 M | Metachronous bilateral parotid | n/a (redo) | 120 (1st); 4 (second) | 11 | n/a | Y/Y | Pain, ear drainage | Abx, HBO |
| 14 | 61 M | BOT | 69 | 10 | 0 | Bacteria | N/Y | Trismus, pain, otalgia, rubor | Abx, I&D, HBO |
“Demographics” gives the age in years and sex of patient, “location” is the site of the primary squamous cell carcinoma. “Radiation” is the total dose of radiation that the patient received. “Time to ORN” is the number of months from finishing radiation to the first signs of ORN by imaging or clinically. “Time to infection” is the number of months from the first signs (imaging or clinical) of ORN to the development of infection. “Known diagnosis ORN/ID” is if the patient's diagnosis of ORN or infection was known clinically at the time of imaging, which confirmed or revealed the diagnosis. “Presenting symptom” is the first complaints that the patient had at presentation with infection. “Treatment” is the means of therapy given to the patient.
Abx, antibiotics; Bacteria, bacterial infection; BOT, base of tongue; F, female; Fungal, fungal infection; Gy, Grey (unit of radiation); HBO, hyperbaric oxygen; I&D, incision and drainage; M, male; mo, months; N, no; n/a, not available; ORN, osteoradionecrosis of the mandible; OT, oral tongue; redo, reirradiated; Seq, sequestrectomy; Sx, surgery (some form of segmental mandibulectomy); T, primary tumor stage; Y, yes.
Figure 1.
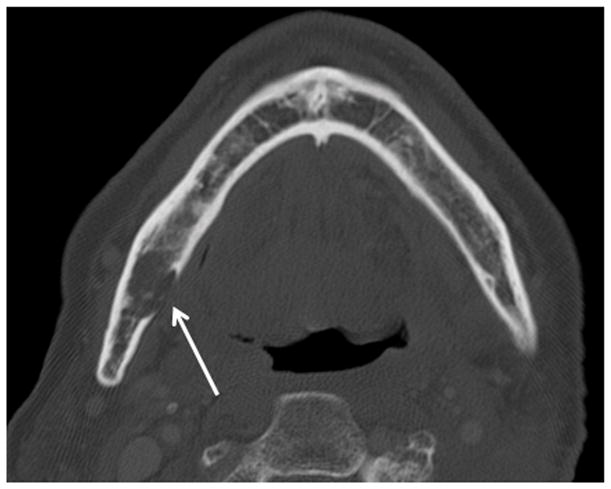
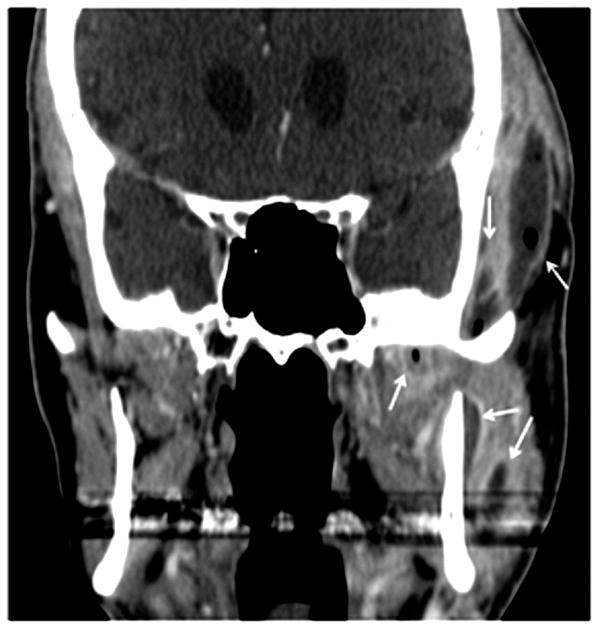
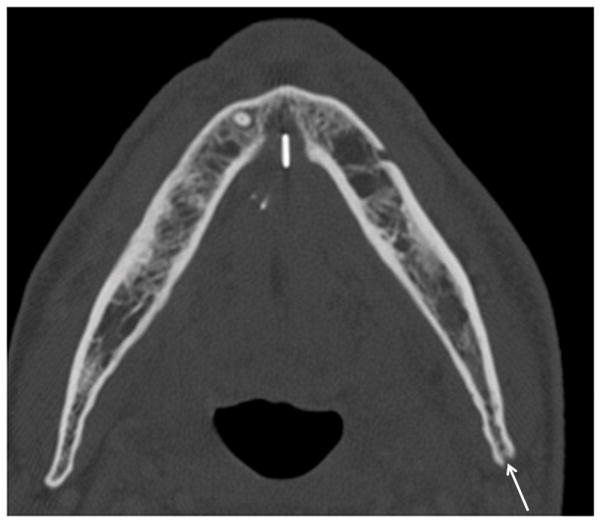
Patient #7 (see table 1) presented with worsening trismus and skin erythema. The diagnosis of ORN was unknown clinically until presenting with of Staph Aureus infection. Figure 1a, axial contrast enhanced CT in soft tissue windows demonstrates mucosal ulceration (straight arrow), soft tissue swelling (myositis and phlegmon) and a subtle periosteal abscess (small double arrows) along the lateral aspect of the right mandibular ramus, contralateral to the primary tumor site. Figure 1b, axial CT image in bone algorithm illustrates osseous cortical defects and fragmentation (arrow) involving the right mandibular ramus. Figure 1c, axial CT image in bone algorithm nearly four years prior demonstrates the subtle cortical defect along the lingual surface adjacent to the last molar (arrow) with trabecular disorganization, as early signs of ORN.
Figure 2.
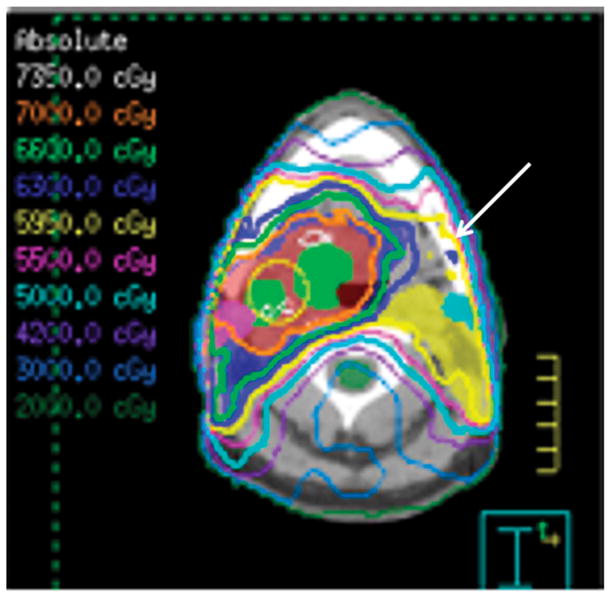
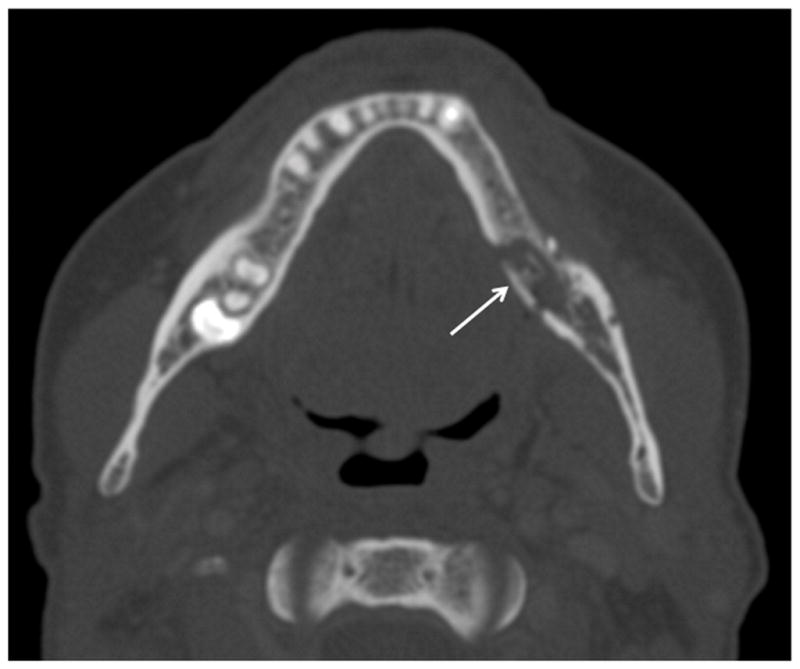
Patient #1 has radiation treatment plan (Figure 2a) superimposed on axial CT image for the tumor demonstrates that >59 Gy was given to the contralateral left mandible (arrow). Figure 2b, two years after treatment the patient developed pain, drainage and skin redness. Axial CT image in bone windows demonstrates left mandibular ORN with cortical defect and fracture on the lingual aspect with cortical disorganization (arrow). Figure 2c, coronal post-contrast CT reformation in soft tissue windows reveals multiple subperiosteal and soft tissue abscesses (small arrows) surrounding the contralateral left mandible, skull base and muscles of mastication due to a superimposed infection with multiple bacterial species and Candida.
Results
Timing and Presentation
Early osseous radiologic findings of ORN developed a mean of 19.8 months after their most recent radiation therapy completion (range 2–60 months). The three patients who were re-irradiated were 5, 11, and 14 years from their first radiation but all developed ORN within 12 months of re-irradiation. The development of subsequent superimposed infection by clinical exam or imaging sign (discussed below) averaged 13.7 months after the first signs of developing ORN (range 0–53 months). The intervals between imaging were variable ranging from 2 weeks to 6 months but tended to be shorter periods during the time of superinfection. Clinical visits were generally within two weeks of imaging.
In 4 of 14 patients (29%) the diagnosis of ORN was unknown until the time of presentation of infection and imaging. Pain was the most common presenting symptom of infection and ORN. Often the patients initially had pain presumably due to ORN but the pain worsened with infection. Specific complaints for pain included new or worsening trismus, odynophagia, otalgia or paresthesias. In 10 of 14 patients (71%) there were additional signs of infection (erythema, swelling or purulent drainage). The remaining 4 patients had non-specific pain at presentation which clinically could have also been interpreted as a manifestation of tumor recurrence. PET standard uptake values ranged from 3 to 10. Of the four patients with SUV values greater than 5, two were misinterpreted as tumor recurrence (Figure 3a). The osseous erosions on CT were what pointed to the correct diagnosis for the PET imaging.
Figure 3.
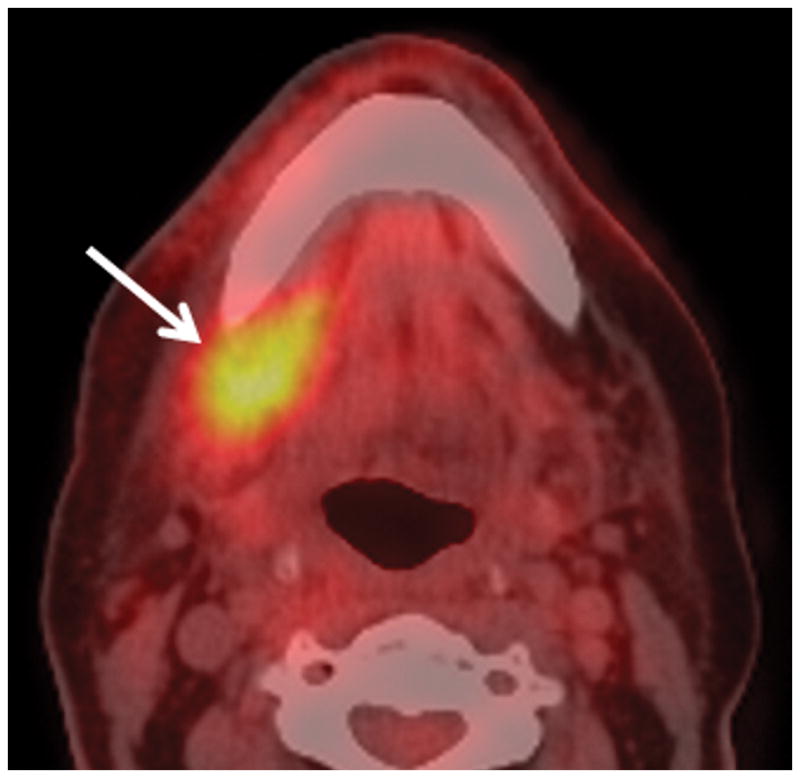
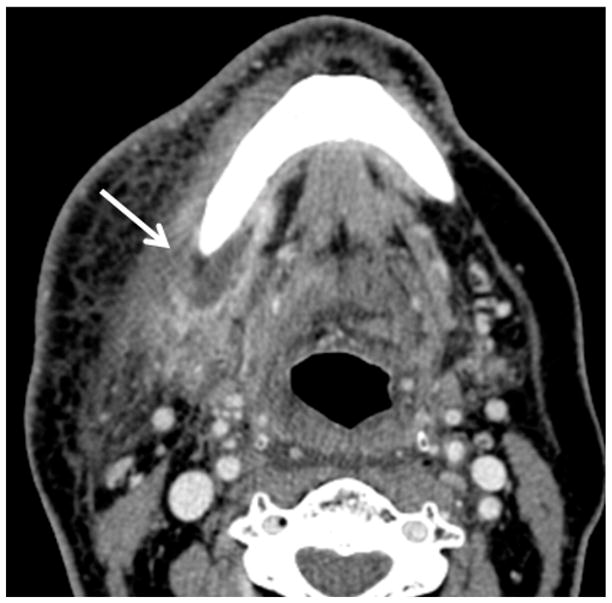
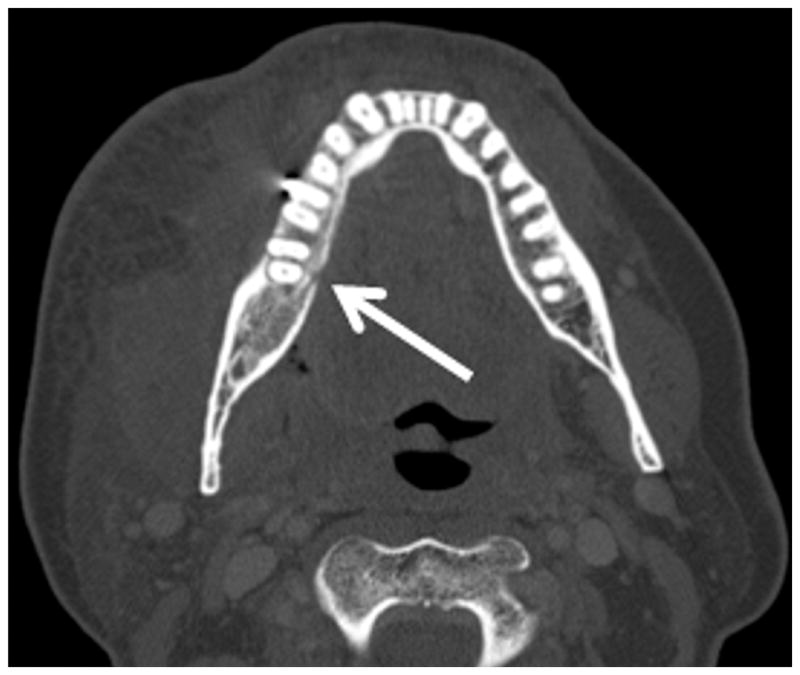
Patient #14 presented 10 months after radiation therapy presents with pain (trismus/otalgia) and skin erythema Figure 3a, axial PET CT image reveals significant (SUV=10) uptake in the soft tissues of the submandibular space. This was interpreted as possible necrotic tumor recurrence and the patient underwent a negative biopsy. Figure 3b, axial contrast enhanced CT image in soft tissue windows obtained 6 days later demonstrates a ring enhancing fluid collection surrounding the mandible from a subperiosteal abscess (white arrow). There has been interval increase in the soft tissue stranding of the subcutaneous tissues from cellulitis and thickening of the mylohyoid from myositis. Figure 3c, axial CT image in bone windows at a slightly more cranial level shows a subtle cortical defect involving the lingual surface of the mandible near the last molar. This was not present 3 months prior and indicates the true diagnosis of superimposed infection on mandibular ORN.
Osseous Imaging Findings Associate with Osteoradionecrosis
The earliest osseous imaging finding of ORN was cortical defects which were found in 13 of 14 patients (93%). As would be expected, most patients (10 of 14; 71%) developed the ORN ipsilateral to the tumor (original site), with 2 bilateral and 2 contralateral cases (Figures 1 and 2). The majority of the cortical defects were in a characteristic location along the lingual surface, near the last mandibular molar (Figures 1b, 2b and 3c). Two additional early defects were found along the posterior border of the mandibular angle or ramus (Figure 4b) and one within the mandibular condyle, in the parotid cancer patient (Patient #13). Osseous imaging findings for ORN in the later stages included trabecular disorganization, air within the bone marrow space, pathologic fracture and fragmentation (Figures 1b, 2b and 4b). Only 4 patients had normal appearing posterior mandibular molars on the side of ORN.
Figure 4.
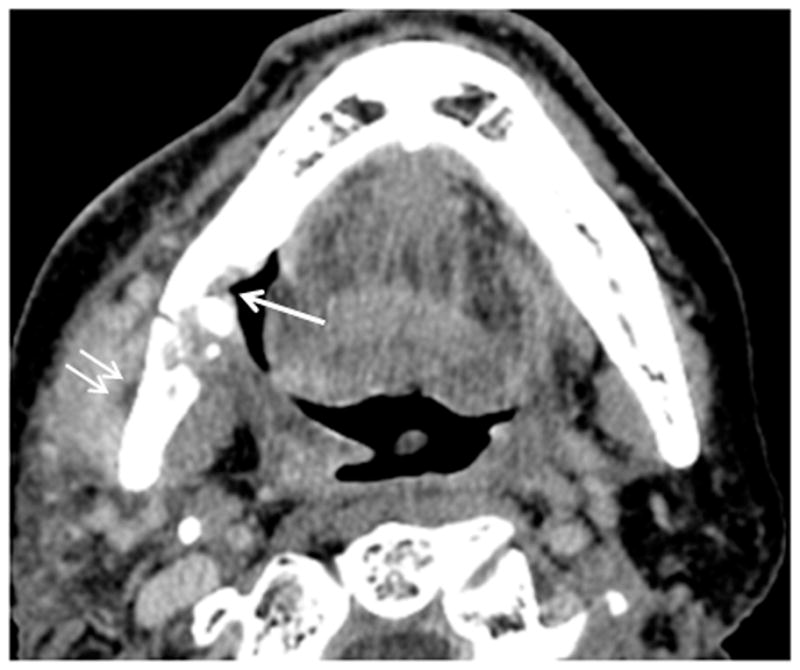
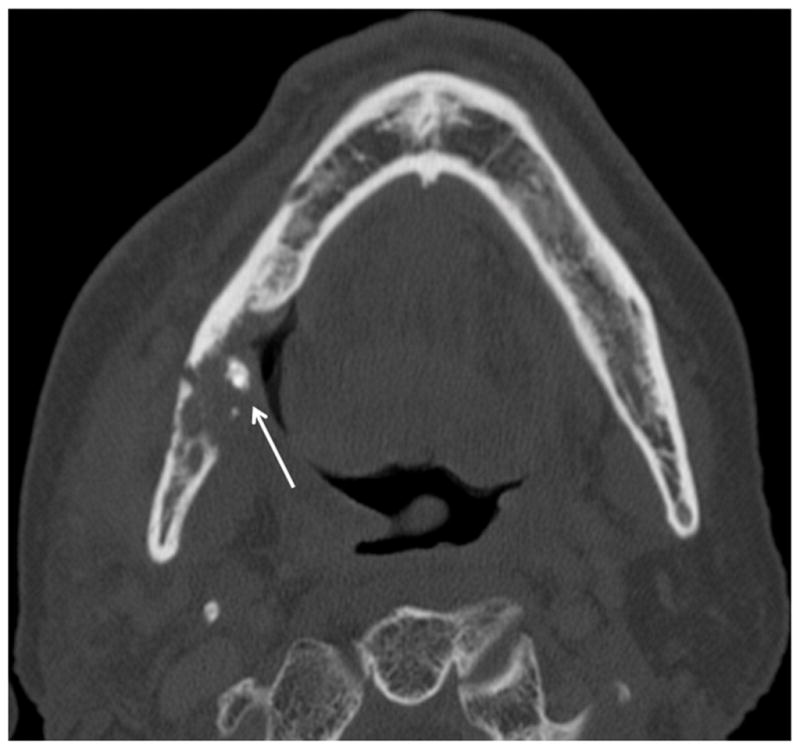
Patient #11 presented with pain and drainage from Streptococcal infection without a known diagnosis of ORN. Figure 4a, axial CT image in soft tissue window demonstrates portions of a draining cutaneous fistula from the periosteum of the mandible body to the skin of the left neck below (arrows). Extensive phlegmon involves bilateral deep spaces of the neck and the subcutaneous tissues of the left face with bilateral cutaneous thickening. In Figure 4b, axial CT image in bone windows at a slightly more cranial level, demonstrates a pathologic fracture through the left mandible (large arrow), multiple cortical defects (small arrows) and trabecular disorganization. In retrospect (Figure 4c, 3.5 years before Figures 4a and 4b), the earliest sign of ORN was a subtle cortical defect truncating the left posterior mandibular angle (arrow) which occurred 13 months after completion of radiation therapy. The marrow pattern at that time had not significantly changed when compared to pre-treatment imaging.
Imaging Findings of Soft Tissue Infection
The most characteristic and common finding of superimposed infection was subperiosteal abscess formation (Figures 1a, 2c, and 3b) seen in all but two cases (86%). These characteristically are fluid collections with an incomplete ring of enhancement ending at the bone. Other common soft tissue findings included soft tissue abscesses (Figure 2c), soft tissue phlegmon (Figure 4a), cellulitis (Figure 3b), myositis (Figures 1a, 3b and 4a), gingivitis, and intra-oral or orocutaneous fistulae (Figure 4a). The minority of patients (4 of 13 patients; 31%) required surgical drainage of large abscesses involving the masticator space (Figure 2c) or subcutaneous tissues. All but two patients had mucosal ulceration or exposed bone by CT or clinical exam which occurred either before or during the time of infection (Figures 1).
Infected ORN Therapy
Conservative therapy consisted of several treatment strategies including antimicrobial therapy, oral rinses, hyperbaric oxygen and local surgical procedures (incision and drainage of abscesses, sequestrectomy, and/ or tooth extractions). Bacterial species were typical for respiratory, oral and skin bacterial flora. Four patients also exhibited Candida yeast species and one had actinomyces. Most patients (9 of 14; 64%) eventually failed conservative management and required some form of segmental mandibulectomy and reconstruction with bone free flaps. Three of the patients who are reported to have responded to conservative management (Patients 12–14) have less than one year of follow-up after ORN progression, so the true number of conservative treatment failures may be higher.
Discussion
Although it can be difficult to differentiate sterile and super-infected ORN, the finding of abscess is helpful and was common though many were too small to warrant surgical drainage. The authors have not seen any cases to date where sterile ORN resulted in development of fluid collections in the periosteal space or adjacent soft tissues, thus this appears to be a specific sign of superimposed infection. So abscess maybe a common and useful sign for predicting superimposed infection. Also, extension of cellulitis and phlegmon distant to the bone could support the diagnosis of a superimposed infection. However, in practice it may be difficult, especially without prior post-treatment imaging, to differentiate infectious phlegmon and fat stranding related to the following: 1) fat stranding related to radiation effects; 2) enhancing masses from recurrent tumor; or 3) phlegmon-like changes adjacent to the bone associated with the inflammation from ORN10. Of course, the clinical signs of new or worsening pain or more specific sign of infection would also suggest the superimposed infection and need for anti-microbial therapy.
Infection and ORN are known to coincide, so that one of the initial therapies is empiric antibiotics. However, given the long delay (>1 year) between the start of ORN and the subsequent infection in most of our patients, it would be problematic to empirically continue antibiotics long-term, without any signs of infection. Imaging follow-up can be useful as 4 patients (29%) did not have signs of infection, other than pain which is non-specific.
In over a quarter of our patients the diagnosis of ORN was unknown prior to detection of infection; therefore when a head and neck SCC patient previously treated with radiation has new symptoms of infection or pain, the bone should be carefully scrutinized for signs of ORN. Similarly, if a patient with known diagnosis of ORN presents with new or worsening pain, infection should be considered. In 2 of the 6 cases with PET- CT the high uptake values were misinterpreted as recurrent tumor in or surrounding the mandible. This was interpreted as osseous invasion by tumor or necrotic tumor surrounding the bone (Figure 3). However, scrutiny of the bone by CT revealed cortical erosions or frank cavitation making the diagnosis of superimposed infection on ORN clear.
Careful evaluation of the mandible is important in this patient population. An early imaging finding of ORN, in this group of patients with mostly oral tongue and base of tongue cancers, was a lingual surface cortical defect adjacent to the last molar. Previous literature has also reported defects in the cortex as the earliest radiographic sign of ORN6 with the endosteum, periosteum and vascular channels affected first15. It is postulated that the location is due to the proximity of this area to the tumor which receives relatively larger doses during radiation therapy. This also explains why the single patient re-irradiated for parotid tumors had erosions of the mandibular condyle, located superior to any of the other cases (Patient #13). The radiation treatment planning may include relatively larger doses through the junction of the posterior mandibular body and inferior ramus, as radiation therapists often try to avoid significant radiation through the spinal cord, brain stem, and major salivary glands during three-dimensional radiation planning. This differs from the locations of other series where the location near the last molar is similar but it was the buccal side which was more often affected6. This may reflect differences in tumor location and/or radiation technique.
An alternative explanation for the location of the early cortical erosions is that posterior molars are a frequent site of abnormality and surgical extraction. This has been debated as a risk factor for ORN in the literature7,16 but an association is supported by the fact that only a minority of our patients had normal appearing dentition. Dental loss or extraction could act much like ulceration in providing oral cavity flora access to the devitalized bone.
Many bacterial species DNA have been found in mandibular ORN 17. Only 1 actinomyces infections was found in our series, despite reports that this was a common pathogen18. The relationship of bacterial infection and mandibular ORN is debatable as bacteria have been deemed: 1) a factor for developing ORN7; 2) an incidental contaminant19; and 3) a late sequelae of ORN8. Our series supports the theory that bacterial infection is a predominantly a late stage manifestation of ORN and coincides with “development of denuded bone and fistulous tracts”8. In most patients (11 out of 14; 79%) there was a time delay between the earliest findings of ORN and subsequent development of infection. Two of the remaining three patients had the infection at presentation to our institution, so the timing cannot be well established. Therefore, only one patient (Patient #14, Figure 3) to our knowledge had proven infection coinciding with the onset of ORN. Admittedly, it is difficult to prove in this retrospective study that there was not infection at the original time of ORN diagnosis given the proclivity of bacterial contamination in the oral cavity. However, given the lack of clinical or radiographic signs the authors believe that infection was delayed in these patients. Additionally, infection as late sequelae of ORN may explain failure of conservative management in our series, a finding which was also reported in other series of mandible ORN patients20,21. Subgroup analysis in one study showed that patients with infection and ORN were less likely to respond to conservative management than ORN alone8. A possible explanation would be that the radiation converted fatty marrow4 becomes rotten and acts as a culture medium, if bacteria are introduced through mucosal ulceration/ denuded bone or a fistulous tract8. Further data is needed to determine if earlier conservative management of ORN could improve outcomes, such as preventing subsequent infection or need for surgical intervention.
One retrospective review of head and neck osteomyelitis showed that the mandible was the most commonly affected facial bone. Of mandibular osteomyelitis patients, the most common cause was mandibular ORN (in 41%)9. One study had an incidence of 22% of mandible ORN patients having superimposed infections8. However, the true incidence of bacterial superinfection of mandibular ORN will likely remain difficult to accurately estimate because of several factors: 1) the wide range of incidence of mandibular ORN related to radiation (2–22%)8 due to many reasons including radiation dose, dose planning, and host factors; 2) variable timing and presentation of both ORN and superimposed infection; 3) variable response to conservative management of ORN and the timing of such management which could prevent exposure of bone/ bacterial seeding; and 4) the debate over incidental bacterial contamination versus true infection. Without better defining the incidence of mandibular superinfection, it may be difficult to evaluate the sensitivity of any radiographic sign for discerning infected from sterile ORN.
In conclusion, soft tissue finding such as abscess formation are useful in differentiating super-infection from sterile ORN. In previously irradiated patients with new or worsening pain or signs of infection, the mandible should be carefully scrutinized for subtle signs of ORN. Our findings support the theory that infection is commonly a late manifestation of mandibular ORN.
Acknowledgments
Funding: Made possible by MD Anderson Core Grant CA16672
Footnotes
The authors have no financial interests to disclose.
This work was presented at the 44th annual meeting of the American Society of Head and Neck Radiology, San Diego, California, September 2011
References
- 1.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 2.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–44. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 3.Debnam JM, Garden AS, Ginsberg LE. Benign ulceration as a manifestation of soft tissue radiation necrosis: imaging findings. AJNR Am J Neuroradiol. 2008;29:558–62. doi: 10.3174/ajnr.A0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabin BM, Meyer JR, Berlin JW, Marymount MH, Palka PS, Russell EJ. Radiation-induced changes in the central nervous system and head and neck. Radiographics. 1996;16:1055–72. doi: 10.1148/radiographics.16.5.8888390. [DOI] [PubMed] [Google Scholar]
- 5.Sukh S, Rayatt MAMM, Stefan O, Hofer P. Osteoradionecrosis of the mandible: Etiology, prevention, diagnosis and treatement. Indian journal of plastic surgery. 2007;40:65–71. [Google Scholar]
- 6.Bras J, de Jonge HK, van Merkesteyn JP. Osteoradionecrosis of the mandible: pathogenesis. Am J Otolaryngol. 1990;11:244–50. doi: 10.1016/0196-0709(90)90084-9. [DOI] [PubMed] [Google Scholar]
- 7.Vanderpuye V, Goldson A. Osteoradionecrosis of the mandible. J Natl Med Assoc. 2000;92:579–84. [PMC free article] [PubMed] [Google Scholar]
- 8.Store G, Boysen M. Mandibular osteoradionecrosis: clinical behaviour and diagnostic aspects. Clin Otolaryngol Allied Sci. 2000;25:378–84. doi: 10.1046/j.1365-2273.2000.00367.x. [DOI] [PubMed] [Google Scholar]
- 9.Prasad KC, Prasad SC, Mouli N, Agarwal S. Osteomyelitis in the head and neck. Acta Otolaryngol. 2007;127:194–205. doi: 10.1080/00016480600818054. [DOI] [PubMed] [Google Scholar]
- 10.Chong J, Hinckley LK, Ginsberg LE. Masticator space abnormalities associated with mandibular osteoradionecrosis: MR and CT findings in five patients. AJNR Am J Neuroradiol. 2000;21:175–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo JS, Rosenthal DI, Mitchell K, Ginsberg LE. Osteoradionecrosis of the hyoid bone: imaging findings. AJNR Am J Neuroradiol. 2010;31:761–6. doi: 10.3174/ajnr.A1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debnam JM. Imaging of the Head and Neck following Radiation Treatment. Patholog Res Int. 2011;2011:607820. doi: 10.4061/2011/607820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glastonbury CM, Parker EE, Hoang JK. The postradiation neck: evaluating response to treatment and recognizing complications. AJR Am J Roentgenol. 2010;195:W164–71. doi: 10.2214/AJR.09.4122. [DOI] [PubMed] [Google Scholar]
- 14.Quek ST, Poddar S, Khoo JB. Clinics in diagnostic imaging (85). Mandible osteoradionecrosis complicated by infection. Singapore Med J. 2003;44:269–73. [PubMed] [Google Scholar]
- 15.Store G, Granstrom G. Osteoradionecrosis of the mandible: a microradiographic study of cortical bone. Scand J Plast Reconstr Surg Hand Surg. 1999;33:307–14. doi: 10.1080/02844319950159280. [DOI] [PubMed] [Google Scholar]
- 16.Oh HK, Chambers MS, Garden AS, Wong PF, Martin JW. Risk of osteoradionecrosis after extraction of impacted third molars in irradiated head and neck cancer patients. J Oral Maxillofac Surg. 2004;62:139–44. doi: 10.1016/j.joms.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Aas JA, Reime L, Pedersen K, et al. Osteoradionecrosis contains a wide variety of cultivable and non-cultivable bacteria. J Oral Microbiol. 2010:2. doi: 10.3402/jom.v2i0.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen T, Kunkel M, Kirkpatrick CJ, Weber A. Actinomyces in infected osteoradionecrosis--underestimated? Hum Pathol. 2006;37:61–7. doi: 10.1016/j.humpath.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Balogh JM, Sutherland SE. Osteoradionecrosis of the mandible: a review. J Otolaryngol. 1989;18:245–50. [PubMed] [Google Scholar]
- 20.Oh HK, Chambers MS, Martin JW, Lim HJ, Park HJ. Osteoradionecrosis of the mandible: treatment outcomes and factors influencing the progress of osteoradionecrosis. J Oral Maxillofac Surg. 2009;67:1378–86. doi: 10.1016/j.joms.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Notani K, Yamazaki Y, Kitada H, et al. Management of mandibular osteoradionecrosis corresponding to the severity of osteoradionecrosis and the method of radiotherapy. Head Neck. 2003;25:181–6. doi: 10.1002/hed.10171. [DOI] [PubMed] [Google Scholar]