Abstract
Arterial aging is the major contributing factor to increases in the incidence and prevalence of cardiovascular disease, due mainly to the presence of chronic, low-grade, “sterile” arterial inflammation. Inflammatory signaling driven by the angiotensin II cascade perpetrates adverse age-associated arterial structural and functional remodeling. The aged artery is characterized by endothelial disruption, enhanced vascular smooth muscle cell migration and proliferation, extracellular matrix deposition, elastin fracture, and matrix calcification/amyloidosis/glycation. Importantly, the molecular mechanisms of arterial aging are also relevant to the pathogenesis of hypertension, and atherosclerosis. Age-associated arterial proinflammation is, to some extent, mutable, and interventions to suppress or delay it may have the potential to ameliorate or retard age-associated arterial diseases.
Keywords: Proinflammation, angiotensin II, central arterial aging, hypertension, atherosclerosis
Chronic inflammation and arterial aging
The demographics of modern society predict an exponential increase in the number of older persons in the future that could produce an epidemic of chronic arterial hypertension and atherosclerosis [1–6]. Central arterial aging is a hallmark of systems aging, and can be viewed as the failure of critical signaling pathways to execute crucial functions [5]. These aging-related changes in the molecular and cellular functions of key signaling systems facilitate adverse central arterial remodeling, such as diffuse intimal-medial thickening, enhanced stiffening and endothelial dysfunction [2–6] (Figure 1). Arterial wall aging begins with a chronic pro-inflammation, a form of “sterile-like” inflammation that occurs in the absence of any microorganisms and with little or no white blood cell infiltration. Phenotypic shifts in arterial endothelial and vascular smooth muscle cells (VSMCs) promote pathogenic inflammation [2–6]. This is why arterial aging dwarfs other risk factors for clinical manifestations and severity of hypertension and atherosclerosis.
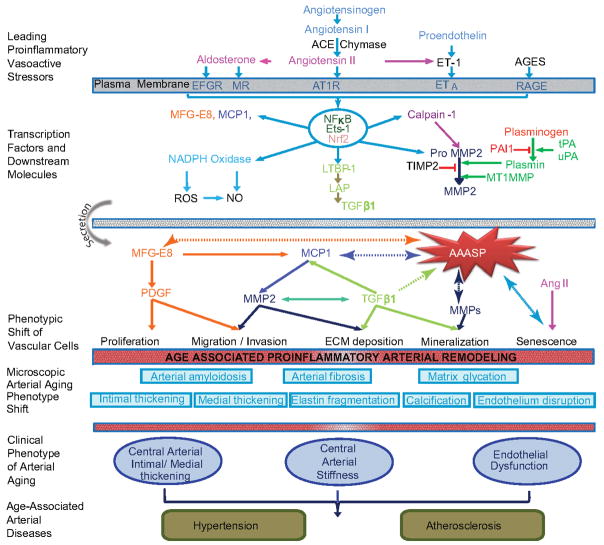
Figure 1. Diagram of age-associated proinflammatory arterial remodeling.
The chronic proinflammatory profile within aged central arteries is characterized by alterations in signaling systems that include Ang II signaling via its receptor AT1, as well as MR, and ET-1/ETA signaling. AGEs recruit inflammatory molecules by interaction with their cellular transduction receptor for AGEs (RAGE). NF-κB and Ets-1 are activated within the aging arterial wall, whereas protective factors such as Nrf-2 are reduced. Downstream signaling molecules include MFG-E8, MMPs, calpain-1, MCP-1 and TGF-β1. Activation of calpain-1, MMPs, TGF-β1, NADPH oxidase increases whereas NO bioavailability decreases with advancing age. Old VSMCs secrete increased amounts of MFG-E8, MCP-1, MMP-2 and TGF-β1 and concurrent proinflammatory proliferation, migration, secretion, senescence, and extracellular matrix deposition within the aged arterial wall is observed. Disruption of the endothelium, intima-media thickening, arterial amyloidosis, fibrosis, calcification, elastin fracture, and matrix glycoxidative modifications are consequences of the enhanced signaling via these receptor signaling cascades. Some can lead to changes in arterial clinical phenotype that can be measured non-invasively in humans. A significant interaction exists between aging and hypertension and the frequency of arterial atherosclerosis markedly increases in old vs. young humans.
Abbreviations: Angiotensin II: Ang II, aldosterone/mineralocorticoid receptor: MR, endothelin-1: (ET-1), endothelin-1 receptor A: ETA, Nuclear factor kappa-light-chain-enhancer of activated B cells: NF-κB, v-ets erythroblastosis virus E26 oncogene homolog 1: Ets-1, NF-E2-related factor 2: Nrf-2, milk fat globule epidermal growth factor-8: MFG-E8, matrix metalloproteases: MMPs, monocyte chemo-attractant protein-1: MCP-1, transforming growth factor β1: TGF-β1, nicotinamide adenine dinucleotide phosphate-oxidase: NADPH oxidase, nitric oxide: NO, age-associated arterial secretory phenotype: AAASP.
This review describes the chronic proinflammatory state that develops within the central arterial wall as we age, with focus on angiotensin II (Ang II)-mediated proinflammation and the role of VSMCs (see glossary). The potential to retard age-associated arterial diseases by ameliorating or delaying chronic arterial wall inflammation is discussed.
Proinflammatory signaling molecules within the aged arterial wall
The chronic proinflammatory profile within aged central arteries is characterized by alterations in major signaling cascades that include the renin/Ang II, the aldosterone/mineralocorticoid receptor (Aldo/MR) systems, and the endothelin-1 (ET-1)/endothelin-1 receptor A (ETA) system (Figure 1). The expression and/or activity of several proinflammatory transcription factors that lie downstream of these pathways are increased, whereas levels of protective factors are reduced (Figure 1). As a result, augmented VSMC migration, proliferation or senescence, along with extracellular matrix (ECM) deposition, elastin fracture, and matrix glycoxidative modifications, disrupt the endothelium and contribute to arterial aging (Figure 1). The main signaling systems and the alterations that take place in aging central arteries are discussed below.
Leading proinflammatory vasoactive stressors
Ang II and its receptors
The renin angiotensin system was initially recognized as a potent vasoconstriction system. The proinflammatory profile of the Ang II signaling cascade plays a dominant role in the process of adverse arterial remodeling with aging in numerous species, including humans (Table 1) [2–6]. The transcription, translation, and activity of the angiotensin-converting enzyme-1 (ACE-1) markedly increase within both endothelial cells (ECs) and VSMCs with aging [7–10]. Additionally, expression of chymase, an alternative angiotensin convertase, within the arterial wall, increases with aging [8]. As a result, the cleaved product, Ang II protein, becomes markedly increased, particularly in the thickened intima of rats, nonhuman and human primates [7, 8, 11, 12]. The Ang II receptor, AT1, is up-regulated within the old arterial wall, and angiotensinogen, a precursor of Ang II, is also increased in the old coronary artery [7, 11, 13].
Table 1.
Molecular and cellular remodeling
| Aging
|
Hypertension | Atherosclerosis | Ang II Signaling | |||||
|---|---|---|---|---|---|---|---|---|
| Humans >56 yrs |
Monkeys 15–20 yrs |
Rats 24–30 mo |
Rabbits 2–6 yrs |
|||||
| Inflammatory Molecules | Local Ang II | ↑ | ↑ | ↑ | ? | ↑ | ↑ | ↑ |
| MMPs | ↑ | ↑ | ↑ | ? | ↑ | ↑ | ↑ | |
| Calpain-1 | ↑ | ↑ | ↑ | ? | ↑ | ↑ | ↑ | |
| MCP-1/CCR2 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| TGF-β1 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| NADPH Oxidase | ↑ | ↑ | ↑ | ↑ | ↑ | ? | ↑ | |
| NO Bioavailability | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| TNF-α | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| ICAM | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| MFG-E8 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| VEGF | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
|
| ||||||||
| Cellular-Matrix structure and function | EC dysfunction | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| Diffuse IMT | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Stiffness | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Matrix | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Calcification | ↑ | ↑ | ↑ | ? | ↑ | ↑ | ↑ | |
| FN/Collagen | ↑ | ↑ | ↑ | ↑ | ↑ | ? | ↑ | |
| VSMC migration | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
| VSMC proliferation | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
|
| ||||||||
| Hypertension prevalence | ↑ | ↑ | ↑ | ? | ↑ | ? | ↑ | |
| Atherosclerosis prevalence | ↑ | - | - | - | ? | ↑ | ↑ | |
↑: increase; ↓: decrease; -: not present; Ang II: angiotensin II; MMPs: matrix metalloproteases; MCP -1: monocyte chemo-attractant protein-1; CCR2: C-C chemokine receptor type 2; TGF-β1: transforming growth factor β1; NADPH oxidase: nicotinamide adenine dinucleotide phosphate-oxidase; NO: nitric oxide; TNFα: tumor necrosis factor alpha; ICAM: intercellular adhesion molecule; MFG-E8: milk fat globule epidermal growth factor-8; VEGF: vascular endothelial growth factor; EC: endothelial cell; IMT: intima-media thickening; FN: fibronection; VSMC: vascular smooth muscle cell. Modified from references [2–6].
Aldo, MR, and epidermal growth factor receptors
Aldo is a hormone secreted by the adrenal gland, which binds to the MR and regulates blood pressure in the cardiovascular system. The arterial wall Aldo/MR signaling pathway is increased with aging [13, 14]. The increased sensitivity of MR to Aldo and thus increased MR activity, in aged rats, promotes a proinflammatory phenotype via an extracellular signal-regulated kinase 1/2/mitogen-activated protein kinase/epidermal growth factor receptor (ERK/MAPK/EGFR) -dependent pathway, contributing to the phenotypic shift of VSMCs within the aging wall [14]. In addition, Aldo mediated increase in the expression of EGFR in VSMCs with aging, reinforces its inflammatory effects [14].
ET-1 and its receptor
Aortic expression of both the vasoconstriction peptide pro-ET-1 and endothelin converting enzyme-1 (ECE-1) mRNA become increased with advancing age [15, 16]. Consequently, both pro-ET-1 (1–31) and active ET-1(1–21) levels are increased in old vs. young arteries [15, 16]. The catalytic action of matrix metalloprotease type 2 (MMP-2), enzymes that are involved in the breakdown of extracellular matrix, increases with aging as well, and resembles that of ECE-1 [8, 11, 16, 17, 18]. MMP-2 enhancesthe conversion ofthe inactive pro -ET-1 to the active ET-1, that increases blood pressure and augments inflammation via the transcription factor v-ets erythroblastosis virus E26 oncogene homolog 1 (Ets-1) [16].
Transcription factors involved in inflammation
The leading inflammatory triggers Ang II, Aldo, and ET-1 increase the activation of inflammatory transcription factors within the arterial wall, with aging (Figure 1). Elevated Ets-1 activity is closely associated with increased transcription of ET-1, monocyte chemoattractant protein-1 (MCP-1), transforming growth factor beta 1 (TGF-β1), and MMP-2, within the old arterial wall [16, 19]. These proinflammatory triggers also increase the activation of the key inflammatory marker Nuclear Factor-κB, thus initiating an inflammatory signaling loop. Activated NF-κB regulates the activity of MMP-2/-9, calpain-1, MCP-1, TGF-β1, and reactive oxygen species (ROS), which deliver multiple signals and potentially drive arterial aging [20–22]. The transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2) that acts as key cellular defense against the cytotoxic effects of oxidative stress by increasing the expression of antioxidant enzymes, is inactivated by ROS in the old vasculature, leading to down-regulation of detoxifying and antioxidant genes [20]. Interestingly, Nrf2 activity is negatively regulated by NF-κB [20]. Indeed, old monkey arteries exhibit significant increases in oxidative markers such as 8-iso-prostaglandin F2α and 4-hydroxy-2-nonenal. In contrast, levels of the antioxidant enzyme glutathione are reduced, compared with young animals [20]. Unlike the case in young arteries, however, increased oxidative stress in old animals is associated with a reduction in Nrf2 activity modulated by NF-κB signaling [20].
Downstream molecules of activated transcription factors
Milk fact globule EGF-8 (MFG-E8) and integrins
A high-throughput proteomic screening identified MFG-E8, a cell adhesion protein, as a novel biomarker of aging arterial walls [12, 23, 24]. Importantly, MFG-E8 is a key molecule for the pathogenesis of chronic arterial inflammation [24]. Levels of arterial MFG-E8 and its degradation fragment, medin, both increase and accumulate in the aorta with aging [12, 23–25]. MFG-E8 is induced by Ang II and itself induces the expression of MCP-1 in VSMCs within the old rat aortic wall [12]. This places MFG-E8 within the Ang II/MCP-1/VSMC invasion signaling cascade. Finally, during aging, MFG-E8 also interacts with integrin avβ5 to further facilitate endothelial cell apoptosis and a VSMC phenotypic shift, via an integrin/ERK1/2 signaling pathway [23].
MMP-2
MMPs are proteins that are involved in the breakdown of ECM in both physiological and disease processes. They are produced within vascular cells and are secreted. The activity of MMP-2 becomes increased within the aortic wall with advancing aging and cleaves and activates TGF-β1 and pro-ET-1, contributing to their increased bioavailability in the vascular cells [7, 8, 11, 13, 16–18, 26, 27]. The increased MMP-2 activity is due not only to enhanced transcription and translation, but also to a shift in the ratio of its activator membrane type-1 MMP (MT1-MMP) and tissue inhibitor of MMP-2 (TIMP-2) [8, 17]. Activated MMP-2 has a high affinity for and cleaves both elastin fibers and cellular basement membrane, thus facilitating the age-associated increase in VSMC migration/invasion, elastin fragmentation and calcification. In addition, other members of the MMP family such as MMP-9 and MMP-13 are also increased in the arterial wall with aging [16].
Calpain-1
Calpain-1 is an intracellular calcium-activated proteinase that regulates cytoskeletal protein remodeling. The transcription, translation, and activity of calpain-1 are significantly up-regulated in old compared to young rat aortae, and this up-regulation is recapitulated in the aorta of young rats infused with Ang II [28, 29]. Importantly, calpain-1 activation may lead to the induction and increased secretion of active MMP-2, partly by increasing the ratio of membrane type 1 MMPs to tissue inhibitor of MMP-2 [29]. Thus, calpain-1 is key in the Ang II/MMP-2/collagen types I, II, or III production cascade in the arterial wall that contributes to arterial fibrosis and calcification [28, 29].
MCP-1 and C-C chemokine receptor type 2 (CCR2)
MCP-1 is a notorious inflammatory cytokine. It belongs to the CC chemokine family and originally functions by recruiting immune cells to sites of inflammation. Transcription and translation of MCP-1, and its cognate receptor, CCR2, become enhanced in the aged arterial wall [18, 30, 31]. Importantly, a striking MCP-1 protein gradient exists across the aortic wall from the intima to media that is increased with aging, and which likely is a key driving force for medial VSMC invasion of the intima [7].
TGF-β1
TGF-β1 is a powerful profibrotic cytokine, which is secreted by many cell types in a latent form. The cytokine is in a complex with two other proteins, latent TGF binding protein-1 (LTBP-1) and latency-associated peptide (LAP). Inflammatory stimuli are required to promote the release of active TGF-β1 from this complex and binding to its receptor. Activated TGF-β1, LAP, and LTBP-1 all increase in the arterial wall with aging [26]. Importantly, downstream signaling molecules of activated TGF-β1 signaling such as arterial p-SMAD-2/-3/-4 are increased, while the antagonistic or inhibitoryp -SMAD-7 is decreased in the arterial wall with aging [26]. Consequently, the activated SMAD complex translocates to the nucleus to mediate extracellular matrix gene transcription [26].
ROS and nitric oxide (NO) bioavailability
ROS are major modulators of NO bioavailability. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases are membrane-bound enzymes and a major source of ROS generation [20, 32]. The decrease in endothelial NO bioavailability with aging occurs concurrently with an increase in arterial wall O2- production [33]. An interaction of NO with ROS results in subsequent formation of peroxynitrite (ONOO-), an oxidant that can damage proteins as well as DNA [33]. An increased deposition of 3-nitrotyrosinated proteins, a marker of ONOO-, is observed in old compared to young aortae [33]. Notably, the decreased bioavailability of NO contributes to the decline of endothelium-dependent arterial relaxation with increasing age [32].
Proinflammatory signals drive the phenotypic shift of vascular cells in the aging arterial wall
Chronic infusion of a physiologically relevant dose of Ang II to young rats increases activity of molecules that comprise the proinflammation profile, e.g. MMP-2, MCP-1, calpain-1, TGF-β1, and NADPH oxidase [11, 13, 28]. An ACE inhibition and AT1 blockade, beginning at an early age, markedly inhibit the proinflammatory molecules and delays the progression of age- associated aortic remodeling [9, 10, 35]. The combined proinflammation within arterial wall cells and matrix consequently drives adverse arterial restructuring via modifications of arterial cells with aging (Figure 1). The phenotypic proinflammatory shift of the aging arterial cells, including ECs and VSMCs, isan important cellular event.
ECs
ECs, the frontline cells of the arterial wall, rest on the basement membrane and shoulder the component of the proinflammatory burden that originates within the circulation. The Ang II, MCP-1, and MFG-E8 inflammatory load is increased in the old endothelia [7, 11, 12, 30]. This age-associated proinflammation may enhance ROS generation, which damages endothelial mitochondrial DNA and interferes with the mitochondria life cycle [2–6, 21, 36]. These responses initiate and promote EC senescence and apoptosis [2–6, 20, 24]. Old ECs become enlarged and likely detach from the vulnerable basement membrane by enhanced activation of MMPs that break down the ECM [2–6, 37]. The disrupted basement membrane likely recruits and concentrates bioactive factors such as Ang II and MFG-E8, which form the local inflammatory niche that is hostile to EC residents [2–4]. These cellular events and micro-environments lead to the dysfunction of aged inflamed endothelia, and are the culprits of enhanced permeability, infiltration, pro-thrombosis or coagulation, that facilitate arterial damage with aging[2–4].
VSMCs
Old VSMCs become stiffened and develop heterogeneous phenotypes within the arterial wall, with some cells functioning as “pluripotent cells”, similar to the phenotypic shifts of VSMCs also observed in hypertension, restenosis, and atherosclerosis, or in cell culture [2–6, 38–40]. Concurrent VSMC proinflammatory secretion, senescence, proliferation, migration, and ECM deposition are characteristic features of arterial aging.
Senescence and Secretion
Substantial heterogeneity among VSMC phenotypes within the old arterial wall suggests that both proliferative and senescent VSMC subsets may coexist. Indeed, some old VSMC are likely to enter an irreversible growth arrest called cellular senescence. Senescence-associated β-gal activity in the old rat arterial wall is detected in VSMCs enriched in the cyclin-dependent kinase inhibitor p16 and NADPH oxidase 4 (NOX4), which is likely linked to increasing Ang II signaling [41, 42]. Indeed, Ang II induces an irreversible growth arrest of VSMCs through stress-induced premature senescence (SIPS) or telomere shortening [43, 44]. Both stress and progressive telomere shortening consequently leads to activation of the DNA damage machinery and p53, leading to senescence [36, 43, 44].
Importantly, the age-associated arterial secretory phenotype (AAASP) is observed in the cytokine secretion profile of primary VSMCs derived from old nonhuman primates [2, 45]. Old cells secrete more interleukin-1β, interleukin-6, MCP-1, and tumor necrosis factor α, mimicking the fibroblast senescence-associated secretory phenotype (SASP) [45]. Actually, young VSMCs treated with Ang II secrete a large amount of proinflammatory factors, including MFG-E8, similar to the AAASP of old untreated cells [2, 12, 46]. Emerging evidence indicates that the AAASP likely delivers signals to the neighboring VSMC, enhancing the phenotypic shift with aging in a paracrine/juxtacrine-manner [47].
Proliferation
VSMC subsets within the aged aortic wall have an enhanced proliferation capacity [23]. The replication rate in old cultured VSMCs is increased, compared to young [23]. Old cultured VSMCs have a greater percentage of cells in the S and G2/M phases and a lower percentage in the G0/G1 phase, than young [23]. In young cultured VSMCs, MFG-E8 triggers phosphorylation of ERK1/2, augments levels of proliferative cellular nuclear antigen (PCNA), CDK4 and platelet derived growth factor (PDGF) signaling, increases 5-bromo-2′-deoxyuridine (BrdU) incorporation, and promotes proliferation via αvβ5 integrin signaling [23]. MFG-E8 silencing, integrin inhibition, or the blockade of ERK1/2 phosphorylation in young cells reduces PCNA and CDK4 levels and decelerates the cell cycle S phase, conferring a reduction in proliferative capacity [23]. Collectively, these results indicate that MFG-E8 coordinates the expression of cell cycle molecules and facilitates proliferation in old VSMC via integrin/ERK1/2 signaling.
Migration/Invasion
The migration/invasion of VSMC from the arterial media to the intima is a key cellular event in the age-associated diffuse intimal thickening. With advancing passage in culture, the capacity of young VSMC invasion increases up to that of old cells, via increased activation of MMP-2/-9 [2–6, 48]. Old cultured VSMCs exhibit an exaggerated migration/invasion capacity compared to the young [11, 12, 18, 23, 28, 30, 48]. Exposure of early passage young VSMCs to Ang II, calpain-1, MFG-E8, PDGF-bb, or MCP-1 enhances invasive capacity to levels observed in untreated old cells [7, 11, 12, 18, 28, 30, 48]. MFG-E8 silencing RNA substantially reduces MCP-1, PDGF, its receptor expression and VSMC invasion capacity [12, 23]. These findings indicate that MFG-E8 is a pivotal relay element within the Ang II/MCP-1/PDGF/VSMC invasion signaling cascade.
ECM deposition
VSMCs produce and maintain a complex meshwork of ECM, including collagen types I, II and III. Enhanced collagen deposition is a salient feature of ECM remodeling of the arterial wall with aging. Increased active TGF-β1 signaling governs the production of collagen molecules by VSMCs [26, 29]. MMP-2-activated TGF-β1signaling is also intimately involved in the increased collagen I, II, and III production by old VSMCs [26, 29]. In addition, calpain-1/TGF-β1 activation also increases collagen production by old VSMCs mediated by MMP-2 activation [29].
Intervention of proinflammatory-driven age-associated adverse remodeling
MMP inhibition, elastin fragmentation, and ECM deposition
Chronic administration of a broad-spectrum MMP inhibitor, PD166739, markedly blunts the age-associated increases in aortic gelatinase and interstitial collagenase activity, and reduces the elastic fiber degeneration, collagen deposition, MCP-1 expression, TGF-β1 activation, and SMAD-2/3 phosphorylation [16]. Interestingly, MMP inhibition also substantially diminishes pro-ET-1 activation and down-regulates Ets-1 expression [16].
MFG-E8 and amyloidosis
Increased amyloid deposition is a characteristic of the aged arterial wall [5, 24, 25]. A specific amyloid protein, known as medin, is deposited in the aortic media in the majority of Caucasians over 50 years [5, 24, 25, 49]. The medin fragment is 5.5 kDa and is cleaved from the C2-like domain of MFG-E8 [5, 25]. In addition, both medin and MFGE8, in an amyloid protein complex, bind to tropoelastin, and regulate the amyloid interaction with tropoelastin [5, 25]. Thus, MFG-E8/medin amyloid may likely be a factor in the increased aortic stiffness that accompanies advancing age. Indeed, serum MFG-E8 levels and pulse wave velocity (PWV), an index of arterial stiffening, correlate with cardiovascular risk factors in old humans [50].
Calpain-1 and calcification
Arterial calcification is a salient feature of age-associated arterial remodeling. Old cultured VSMCs, like osteoblasts, are able to produce large amounts of bone-like substrates, including collagen II, which become bio-mineralized as calcification [29]. The over-expression of calpain-1 reduces the calcification inhibitors, osteonectin and osteopontin (OPN), and induces alkaline phosphatase activity in young VSMC, mimicking old cells [29]. Importantly, both calpain-1 activity and collagen II are increased within the human calcified aortae [29]. In addition, the activity of tissue transglutaminase (TG2), a protein crosslinking enzyme, increases in the old arterial wall [51]. Activated TG2 up-regulates calcification promoter genes, i.e. Runx2 and down-regulates the expression of calcification inhibitor genes, i.e. OPN within VSMCs [51]. Thus, TG2 activation is also a key molecular event of arterial calcification.
Advanced glycation end-products (AGEs) and arterial stiffening
With aging, advanced non-enzymatic glycation of proteins via the Maillard reaction occurs within the arterial matrix and produces cross-linking of collagen known as AGEs. ALT-711, a non-enzymatic cross-link breaker, improved arterial compliance in old nonhuman primates and humans [52, 53]. Thus, increased AGEs are an important molecular event of age-associated arterial stiffening. Additionally, AGEs recruit inflammatory molecules TGF-β1 and MCP-1 by interaction with their cellular transduction receptor for AGEs (RAGE) [54]. Notably, a soluble RAGE (sRAGE) contributes to the removal/detoxification of AGEs. Circulating sRAGE levels become decreased withaging and are negatively associated with arterial stiffening [55].
Caloric restriction (CR) and oxidation
The expression of SIRT1, a longevity gene, decreases with aging within the arterial wall, contributing to arterial dysfunction [21, 56, 57]. CR retards aging and increases lifespan in rodents by elevating SIRT1 activity [21]. Resveratrol, an activator of SIRT1, mimics CR, and improves arterial health in rodents fed a high fat diet [56–58]. Importantly, overexpression of SIRT1 inhibits both VSMC AT1 expression and NADPH oxidase activation [57, 59]. These findings suggest that CR/resveratrol treatment retards aging likely via an inhibition of Ang II-driven oxidation.
Physical Conditioning and inflammation
It is well established in humans that habitual exercise leads to improvement in vascular structure and function with aging [60]. Several studies in both aging mice and humans have demonstrated that vascular health is improved with voluntary exercise through a pronounced reduction of the inflammation markers NF-κB, NADPH oxidase, and TGF-β1 [61–64].
Concluding remarks and future perspectives
A chronic increase in production of inflammatory signals is the key to age-associated adverse arterial structural remodeling, including diffuse intimal-medial thickening, increased stiffening and VSMC migration/proliferation/senescence. Under the microscope, the aged artery is characterized by the disruption of the endothelium, extracellular matrix deposition, elastin fracture, and matrix calcification/amyloidization/glycation. These adverse arterial cellular and molecular events are recapitulated in experimental young animals under stressful Ang II infusion, and are attenuated in old animals via interference of inflammatory signals by blockade of Ang II receptor signaling and inhibition of MMPs. Arterial age is intertwined with hypertension and atherosclerosis at the molecular and cellular levels (see boxes) because the aged arterial wall is fertile soil for their pathogenesis. If aging is a form of sub-clinical disease, then disease as originally applied to those conditions may be considered archaic. Thus, early and effective strategies to suppress age-associated arterial inflammation via pharmacologic, e.g., ACE inhibitors, MMP inhibitors, and protein cross-link breaker drugs, or non-pharmacologic, e.g., dietary restriction, and physical exercise, approaches may be realistic strategies to curb the initiation and progression of age-associated cardiovascular diseases.
Box 1. The age-associated epidemic of hypertension.
Growing evidence in humans indicates that significant interactions exist between aging and hypertension. The prevalence of hypertension exponentially increases with aging: 6.8% in 18–39 yrs, 30.4% in 40–59 yrs, and greater than 70% for 60 yrs and over in humans [65]. Sustained hypertension in young humans and rats contributes to functional and structural changes within the arterial wall that mimic those in old normotensives [2–6]. These manifestations, including decreases in endothelial-dependent vascular dilatation and increases in PWV and intimal-medial thickness (IMT), are considered to be a “silent arterial syndrome” [2–6]. Further, hypertension accelerates age-associated remodeling of the arterial wall and modifications of its mechanical properties and vascular events [2–6].
The aging process and essential hypertension share similar functional and/or structural, and molecular modifications of the central arterial wall (Table 1). Chronic administration of Ang II in vivo at a pressor dose in young FXBN rats increases blood pressure and activity of MMP-2, TGF-β1, and calpain-1 as well as collagen production within the arterial wall [11, 28], which are similar to what occurs in untreated old animals [11, 28]. Interestingly, a recent study demonstrates that the absence of kidney ACE substantially blunts the hypertension induced by Ang II infusion [66]. Further, chronic infusion of phenylephrine, an α-adrenergic transmitter that increases with age, into young rats markedly increases blood pressure and arterial wall Ang II levels, and partially recapitulates age-associated remodeling [11]. In addition, downstream molecules of the AT1 receptor signaling, e.g. TGF-β1, ROS, PDGF, p-ERK1/2, and PCNA, are markedly increased with aging in spontaneously hypertensive rats (SHR) compared to normotensive Wistar Kyoto rats (WKY) [2–6, 67]. Further, lifelong treatment of young stroke-prone spontaneously hypertensive rats (SHR-sp) with the AT1 blocker, fonsartan, doubles the lifespan to 30 months by alleviating complications of hypertension, and this is related to the decreases in circulating arterial and cardiac ACE activity and increases in arterial endothelial nitric oxide synthase (eNOS) activity [68].
Box 2. The age-associated epidemic of atherosclerosis.
The current frequency of arterial atherosclerosis markedly increases in old vs. young humans [1–6, 65]. Arterial aging and atherosclerosis share similar functional, structural, and molecular profiles (Table 1). The interaction of aging and atherosclerosis in humans is demonstrated by recent studies of ancient Egyptian mummies, which indicate that the prevalence of atherosclerosis, as evidenced by intimal calcification, is similar to that in the modern era and its severity is closely associated with aging [69, 70]. The ancient human population had widely differing diets and physical activity but had the same incidence and age profile of atherosclerotic calcification as the current population [69, 70]. It indicates that atherosclerosis is likely “a manifestation of arterial aging”, and not a modern disease attributable to lifestyle components of diet and physical activities. The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) studies demonstrate that in young human subjects abnormal plasma cholesterol levels are associated with increased arterial thickening, VSMC proliferation, and collagen deposition, mimicking those that occur in the grossly normal arterial wall of the elderly with normal plasma lipid levels [7, 71, 72]. In addition, animal studies show that the prevalence and severity of atherosclerosis and its complications are more common in old mice, rabbits, and nonhuman primates although the plasma lipid profile is essentially unchanged with aging [73–75]. Finally, proteomic studies have identified over 800 proteins from human coronary atherosclerotic plaque that may be actively involved in arterial aging and atherosclerosis [76]. Many of the elevated proteins in the atherosclerotic intimae also increase in the aged arterial wall of all species, including TGF-β1, MFG-E8, and MMPs [12, 76]. For example, atherosclerotic smooth muscle cells, macrophages and pericytes express abundant MFG-E8, which regulates pathologic angiogenesis by interacting with integrins [24, 76–78]. Thus, the age-associated increase in atherosclerosis is likely mediated, in part, by enhanced MFG-E8 signaling.
BOX 3. Emerging questions and trends.
Questions
What specific signals determine the fate of arterial cells to develop heterogeneous phenotypes, i.e., senescence, proliferation, invasion or apoptosis that develop with aging within the arterial wall?
What is the feasibility, and time/dose window, of potential pharmacologic or lifestyle interventions aimed at retarding potentially mutable aspects of arterial aging in humans?
Trends
Arterial aging causes chronic arterial inflammation.
Age-associated arterial wall chronic inflammation is the key that drives arterial cell phenotypic shifts and adverse arterial wall remodeling.
The inflamed arterial wall that accompanies advancing age confers the major risk for hypertension and atherosclerosis.
Interventions to suppress chronic arterial inflammation may beneficially impact the rampant epidemic of age-associated arterial diseases in our society.
Highlights of Proinflammation: The key to Arterial Aging.
Arterial aging causes chronic arterial inflammation.
Age-associated arterial wall chronic inflammation drives arterial cell phenotypic shifts and adverse arterial wall remodeling.
The inflamed arterial wall in advancing age confers the major risk for hypertension and atherosclerosis.
Interventions to suppress chronic arterial inflammation may beneficially impact age-associated arterial diseases in our society.
Acknowledgments
Sources of Funding
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
GLOSSARY
- Age-associated arterial secretory phenotype (AAASP)
a hostile extracellular microenvironment enriched with the secretion of proinflammatory molecules such as MCP-1 from old vascular cells, which share features of the fibroblast senescence-associated secretory phenotype (SASP)
- Aldosterone
a steroid hormone and ligand for MR. It plays a central role in the regulation of blood pressure. Dysregulated aldosterone (and MR) in the heart and blood vessels negatively affects the cardiovascular system
- Angiotensin II (Ang II)
an element of the renin-angiotensin system (RAS), which is derived from angiotensin I by the enzyme angiotensin converting enzyme (ACE). Ang II regulates blood pressure via vasoconstriction and fluid balance. Pharmaceutical interventions of different steps in RAS are used to treat hypertension, heart and kidney failure
- C-C chemokine receptor type 2 (CCR2)
belongs to the G-protein coupled receptor 1 family and acts as a receptor for the MCP-1, MCP-3 and MCP-4 chemokines
- Collagen
a protein present in the normal arterial wall that offers tensile strength. Collagen types I and III are predominant in the arterial wall
- Endothelin-1
a protein that constricts blood vessels and raises blood pressure via the endothelin receptor. Overexpression of endothelin-1 or its receptor contributes to hypertension and heart disease
- Milk fat globule-EGF factor 8 protein (MFG-E8)
initially identified as a principle component of the milk fat globule, which is functionally identified as an “eat-me” signal or a bridging molecule between apoptotic cells and phagocytes
- Matrix metalloproteinases (MMPs)
zinc-dependent endopeptidases capable of degrading extracellular matrix proteins and bioactive molecules. MMPs not only digest arterial structural proteins, but more importantly, cleave extracellular bioactive factors, including TGF-β1 and pro-ET-1, increasing their bioavailability to vascular cells
- Mineralocorticoid receptor (MR)
also known as aldosterone receptor belonging to the nuclear receptor superfamily. MR binds mineralocorticoids such as aldosterone and glucocorticoids. Upon binding of aldosterone, MR is translocated to the nucleus, binds to the promoter of target genes as a homodimer and activates transcription
- Monocyte chemotactic protein-1 (MCP-1)
MCP-1 is a small cytokine that belongs to the CC chemokine family. MCP-1 specifically mediates monocyte chemotaxis to the sites of inflammation produced by tissue injury. CCR2 is a cognate chemokine receptor for MCP-1
- Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)
a ubiquitous transcription factor responsible for cytokine production and cell survival. It is activated by stimuli such as stress, free radicals, oxidized LDL, and bacterial or viral antigens
- Reactive oxygen species (ROS)
are chemically reactive molecules such as oxygen ions and peroxides, which form as a natural byproduct of normal metabolism. ROS have important roles in cell signaling and homeostasis. However, ROS are capable of damaging cell structures, especially during times of micro-environmental stress
- Transforming growth factor beta 1 (TGF-β1)
is a polypeptide member of the transforming growth factor beta superfamily of cytokines. It is a secreted protein that has multiple cellular functions, including cell proliferation, cell differentiation and apoptosis
- Senescence-associated secretory phenotype (SASP)
is a secreted niche of proinflammatory molecules such as MCP-1 and interlukin-1/6/8 by senescent cells. The SASP has powerful paracrine inflammatory activities
- Stress-induced premature senescence (SIPS)
is a cellular senescent phenotype mediated by genomic and epigenomic damage under subcytotoxic stressors such as toxins, irradiation, or the activation of certain oncogenes
Footnotes
Conflicts of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edwards RD. Population aging, the dependency burden, and challenges facing preventive medicine. Prev Med. 2012;55:533–534. doi: 10.1016/j.ypmed.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 2.Wang M, et al. Arterial aging: a journey into subclinical arterial disease. Curr Opin Nephrol Hypertens. 2010;19:201–207. doi: 10.1097/MNH.0b013e3283361c0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Lakatta EG. Central arterial aging: humans to molecules. In: Safar M, editor. Handbook of Hypertension: Arterial Stiffness in Hypertension. Elsevier; 2006. pp. 137–160. [Google Scholar]
- 4.Wang M, et al. Central Arterial Aging and Angiotensin II Signaling. Curr Hypertens Rev. 2010;6:266–281. doi: 10.2174/157340210793611668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakatta EG. The reality of aging viewed from the arterial wall. Artery Res. 2013;7:73–80. doi: 10.1016/j.artres.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakatta EG, et al. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. The Medical clinics of North America. 2009;93:583–604. doi: 10.1016/j.mcna.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, et al. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, et al. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41:1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 9.Michel JB, et al. Effect of chronic ANG I-converting enzyme inhibition on aging processes. II. Large arteries. Am J Physiol. 1994;267:R124–135. doi: 10.1152/ajpregu.1994.267.1.R124. [DOI] [PubMed] [Google Scholar]
- 10.Basso N, et al. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293:H1351–1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, et al. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. The American journal of pathology. 2005;167:1429–1442. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Z, et al. Milk fat globule protein epidermal growth factor-8: a pivotal relay element within the angiotensin II and monocyte chemoattractant protein-1 signaling cascade mediating vascular smooth muscle cells invasion. Circ Res. 2009;104:1337–1346. doi: 10.1161/CIRCRESAHA.108.187088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M, et al. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol. 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krug AW, et al. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension. 2010;55:1476–1483. doi: 10.1161/HYPERTENSIONAHA.109.148783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goel A, et al. Increased endothelial exocytosis and generation of endothelin-1 contributes to constriction of aged arteries. Circ Res. 2010:242–251. doi: 10.1161/CIRCRESAHA.109.210229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, et al. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension. 2012;60:459–466. doi: 10.1161/HYPERTENSIONAHA.112.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M, Lakatta EG. Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension. 2002;39:865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- 18.Wang M, et al. A local proinflammatory signalling loop facilitates adverse age-associated arterial remodeling. PLoS One. 2011;6:e16653. doi: 10.1371/journal.pone.0016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhan Y, et al. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest. 2005;115:2508–2516. doi: 10.1172/JCI24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ungvari Z, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Csiszar A, et al. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ungvari Z, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M, et al. MFG-E8 activates proliferation of vascular smooth muscle cells via integrin signaling. Aging Cell. 2012;11:500–508. doi: 10.1111/j.1474-9726.2012.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, et al. Milk Fat Globule Epidermal Growth Factor VIII Signaling in Arterial Wall Remodeling. Curr Vasc Pharmacol. 2013;11:768–776. doi: 10.2174/1570161111311050014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsson A, et al. Signs of cross-seeding: aortic medin amyloid as a trigger for protein AA deposition. Amyloid. 2011;18:229–234. doi: 10.3109/13506129.2011.630761. [DOI] [PubMed] [Google Scholar]
- 26.Wang M, et al. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Patron C, et al. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85:906–911. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]
- 28.Jiang L, et al. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS One. 2008;3:e2231. doi: 10.1371/journal.pone.0002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang L, et al. Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension. 2012;60:1192–1199. doi: 10.1161/HYPERTENSIONAHA.112.196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spinetti G, et al. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- 31.Song Y, et al. Aging enhances the basal production of IL-6 and CCL2 in vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:103–109. doi: 10.1161/ATVBAHA.111.236349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trott DW, et al. NAD(P)H oxidase-derived reactive oxygen species contribute to age-related impairments of endothelium-dependent dilation in rat soleus feed arteries. J Appl Physiol. 2011;110:1171–1180. doi: 10.1152/japplphysiol.01037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Loo B, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Celermajer DS, et al. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 35.Min LJ, et al. Angiotensin II type 2 receptor-interacting protein prevents vascular senescence. J Am Soc Hypertens. 2012;6:179–184. doi: 10.1016/j.jash.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–259. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- 37.Asai K, et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:1493–1499. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- 38.Qiu H, et al. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res. 2010;107:615–619. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y, et al. Temporal analysis of vascular smooth muscle cell elasticity and adhesion reveals oscillation waveforms that differ with aging. Aging Cell. 2012;11:741–750. doi: 10.1111/j.1474-9726.2012.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell JH, Campbell GR. Smooth muscle phenotypic modulation--a personal experience. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1784–1789. doi: 10.1161/ATVBAHA.111.243212. [DOI] [PubMed] [Google Scholar]
- 41.Yang D, et al. Increased polyploidy in aortic vascular smooth muscle cells during aging is marked by cellular senescence. Aging Cell. 2007;6:257–260. doi: 10.1111/j.1474-9726.2007.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCrann DJ, et al. Upregulation of Nox4 in the aging vasculature and its association with smooth muscle cell polyploidy. Cell Cycle. 2009;8:902–908. doi: 10.4161/cc.8.6.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kunieda T, et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- 44.Herbert KE, et al. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res. 2008;102:201–208. doi: 10.1161/CIRCRESAHA.107.158626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Csiszar A, et al. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. doi: 10.1093/gerona/glr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao BB, et al. Label-free quantitative analysis of one-dimensional PAGE LC/MS/MS proteome: application on angiotensin II-stimulated smooth muscle cells secretome. Mol Cell Proteomics. 2008;7:2399–2409. doi: 10.1074/mcp.M800104-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan SJ, et al. Stress-induced senescence exaggerates postinjury neointimal formation in the old vasculature. Am J Physiol Heart Circ Physiol. 2010;298:H66–H74. doi: 10.1152/ajpheart.00501.2009. [DOI] [PubMed] [Google Scholar]
- 48.Pauly RR, et al. Experimental models that mimic the differentiation and dedifferentiation of vascular cells. Circulation. 1992;86:68–73. [PubMed] [Google Scholar]
- 49.Haggqvist B, et al. Medin: an integral fragment of aortic smooth muscle cell-produced lactadherin forms the most common human amyloid. Proc Natl Acad Sci U S A. 1999;96:8669–8674. doi: 10.1073/pnas.96.15.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng M, et al. Correlation between serum lactadherin and pulse wave velocity and cardiovascular risk factors in elderly patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2012;95:125–131. doi: 10.1016/j.diabres.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 51.Johnson KA, et al. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kass DA, et al. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 53.Vaitkevicius PV, et al. A cross-link breaker has sustained effects on arterial and ventricular properties in older rhesus monkeys. Proc Natl Acad Sci U S A. 2001;98:1171–1175. doi: 10.1073/pnas.98.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torreggiani M, et al. Advanced glycation end product receptor-1 transgenic mice are resistant to inflammation, oxidative stress, and post-injury intimal hyperplasia. The American journal of pathology. 2009;175:1722–1732. doi: 10.2353/ajpath.2009.090138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee D, et al. The effect of soluble RAGE on inhibition of angiotensin II-mediated atherosclerosis in apolipoprotein E deficient mice. PLoS One. 2013;8:e69669. doi: 10.1371/journal.pone.0069669. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Zarzuelo MJ, et al. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol. 2013;85:12388–1296. doi: 10.1016/j.bcp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 57.Donato AJ, et al. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. 2011;589:4545–4554. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyazaki R, et al. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1263–1269. doi: 10.1161/ATVBAHA.108.166991. [DOI] [PubMed] [Google Scholar]
- 60.Najjar SS, et al. Cardiovascular aging: The next frontier in cardiovascular prevention. Saunders; 2011. pp. 415–432. [Google Scholar]
- 61.Pierce GL, et al. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10:1032–1037. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lesniewski LA, et al. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol. 2011;301:H1025–H1032. doi: 10.1152/ajpheart.01276.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fleenor BS, et al. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol. 2010;588:3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Durrant JR, et al. Voluntary wheel running restores endothelial function in conduitarteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lloyd-Jones D, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez-Villalobos RA, et al. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;123:2011–2023. doi: 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Behbahani J, et al. Resveratrol and small artery compliance and remodeling in the spontaneously hypertensive rat. Am J Hypertens. 2010;23:1273–1278. doi: 10.1038/ajh.2010.161. [DOI] [PubMed] [Google Scholar]
- 68.Linz W, et al. Long-term angiotensin II type 1 receptor blockade with fonsartan doubles lifespan of hypertensive rats. Hypertension. 2000;35:908–913. doi: 10.1161/01.hyp.35.4.908. [DOI] [PubMed] [Google Scholar]
- 69.Thompson RC, et al. Atherosclerosis across 4000 years of human history: the Horus study of four ancient populations. Lancet. 2013;381:1211–1222. doi: 10.1016/S0140-6736(13)60598-X. [DOI] [PubMed] [Google Scholar]
- 70.Finch CE. Atherosclerosis is an old disease: Summary of the Ruffer Centenary Symposium, The Paleocardiology of Ancient Egypt, a meeting report of the Horus Study team. Exp Gerontol. 2011;46:843–846. doi: 10.1016/j.exger.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 71.McGill HC, et al. Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Circulation. 2008;117:1216–1227. doi: 10.1161/CIRCULATIONAHA.107.717033. [DOI] [PubMed] [Google Scholar]
- 72.Strong JP, et al. Lipoproteins and atherosclerosis in children: an early marriage? Nutr Metab Cardiovasc Dis. 2001;11(Suppl 5):16–22. [PubMed] [Google Scholar]
- 73.Clarkson TB, Adams MR, Weingand KW, Miller LC, Heydrick S. Effect of age on atherosclerosis progression in nonhuman primates. In: Bates SR, Ganghoff EC, editors. Atherogenesis and Aging. NY: Springer-Verlag; 1987. pp. 57–71. [Google Scholar]
- 74.Spagnoli LG, et al. Aging and atherosclerosis in the rabbit. 1. Distribution, prevalence and morphology of atherosclerotic lesions. Atherosclerosis. 1991;89:11–24. doi: 10.1016/0021-9150(91)90003-l. [DOI] [PubMed] [Google Scholar]
- 75.Eto H, et al. The long-term effect of angiotensin II type 1a receptor deficiency on hypercholesterolemia-induced atherosclerosis. Hypertens Res. 2008;31:1631–1642. doi: 10.1291/hypres.31.1631. [DOI] [PubMed] [Google Scholar]
- 76.Bagnato C, et al. Proteomics analysis of human coronary atherosclerotic plaque: a feasibility study of direct tissue proteomics by liquid chromatography and tandem mass spectrometry. Mol Cell Proteomics. 2007;6:1088–1102. doi: 10.1074/mcp.M600259-MCP200. [DOI] [PubMed] [Google Scholar]
- 77.Motegi S-i, et al. Pericyte-derived MFG-E8 regulates pathologic angiogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2024–2034. doi: 10.1161/ATVBAHA.111.232587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Motegi S-i, et al. Potentiation of platelet-derived growth factor receptor-β signaling mediated by integrin-associated MFG-E8. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2653–2664. doi: 10.1161/ATVBAHA.111.233619. [DOI] [PMC free article] [PubMed] [Google Scholar]