SUMMARY
Enzymatic cleavage of transmembrane anchors to release proteins from the membrane controls diverse signaling pathways and is implicated in over a dozen diseases. How catalysis works within the viscous, water-excluding, two-dimensional membrane is unknown. We developed an inducible reconstitution system to interrogate rhomboid proteolysis quantitatively within the membrane in real time. Remarkably, rhomboid proteases displayed no physiological affinity for substrates (Kd ~190 μM, or 0.1 mol%). Instead, ~10,000-fold differences in proteolytic efficiency with substrate mutants and diverse rhomboid proteases were reflected in kcat values alone. Analysis of gate-open mutant and solvent isotope effects revealed that substrate gating, not hydrolysis, is rate limiting. Ultimately a single proteolytic event within the membrane normally takes minutes. Rhomboid intramembrane proteolysis is thus a slow, kinetically controlled reaction not driven by transmembrane protein-protein affinity. These properties are unlike those of other studied proteases or membrane proteins but strikingly reminiscent of one subset of DNA-repair enzymes, raising important mechanistic and drug-design implications.
INTRODUCTION
Each protein in a living cell will be cleaved by a protease (Doucet et al., 2008; Lopez-Otin and Bond, 2008). The purpose of these enzymatic events ranges from shredding damaged proteins that might otherwise harm the cell, to sculpting signal precursors to initiate cell communication (Lopez-Otin and Bond, 2008). Aside from controlling essential processes in all forms of life, protease inhibition has proven to be a particularly effective therapeutic strategy, especially in hypertension and antiviral treatment (Drag and Salvesen, 2010).
Ultimately deciphering how a protease shapes the signaling characteristics of healthy cells, or targeting it for therapeutic intervention in disease, requires a sophisticated understanding of its enzymatic properties. Kinetic dissection of protease catalysis has been key in revealing these properties (Huntington, 2012; Perona and Craik, 1997; Timmer et al., 2009). Coupled with structural analyses, these studies have established that both cytosolic and extracellular proteases are designed to bind their substrates specifically at discrete sites, with affinity reflected in the Michaelis constant (KM), and endowed with catalytic residues that function in rate enhancement, reflected in the turnover number (kcat). The catalytic efficiency of a protease is the quotient of these two parameters, and typically ranges from 104–107 M−1s−1 (108 reflects enzymes whose activity is limited by diffusion).
Intramembrane proteases, in contrast to these well-studied soluble proteases, are a more recently-discovered class of extraordinary enzymes that evolved independently to catalyze hydrolysis immersed within the membrane (De Strooper and Annaert, 2010; Fluhrer et al., 2009; Makinoshima and Glickman, 2006; Urban and Dickey, 2011; Wolfe, 2009). Despite this complexity, there is significant incentive for understanding how proteolysis is accomplished within these constraints, because intramembrane proteases hold great promise for developing therapies: rhomboid proteases are implicated in Parkinson’s disease and parasite invasion (Urban and Dickey, 2011); γ-secretase in Alzheimer’s disease and leukemia (De Strooper and Annaert, 2010; Wolfe, 2009); signal peptide peptidases in immunity and hepatitis C virus assembly (Fluhrer et al., 2009); and site-2 proteases in the virulence of some of the world’s deadliest bacterial and fungal pathogens (Makinoshima and Glickman, 2006; Urban, 2009).
Major insights into the molecular architecture of these remarkable enzymes has been gained from a series of high-resolution intramembrane protease crystal structures of prokaryotic orthologs (Li et al., 2013; Wolfe, 2009), as led by analyses of the Escherichia coli rhomboid protease GlpG (as summarized in (Urban, 2010). In contrast, analysis of catalysis within the membrane in quantitative terms has not been achieved with any intramembrane protease, making it difficult to decipher their functional properties. Current models are based largely on extrapolations from soluble proteases, which evolved independently and could be different. In fact, the membrane is a fundamentally unusual setting for proteolysis: chemically, the membrane is viscous and excludes water, which is both essential for proteolysis and affects how proteins interact. Spatially, proteins in a membrane exist in a two-dimensional plane and are orientationally-confined relative to each other. Although techniques for studying proteins inside the membrane are scarce, understanding the consequences of this environment, and how intramembrane proteases function within it, requires interrogating the kinetics of proteolysis within its natural membrane setting.
We have overcome multiple inherent limitations to develop the first ‘inducible’ membrane reconstitution system for the quantitative analysis of rhomboid proteolysis occurring within the membrane and in real time. The results reveal that, contrary to expectations, rhomboid proteolysis is a slow reaction that is not driven by affinity of enzyme for substrate. Instead, these insights suggest a completely different mode of action for this ancient and widespread family of enzymes.
RESULTS
Development of an Inducible Co-Reconstitution System
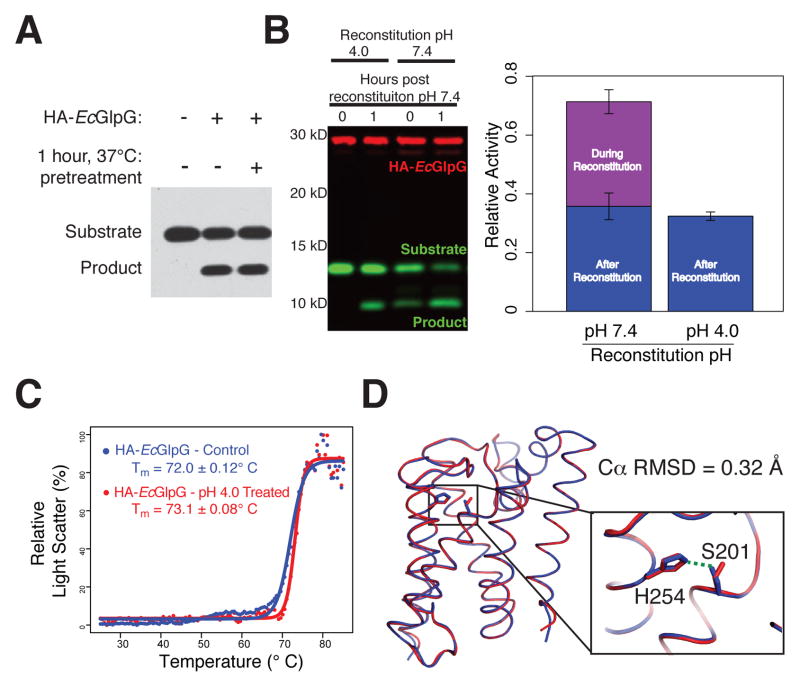
In order to study kinetics of proteolysis directly within the membrane, we faced three obstacles that are inherent to GlpG and substrate both being transmembrane proteins. First, although membrane proteins are prone to aggregation in vitro, an important requirement for kinetic analysis is that enzyme concentration does not change during the course of the reaction. We evaluated this concern and found no loss in activity upon pre-incubating E. coli GlpG at 37ºC for 1 hour prior to initiating the reaction (Figure 1A), revealing GlpG enzyme preparations are robust for kinetic analysis.
Figure 1. An inducible co-reconstitution system for membrane enzyme analysis.
(A) HA-tagged GlpG (HA-EcGlpG) preincubated for 1 hour at 37°C retained full activity against the substrate APP+Spi7-Flag (shown is an anti-Flag western). (B) HA-EcGlpG was inactive during the reconstitution with APP+Spi7-Flag at pH 4, but active when reconstituted at pH 7.4 (t=0 reaction times). Activity was restored upon neutralization to pH 7.4 (see 1h reaction time). Shown is a 2-color western, and quantification (graph) revealing the amount of protease activity in proteoliposomes was indistinguishable whether the protease was subjected to pH shift or not. (C) Thermostability of HA-EcGlpG without and after pretreatment in pH 4 buffer was examined by heating from 25 to 85°C and monitoring differential static light scattering every 0.5°C. (D) X-ray crystal structure comparison of ΔN-EcGlpG at low (red) and neutral (blue) pH. Note that although the overall conformation is indistinguishable (Cα RMSD = 0.32 Å), at low pH the catalytic serine 201 sidechain was no longer hydrogen-bonded to the histidine base (inset), rendering the enzyme catalytically inactive. See Table S1 for structural parameters.
The greatest challenge to kinetic analysis of proteolysis within membranes is that cleavage already begins during the lengthy procedure to reconstitute protease and substrate into the membrane (Osenkowski et al., 2008). To overcome this obstacle, we developed a rapid and reliable co-reconstitution method in which proteolytic activity can be switched off and on. We reasoned that co-reconstituting at lower pH would protonate the catalytic histidine, rendering it catalytically inactive. Then, after collecting the proteoliposomes by ultracentrifugation, we planned to initiate the reaction by raising the pH to the physiological 7.4. Under these conditions, we detected no proteolysis during the lowered pH co-reconstitution, and regained full protease activity relative to untreated controls upon neutralization (Figure 1B). Moreover, treated GlpG was indistinguishable from untreated GlpG in a sensitive and quantitative structural stability assay (Baker and Urban, 2012), arguing that the pH switch did not alter enzyme structure (Figure 1C). In fact, crystallization of GlpG at neutral and low pH revealed its overall architecture was unperturbed (Cα RMSD of ~0.32 Å), yet at low pH the serine sidechain was incompetent for catalysis because it had turned away from the histidine (Figure 1D and Table S1), which is consistent with histidine protonation.
Quantitative Analysis of Intramembrane Proteolysis in Real Time
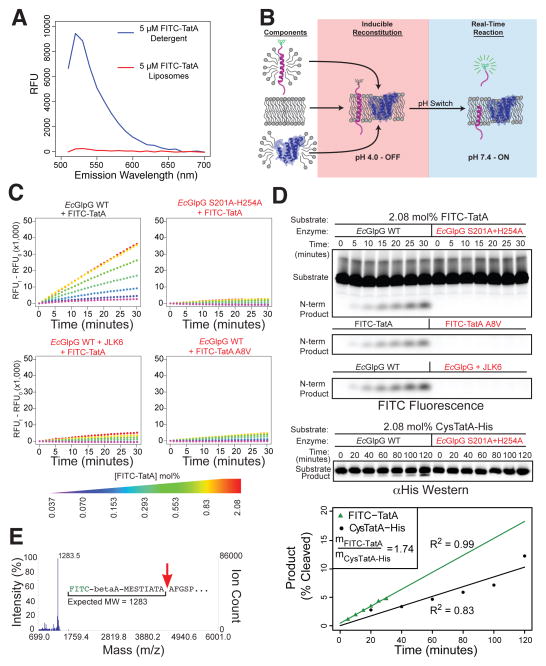
With robust enzyme preparations and this novel reconstitution method established, we next focused on developing a substrate that would permit monitoring intramembrane proteolysis in real time. Unexpectedly, we discovered that a FITC fluorophore attached to the natural amino-terminus of a Providencia stuartii TatA construct, the only known bacterial rhomboid substrate (Stevenson et al., 2007), was robustly quenched when reconstituted into proteoliposomes composed of E. coli lipids (Figure 2A, 2B and S1A). In the presence of GlpG, however, a fluorogenic signal was generated at a rate (Figure 2C) that was perfectly coincident with appearance of the cleaved product as assessed by tricine gel analysis (Figure 2D). Mass spectrometric analysis confirmed TatA was being cut only at the natural rhomboid cleavage site between alanines 8 and 9 (Stevenson et al., 2007) (Figure 2E). Importantly, both fluorescence and the cleaved product were absent when we mutated either the substrate at the alanine preceding the cleavage site, or the GlpG catalytic residues (Figure 2C and 2D). JLK6, an isocoumarin-based rhomboid inhibitor (Vinothkumar et al., 2010), blocked generation of the fluorescence signal and cleavage product (Figure 2C and 2D). Finally, the FITC moiety neither reduced proteolysis (Figure 2D), nor affected the helicity of TatA in membranes as measured by circular dichroism (Figure S1B). Therefore, FITC-TatA cleavage exhibits all known hallmarks of rhomboid intramembrane proteolysis, and permits its monitoring within the membrane in real-time.
Figure 2. Quantifying proteolysis within the membrane in real time.
(A) FITC-TatA fluorescence is quenched in proteoliposomes (red), but not in detergent micelles (blue). Shown is an emission scan: see Figure S1 for absorption/excitation scans. (B) Assay schematic: transmembrane FITC-TatA and HA-EcGlpG in detergent were co-reconstituted into proteoliposomes at pH 4 (red shading), collected by ultracentrifugation, and proteolysis initiated by neutralization (blue shading). Proteoliposomes quench fluorescence, which is relieved by cleavage-mediated release. (C) Real-time progress curves at 37°C (read/minute) of EcGlpG reconstituted with indicated FITC-TatA concentrations (mole % relative to phospholipids). Mutating EcGlpG or TatA, or pretreating with JLK6, abrogated signal to background. (D) Fluorescence scan of gel analysis confirmed linear product accumulation (top R2=0.997), and sensitivity to mutation/inhibition. Also see Figure S2B for gel analysis of extended incubations. Lower gel and graph indicate that the TatA construct lacking the FITC label was not cleaved more efficiently. (E) MALDI-TOF analysis of the N-terminal FITC-TatA cleavage product (red arrow indicates cleavage site).
Steady-State Kinetic Analysis of Proteolysis Within the Membrane
To establish controlled conditions for a Michaelis-Menten kinetic system, we lowered GlpG levels to 4 pmoles while varying substrate from 20 to 1600 pmoles and co-reconstituted both into 200 nm proteoliposomes comprised of E. coli lipids to preserve its physiological environment as much as possible (Figure S2A). Under these low enzyme conditions, <5% of the substrate was converted to product in 30 minutes (Figure 2D), thus satisfying Michaelis-Menten requirements of constant substrate concentrations. Progress curves remained linear for ~60 minutes before the reactions expectedly slowed due to substrate depletion and product accumulation. Extended incubation times allowed FITC-TatA turnover (Figure S2B), arguing that >85% of the substrate is available to the protease. Finally, FITC-TatA reconstituted in both orientations in approximately equal proportions, as quantified by reacting single engineered cysteines with membrane-impermeable dyes (Figure 3A). As such, the effective concentration of FITC-TatA per orientation is half of the total amount reconstituted.
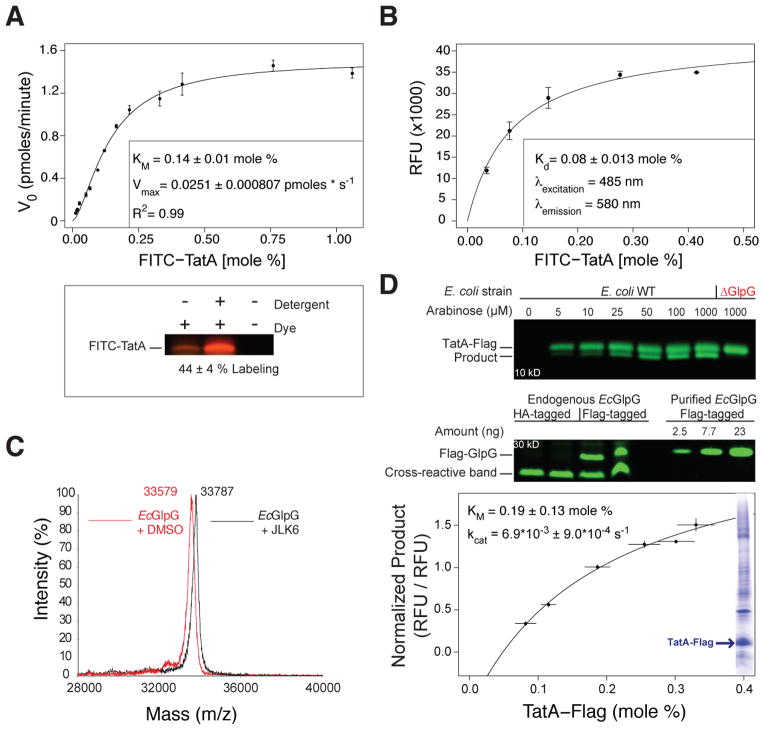
Figure 3. Intramembrane proteolysis kinetics and equilibrium binding parameters inside the membrane.
(A) Real-time kinetics of FITC-TatA cleavage by EcGlpG in proteliposomes was fit with a Michaelis-Menten model (mean ± sem, n=2, inset: fit ± sd). Gel image (below) quantifying FITC-TatA-Cys labeling by a membrane-impermeable thiol-reactive dye, revealing half of TatA reconstituted with its amino-terminus facing the liposome interior. The reconstitution efficiency was consistent between experiments (see Figure S2A). Reactions were also analyzed on 16% tricine gels, products quantified and plotted (see Figure S2C). (B) Binding of FITC-TatA to catalytically inactive TMR-GlpG (C104A+W196TMR+H254A) in proteoliposomes. Plotted is background-subtracted FRET fluorescence intensity versus mole fraction of FITC-TatA (relative to phospholipids). Kd was derived from the curve fit. (C) Mass spectra of EcGlpG incubated with the activity-based inhibitor JLK6 produced a complete mass shift, revealing all purified EcGlpG is active. (D) TatA-Flag cleavage in E. coli cells by endogenous GlpG. TatA expression levels were titrated with arabinose, and cleavage assessed by anti-Flag western blot (upper gel). Deleting genomic GlpG (ΔGlpG) resulted in no cleavage. Levels of endogenous GlpG (Flag-tagged by knock-in) were quantified in duplicate relative to known Flag-GlpG pure protein standards by anti-Flag western blot (lower gel). Cross-reactive bands served as loading controls. Graph: half maximal cleavage by endogenous GlpG occurred when TatA reached 0.19 mole percent (relative to phospholipids). Also shown (inset) is a Coomassie blue-stained SDS-PAGE gel revealing TatA became 20% of the E. coli membrane proteome when induced with 1 mM arabinose (also see Figure S2D).
Plotting the substrate concentration versus reaction velocity (measured over the first 15 minutes) produced a rectangular hyperbola that could be fit exceptionally well (R2=0.99) with a Michaelis-Menten equation (Figure 3A). Remarkably, the resulting data revealed an extraordinarily high KM of 0.14±0.02 mole percent relative to phospholipid (~1 substrate transmembrane segment per 350 monolayer phospholipids). We verified this measurement independently by tricine gel analysis (Figure S2C). However, since KM is more complex than physical affinity alone, we developed a binding assay to measure the Kd between rhomboid and substrate within the membrane directly. We installed a tetramethylrhodamine (TMR) FRET acceptor group onto an extracellular GlpG loop at a site we previously found does not perturb protein structure (Baker and Urban, 2012). Co-reconstituting different amounts of FITC-TatA resulted in an increasing FRET signal from a catalytically-inactive mutant of TMR-GlpG that became saturated, revealing an apparent Kd of 0.08±0.026 mole percent (Figure 3B), which agrees well with the low affinity revealed by kinetic analysis.
To derive the catalytic turnover rate, kcat, from the measured Vmax, we quantified the fraction of active protease in our preparations by incubating with JLK6, which is a ‘suicide inhibitor’ that covalently labels only active GlpG (Vinothkumar et al., 2010). Mass spectrometry revealed that ~100% of GlpG in our preparations is catalytically active (Figure 3C), yielding a kcat of 0.0063±0.00021 cuts per second (s−1), or >2.5 minutes required for a single cleavage event within the membrane when the enzyme is saturated with substrate.
Evaluation of Intramembrane Protease Kinetics in Living Cells
The kinetic parameters governing proteolysis within the membrane were unexpectedly inefficient, raising the concern that our reconstitution system may be missing an unknown component, or may not accurately reflect physiological conditions. In fact, while we have been careful to use lipids purified from growing E. coli cell for forming our liposomes, in vitro membrane systems cannot recapitulate potential bilayer asymmetry of lipids, or potential crowding induced by ‘bystander’ proteins, that may be present in natural membranes. We therefore examined the characteristics of rhomboid proteolysis directly in living E. coli cells by expressing full-length TatA from an arabinose promoter that allows titration of target protein levels. Using this system we achieved TatA levels ranging from undetectable to becoming the most abundant protein in the cell membrane (Figure S2D). As expected, increasing TatA resulted in increased intramembrane proteolysis by endogenous GlpG in the natural membranes of living E. coli cells (Figure 3D). Cleavage was absent in GlpG knockout cells. Remarkably, even when TatA became the most abundant protein in the cell, GlpG was not yet saturated with substrate, confirming low substrate binding affinity. Quantifying TatA levels and cleavage yielded a Michaelis-Menten-like curve, with an ‘apparent in vivo’ KM of 0.19 mole percent, which is remarkably similar to 0.14 mole percent that we measured in our in vitro reconstitution system.
We also estimated the proteolytic rate in vivo by epitope tagging endogenous GlpG in the E. coli chromosome. This was critical because if we overexpressed GlpG even slightly we could titrate out any potential stimulatory cofactor or condition. Quantification of endogenous GlpG levels relative to pure protein standards (Figure 3D) yielded an ‘apparent in vivo’ kcat of 0.0069±0.0009 s−1, which again was remarkably similar to 0.0063±0.00021 s−1 that we measured in our reconstitution system. As such, GlpG exhibits low affinity for substrate and slow catalytic rate even in living cells, validating that our reconstitution system faithfully recapitulates physiological conditions for GlpG proteolysis.
The Membrane Environment Slows Rhomboid Proteolysis
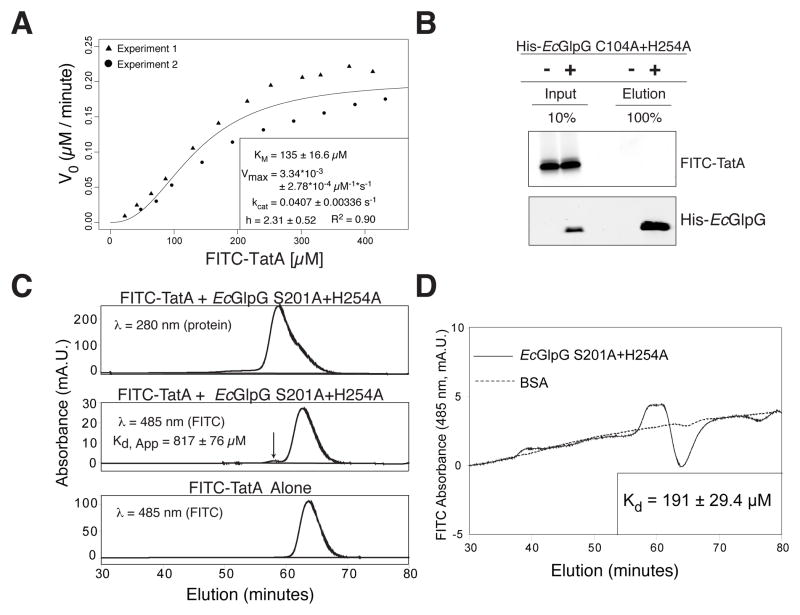
Having discovered that the kinetics of proteolysis within the membrane are inefficient, we sought to test whether this reflects limitations imposed by the membrane environment. We examined proteolysis in detergent micelles that support high rhomboid activity, and found a strikingly high KM of at least 135±16.6 μM, with Vmax approached at >300 μM substrate (Figure 4A). The Vmax we measured is ~10 times faster than previously measured for rhomboid proteases in detergent (Lazareno-Saez et al., 2013), suggesting that prior studies did not saturate enzyme with substrate but instead suffered plateauing for another reason, such as substrate aggregation at higher concentrations and extended incubation times of 2 hours.
Figure 4. Intramembrane proteolysis kinetics and equilibrium binding analyzed in 3-dimensions.
(A) Michaelis-Menten plot of FITC-TatA cleavage by HA-EcGlpG in detergent micelles (inset: fit ± sd). Variation increased at >100 μM due to variable solubility of the transmembrane substrate. (B) Analysis of FITC-TatA coprecipitation with the inactive mutant His-EcGlpG C104A+H254A. Quantification revealed <0.1% of FITC-TatA eluting with His-EcGlpG, which under non-equilibrium conditions corresponds to an apparent Kd ≥ 1 mM. (C) HPLC gel filtration analysis of FITC-TatA mixed with HA-EcGlpG S201A+H254A reveals a small complex peak (arrow), which is absent in the elution profile of FITC-TatA alone. The single A280 (protein absorption) peak is an overlap of HA-EcGlpG (eluting first) and FITC-TatA (right shoulder). (D) HPLC equilibrium gel filtration analysis of FITC-TatA/EcGlpG (inactive S201A+H254A mutant) complex versus FITC-TatA/BSA control run on a column equilibrated with 9.5 μM FITC-TatA. The A485 (FITC absorption) peak corresponds to the GlpG-TatA complex, while the trough results from FITC-TatA depletion from the mobile phase due to GlpG binding (peak/trough absence with BSA indicates no binding).
A way to distinguish between indirect effects and true binding is to measure the Kd directly between rhomboid and substrate, which has never been achieved for any intramembrane protease. Commonly used gel filtration and co-precipitation approaches indicated low affinity of >800 μM (Figure 4B and 4C). Since these approaches separate complex from monomers, they promote dissociation and thus can over-estimate Kd. We therefore performed equilibrium gel filtration (Hummel and Dreyer, 1962) by pre-equilibrating the entire column with substrate. Under these equilibrium conditions, the Kd between rhomboid and substrate was 191±29.4 μM (Figure 4D), which is in excellent agreement with our measured KM. These values indeed suggest that rhomboid does not display even modest natural affinity for substrates in any environment.
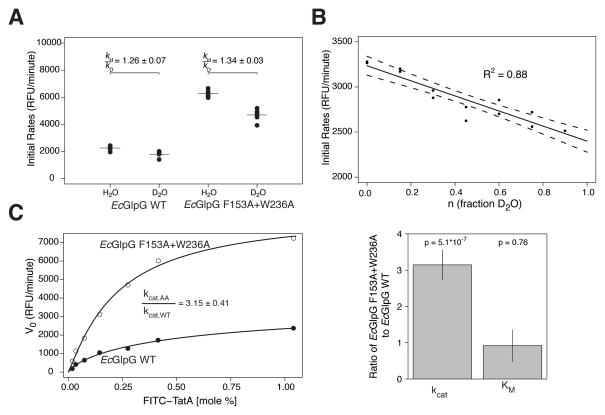
Interestingly however, kcat was ~6.5-fold faster in detergent than in proteoliposomes (Figure 4A), revealing that the membrane environment slows proteolysis. It’s generally accepted that hydrolysis within the membrane, where water is scarce, is rate-limiting for proteolysis. To test this directly, we performed a kinetic solvent isotope effect analysis by substituting deuterium oxide for water. A decrease in rate in deuterium oxide is commonly used to identify a rate-limiting hydrolysis step in a reaction (Fersht, 1999). Surprisingly, hydrolysis itself was not rate-limiting in membranes, since the ratio of the water/deuterium oxide proteolysis rates for GlpG was only a modest 1.26±0.32 (Figure 5A) instead of the expected ≥2 for serine proteases (Elrod et al., 1980; Zhang and Kovach, 2006). In contrast, such mild effects are often caused by deuterium exchange with protein groups, and indeed we observed a gradual effect on proteolysis when we titrated deuterium oxide in the presence of water (Figure 5B). This is diagnostic of an equilibrium effect on protein, rather than a direct effect on hydrolytic rate (Fersht, 1999).
Figure 5. Rate-limiting step for rhomboid proteolysis inside the membrane.
(A) Rate analysis of FITC-TatA cleavage by EcGlpG in proteoliposomes in the presence of H2O versus D2O. The weak (≪2) effect of D2O (expressed as a rates ratio) indicates hydrolysis is not rate limiting. (B) Titration analysis of FITC-TatA cleavage rate by EcGlpG in proteoliposomes in the presence of different proportions of D2O. The linear effect indicates the slowing is due to perturbation of an equilibrium (exchange with protein groups), not a rate (hydrolysis step), effect. (C) Parallel real-time kinetic analysis of FITC-TatA cleavage by wildtype (filled circles) versus gate-open (F153A+W236A, open circles) EcGlpG in proteoliposomes was fit (left panel) with a Michaelis-Menten model (mean ± sem, n=2, inset: fit ± sd). Bar graph comparison (right panel) of the gate-open mutant effect on kcat (~3 fold faster) and KM (no change).
Conversely, we found that substrate gating plays a major role in determining reaction rate in the membrane (Figure 5C). Analysis of gate-open relative to wildtype GlpG revealed a >3-fold increase in kcat without any change in KM (Figure 5C) or cleavage site (Figure S3), arguing that gate-opening is the rate-limiting step for proteolysis within the membrane. While recent structural studies with covalent inhibitors have suggested gating residues contribute to substrate binding (Xue and Ha, 2012), mutants in these residues do not change KM. The membrane environment thus slows proteolytic rate, in part, by restraining protease gate opening, but KM appears to be inherently inefficient in all settings.
Kinetic Analysis of Diverse Rhomboid Proteases Reveals a Common Mechanism
To our knowledge, it’s unprecedented for the KM of a specific protease to play little or no role in driving proteolysis under physiological conditions (Perona and Craik, 1997; Timmer et al., 2009). To evaluate this possibility further, we characterized the effect of both protease and substrate variants.
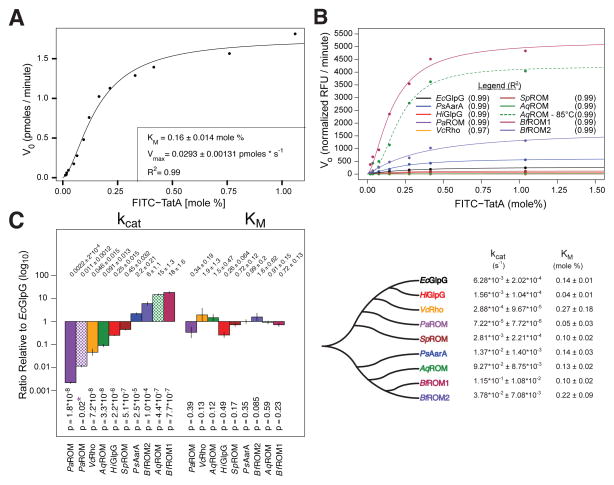
Although E. coli GlpG is currently the best-characterized intramembrane protease, rhomboid proteases vary greatly in sequence (Lemberg and Freeman, 2007) and specific activity (Urban and Wolfe, 2005). We therefore measured the kinetic parameters for a panel of nine diverse rhomboid proteases that share <3% sequence identity (Figure 6A, 6B, and Figure S3). Remarkably, despite varying ~10,000-fold in specific activity, all nine rhomboid proteases displayed indistinguishable KM values (Figure 6C). In fact, even AarA, the natural protease that co-evolved with TatA (Stevenson et al., 2007), had, if anything, a slightly higher (less efficient) KM for TatA than GlpG (Figure 6A–6D). Conversely, all differences in protease activity across this diverse panel of enzymes, when analyzed under identical conditions, were reflected in kcat changes alone, which ranged ~10,000-fold (Figure 6C and 6D).
Figure 6. Kinetics of membrane-immersed proteolysis by 9 diverse rhomboid proteases.
(A) Michaelis-Menten model fit (inset: fit ± sd) to real-time reaction velocity of FITC-TatA processing by HA-Providencia stuartii AarA (HA-PsAarA) in proteoliposomes. (B) Michaelis-Menten graphs of direct pairwise comparisons of eight diverse rhomboid proteases to HA-EcGlpG (HiGlpG: Haemophilus influenzae GlpG, PaROM: Pseudomonas aeruginosa ROM, VcRho: Vibro cholerae Rho, SpROM: Streptococcus pneumoniae ROM, AqROM: Aquifex aeolicus ROM, BfROM1/2: Bacteroides fragilis). See Figure S3 for protein sequence alignment and accession numbers. (C) Comparison of kinetic parameters derived from Michaelis-Menten model fits (above: ratios ± sd, below, p-values of pairwise model fitting with Bonferroni correction of kcat values). Patterned bars indicate AqROM analyzed at 85°C and PaROM analyzed in liposomes composed of DMPC (asterisks denotes comparison to PaROM in E. coli liposomes). (D) A phylogenetic tree of analyzed rhomboid proteases and their best Michaelis-Menten parameters (fitted values ± sd). Data is color-coded throughout the figure.
Comparing a diverse set of rhomboid proteases to E. coli GlpG required maintaining equivalent conditions. As a result, a few rhomboid proteases were assayed under conditions that were physiological for E. coli GlpG, but different from what they would experience normally. Since these enzymes generally appeared less active than GlpG, we re-evaluated their activity under more physiological conditions. Aquifex aeolicus is an extreme thermophilic organism with a growth optimum of 85°C (Deckert et al., 1998). Analysis of AqROM at 85°C revealed a 250-fold increase in protease activity, all of which was reflected in a higher kcat with no corresponding decrease in KM (Figure 6B–D). Similarly, although bacteria do not have phosphatidylcholine, the membranes of Pseudomonas aeruginosa accrue 4% phosphatidylcholine (and potentially other lipid species) as it assimilates choline during infection of its host (Wilderman et al., 2002). Analysis of PaROM revealed a >5-fold stimulation of its protease activity, but not that of other rhomboid proteases, by phosphatidylcholine, that again was accounted for by an increase in kcat (Figure 6C and D). Collectively, these observations strongly support the unexpected discovery that intramembrane proteolysis is fundamentally governed by kcat, with little or no contribution from substrate-binding affinity.
Kinetic Analysis of Substrate Mutants Reveals No Binding Affinity Motif
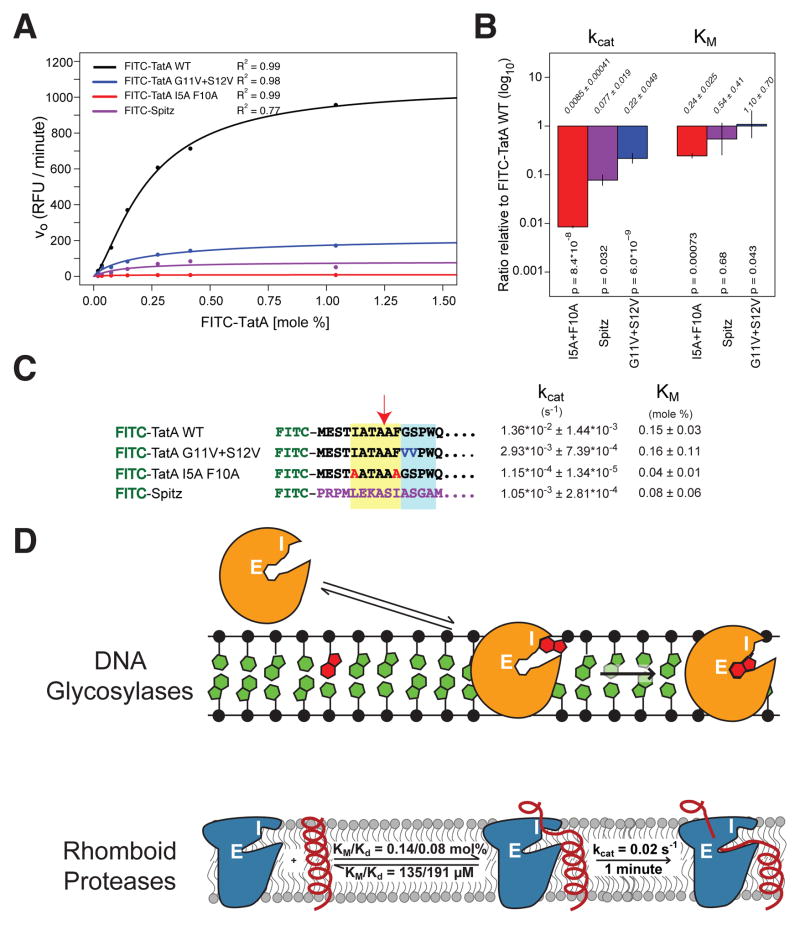
We also examined defined substrate mutants that have been under investigation for over a decade, but the lack of a kinetic system precluded rigorous mechanistic interpretation for how they affect proteolysis (reviewed in (Urban, 2010). TatA itself has been subjected to extensive mutational analysis with >150 mutants, the outcome being interpreted as identification of a ‘recognition motif’ for sequence-specific binding comprised of large residues four residues preceding, and two residues following, the cleavage site (Strisovsky et al., 2009). We therefore examined a double mutant with disallowed alanines at these positions and indeed found proteolysis was decreased >100-fold (Figure 7A). However, contrary to expectation for a sequence-binding motif, the mutant substrate actually decreased KM (p=0.00073), implying 4-fold improved binding (Figure 7B)! This result was also reassuring because it demonstrated that our assay was capable of measuring high affinity. The decrease in processing of the TatA double mutant was instead reflected in a >100-fold decrease in kcat, which itself may account for the lower KM (Figure 7B and 7C). Overall, these findings are completely consistent with prior observations including mutagenesis data, but reveal that mutating apparent sequence motifs in substrates lowers proteolysis not by abrogating binding affinity (KM not increased), but by altering optimal exposure of the peptide bond for efficient hydrolysis (lowered kcat).
Figure 7. Kinetic interpretation of defined intramembrane substrate variants.
(A) Michael-Menten graphs of pairwise comparisons of FITC-TatA mutants and FITC-Spitz compared with FITC-TatA cleavage by HA-PsAarA. (B) Quantification of the kinetic parameters derived from the Michaelis-Menten model fits (above: ratios ± sd, below, p-values of pairwise model fitting with Bonferroni correction of only kcat values). (C) Kinetic parameters for each substrate by HA-PsAarA (fitted values ± sd). The ‘recognition motif’ is shaded in yellow, while the helix-destabilizing center is shaded in blue. (D) Model of rhomboid proteolysis inside the membrane compared to DNA glycosylase excision of damaged bases from duplex DNA. Substrates (damaged base for DNA glycosylase, transmembrane segment for rhomboid) are in red. White letters indicate the enzyme interrogation (I) and inner excision (E) sites. Kinetic parameters that we measured for E. coli GlpG are diagramed.
Second, like all studied rhomboid substrates, TatA contains a region deeper within its transmembrane segment composed of helix-destabilizing residues that facilitate substrate unwinding prior to proteolysis (Moin and Urban, 2012a, b; Strisovsky et al., 2009). A mutant in these residues decreased TatA cleavage, but again reduced kcat without raising KM (Figure 7B and 7C). Lastly, the Drosophila signaling molecule Spitz, which is a less efficient substrate than TatA, also displayed lower kcat without any significant change in KM (Figure 7B and 7C). Together these defined substrate variants further indicate that intramembrane proteolysis is a kinetically controlled reaction that is not driven by affinity between protease and substrate.
DISCUSSION
In summary, we have developed an inducible reconstitution system for the analysis of intramembrane proteolysis in real time. This allowed us to measure for the first time the kinetic parameters of proteolysis occurring directly inside the membrane. Although the rhomboid domain is defined as a transmembrane segment binding moiety (Adrain and Freeman, 2012), all data with nine different rhomboid proteases and five substrate variants reveal protease and substrate have little, if any, meaningful affinity for each other within the membrane. This unexpected conclusion is independently supported by direct measurements of Kd in the membrane using a new FRET-based assay, and in detergent micelles by a variety of approaches including equilibrium gel filtration. While our reconstitution system necessarily uses pure proteins to allow precise measurements, estimating ‘apparent’ kinetic parameters in living E. coli cells indicates that it faithfully recapitulates in vivo rhomboid properties. Lack of need for other cofactors is also consistent with divergent rhomboid enzymes rescuing mutant defects in radically different organisms (Gallio et al., 2002), and no additional components being uncovered in many saturation screens performed over the course of decades in both eukaryotes and prokaryotes (Casci and Freeman, 1999; Rather et al., 1999).
The central implication of the kinetic parameters is that proteolysis within the membrane is not driven by substrate affinity under physiological conditions. It’s important to note that it’s not the absolute KM value itself that leads to this conclusion: while some proteases like chymotrypsin also display high KM values (Wysocka et al., 2008), these digestive proteases encounter food protein at extraordinarily high and thus matched concentrations. Instead, although there is little precedent for interpreting binding affinities within 2-dimensions, a KM of ~0.14 mole percent is extraordinarily high, because the inner membrane of E. coli contains only 1.25–1.67 mole percent (~50% by weight) of total protein (Schnaitman, 1970a, b). Indeed, experimentally we found that substrate has to become nearly the most abundant protein in the E. coli membrane to be near KM, and constitute all protein in a membrane to even approach Vmax. Compared to other signaling proteases, rhomboid proteases are thus at least 2 to 3 orders of magnitude less efficient: the catalytic efficiency (kcat/KM) of E. coli GlpG, the best understood intramembrane protease, is only ~47 M−1s−1 (0.0063 s−1/135 μM) relative to >10,000 M−1s−1 for caspases (Stennicke et al., 2000; Timmer et al., 2009).
Instead, these properties force us to consider that membrane-immersed proteolysis may be organized differently from other forms of proteolysis. All changes we observed involve kcat, revealing intramembrane proteolysis is fundamentally a kinetically-controlled reaction, rather than relying on differences in protein affinity (i.e. not thermodynamically-controlled). Interestingly, these enzymatic properties are unlike those of any other proteases or membrane proteins studied, but strikingly parallel those of one subset of DNA repair enzymes.
DNA glycosylases remove damaged bases from DNA using an intriguing mechanism that involves two different enzyme sites (Figure 7D). Nucleotides flipped out of a DNA double helix first interact with an ‘interrogation site’ on the DNA glycosylase (Friedman and Stivers, 2010). Importantly, a damaged base is not bound with high affinity per se; instead, it is able to spend more time in the dynamic, extrahelical state and thus stay longer in the interrogation complex. This longer residence allows the base to translocate to a second, deeper site – the excision site – where the glycosidic linkage is clipped to excise the base from DNA. The discriminatory mechanism is therefore rate-governed, with a minor contribution from binding affinity to the damaged base itself. The second key property of these DNA glycosylases is a slow kcat, because it ensures catalysis is slower than the residence time of natural bases, kinetically protecting them from hydrolysis (Friedman and Stivers, 2010).
These striking parallels suggest that low substrate affinity and slow rate of rhomboid proteolysis are not defects, but rather features, of this enzyme system. Moreover, they offer a new mechanistic framework for interpreting how membrane-immersed proteolysis is organized (Figure 7D). First, the lack of affinity for substrates and reliance on rates suggests that rhomboid proteases may also use an analogous ‘interrogation’ site to discriminate substrate from non-substrate kinetically. Although the gate has been viewed simply as a point of substrate entry, the crevice created by gate-opening, which is stable in the membrane (Urban and Baker, 2008; Zhou et al., 2012), may actually be an ‘interrogation’ site (Figure 7D). Like with DNA glycosylases, this site is physically separated from the deeper active site, which would force transmembrane helices to reside in the unwound state to reach the catalytic residues for proteolysis to ensue (Figure 7D), instead of returning laterally to the membrane. Our recent spectroscopy analysis of substrates revealed they form inherently less stable helices than non-substrates (Moin and Urban, 2012a), which suggests that they would spend more time in the unfolded state and thus reach the inner active site from the interrogation site.
This model may also explain the infamous property that rhomboid proteases use a catalytically weak dyad instead of evolving a classical triad to enhance catalytic power. While dyads are rare among serine proteases, the reduced catalytic rate would help protect non-substrates kinetically from cleavage by ensuring sufficient time for them to escape back into the membrane before cleavage could occur. In fact, the slow kcat of 0.02 s−1 that we measured for gate-open GlpG is much like the kcat of 0.03 s−1 exhibited by human DNA glycosylase OGG1 (Kuznetsov et al., 2007).
It is thus tempting to speculate that the primordial function of rhomboid proteases was to patrol the membrane looking for unfolded membrane proteins to cleave as a repair mechanism analogous to how DNA glycosylases patrol the genome for damaged bases. Nevertheless, since comparing intramembrane proteases to DNA repair enzymes is entirely new, it requires focused testing to determine to what degree rhomboid function is like that of DNA glycosylases, and where it deviates. Our discovery also has practical implications for inhibitor design; since substrate affinity is low, commonly-used strategies that rely on substrate characteristics to target inhibitor warheads to proteases (Drag and Salvesen, 2010) are unlikely to produce potent compounds against rhomboid. Likewise, caution should be exercised when interpreting covalent inhibitor-bound GlpG structures in what they can teach us about natural substrate binding (Vinothkumar et al., 2010; Xue et al., 2012). On an optimistic note, a robust kinetic system will allow evaluating inhibitors based on precise Ki values and directly within membranes, instead of relying on IC50 concentrations that are condition-dependent.
We focused our analyses on rhomboid proteases, but it should be noted that such quantitative real-time analyses have yet to be realized with other intramembrane proteases. In fact, a major achievement is the recent application of kinetic analysis to γ-secretase (Chavez-Gutierrez et al., 2012), albeit in detergent extracts and with endpoint assays. In this light, a particularly exciting feature of our system is its potential to be applied broadly: all protease catalysis is pH-sensitive, and similar placement of a FITC label should also afford natural quenching of other substrates upon membrane reconstitution. Alternatively, γ-secretase and site-2 proteases could be switched on after reconstitution by washing out reversible inhibitors, or supplying zinc ions, respectively. It is possible that some intramembrane proteases like γ-secretase could exhibit different kinetics, since they evolved extramembraneous domains for substrate binding (Fleig et al., 2012; Li et al., 2009; Shah et al., 2005). However, since these are later adaptations, low intramembrane affinity may be a primordial and common property of intramembrane proteolysis. Whether weak binding at transmembrane sites is important for catalysis inside the membrane, or a deliberate specialization by this class of enzymes, remains to be determined.
EXPERIMENTAL PROCEDURES
Protein Purification
Each HA-rhomboid protein was expressed and purified as described previously (Baker and Urban, 2012; Urban and Wolfe, 2005). Briefly, glutathione-S-transferase (GST) fusion proteins were expressed in E. coli C43 (DE3) cells, purified with glutathione-sepharose (GE Healthcare), and eluted by on-column PreScission cleavage to remove the GST tag. Purity of each enzyme was determined by SDS-PAGE stained with Coomassie colloidal blue and quantified on an Odyssey imager (LiCor Biosciences). To avoid erroneous quantitation from inherent differences in Coomassie staining of different rhomboid proteins, all enzymes were standardized by anti-HA analysis in parallel as quantified on an Odyssey imager.
C-terminal Flag-tagged recombinant substrate APP+Spi7-Flag was expressed and purified from E. coli as described (Baker and Urban, 2012; Baker et al., 2007). Fluorescein isothiocynate (FITC)-TatA (residues 1–33 of 97) and Spitz (residues 135–168 of 186) substrates containing the entire transmembrane segment were synthesized by Fmoc solid-state chemistry, with FITC conjugated to the N-terminus through a β-alanine linkage, and an amidated C-terminus, and resuspended in 50 mM Tris pH 7.4, 150 mM NaCl, 1 mM DTT, 0.2% (w/v) Sarkosyl. The actual final concentration of each substrate preparation was quantified using FITC fluorescence relative to FITC standards using a Synergy H4 Hybrid plate reader (BioTek).
E. coli Liposome Preparation
100 mg of E. coli polar lipid extract in chloroform (Avanti Polar Lipids) was slowly dried into a thin lipid film in a rotary evaporator, and dried under high vacuum overnight on a custom-made glass manifold (Kontes Glass). The film was then resuspended thoroughly in 10 mL of 10 mM HEPES pH 7, 10 mM NaCl, 1 mM DTT, briefly sonicated in a temperature-controlled sonifier (Branson), and extruded through 200nm pore filters to form liposomes of defined size.
Inducible Reconstitution and Real-Time Proteolysis Assay
30 μg of E. coli liposomes were mixed with 50 mM NaAcetate pH 4.0, 150 mM NaCl, 0.05 to 500 pmoles of rhomboid enzyme, and 20 to 1600 pmoles of FITC-substrate. This co-reconstitution mix was diluted 20-fold with 12.5 mM NaAcetate pH 4.0, 37.5 mM NaCl to reduce detergent below its critical micelle concentration and promote reconstitution. Proteoliposomes were collected by ultracentrifugation at 600,000 g for 20 minutes in an Optima MAX-XP ultracentrifuge (Beckman). The supernatant was removed by aspiration, the pellet resuspended in 50 mM Tris pH 7.4, 150 mM NaCl, 1 mM DTT to initiate proteolysis (in the presence of D2O for isotope experiments), rapidly transferred into a pre-warmed black 384-well microtiter plate, covered with film to prevent evaporation, and incubated at 37°C in a Synergy H4 Hybrid plate reader (BioTek). Fluorescence was monitored every minute using monochromators set to wavelengths of 485±20 nm (excitation) and 528±20 nm (emission). Alternatively, time points were quenched with equal volumes of 2X Tricine SDS-sample buffer, resolved on 16% Tricine gels (Life Technologies), and imaged with a blue laser and fluorescein emission filters on a Typhoon Imager (GE Healthcare). APP+Spi7-Flag and HA-EcGlpG were co-reconstituted as above, resolved by SDS-PAGE, detected by 2-color anti-Flag and anti-HA western analysis, and imaged with an Odyssey infrared scanner (LiCor Biosciences).
in vivo TatA-Flag Titration
Expression of TatA-Flag (full-length protein with a C-terminal Flag tag) in log-phase E. coli K12 BW25113 cells was titrated using arabinose-mediated induction from a pBAD plasmid. Induced cultures were grown at 37°C in a shaking incubator for 2 hours. Cleavage by endongenous GlpG was quantified by resolving cell lysates on a 16% Tricine SDS-polyacrylamide gel, followed by anti-Flag western analysis and imaging with an Odyssey infrared scanner (LiCor Biosciences). To quantify expression levels of TatA-Flag, cells were lysed in a French pressure cell (2 passes at 16,000 PSI), and the lysate was clarified to remove unbroken cells at 9,000 g for 8 minutes in a JLA 8.1000 rotor (Beckman). Membranes were collected by ultracentrifugation at 50,000 rpm for 1 hour in a MLA-55 rotor (Beckman). Peripheral and contaminating soluble proteins were removed with a sodium carbonate wash. Total membrane protein from each titration were separated on a 4–20% tris-glycine SDS-polyacrylamide gel, stained with a colloidal Coomassie blue dye (LiCor Biosciences), and quantified with an Odyssey infrared scanner. TatA-Flag expression was converted to molar ratio of membrane proteins by correcting signals for molecular weight, then converted to mole% of membranes. To estimate kcat in vivo, endogenous Flag-EcGlpG levels in membranes prepared by sucrose gradient ultracentrifugation were quantified by western analysis relative to purified Flag-EcGlpG as a standard.
Fitting and Statistical Analysis
All data were analyzed and graphed using the R language and environment. Initial rates were extracted from real-time curves between 4 and 14 minutes using the slope (m) in a linear model: y = mx + b. Initial rates versus substrate concentration of reconstituted reactions were modeled using the Hill-modified Michaelis Menten equation: . Importantly, we did not observe cooperativity with reconstituted reactions analyzed by SDS-PAGE, indicating that cooperativity is not a true feature of the enzyme reaction. P-values in pairwise comparisons were derived from multiple non-linear regression analysis and kcat p-values were corrected for multiple comparisons using the Bonferroni method (KM p-values were not corrected because none achieved significance).
Supplementary Material
1st inducible reconstitution assay for real-time analysis of intramembrane catalysis
reveals low substrate affinity/slow catalysis by rhomboid in vitro and in cells
rate-controlled reaction: rhomboid/substrate mutants change only kcat, KM unperturbed
kinetics expose similarity to DNA repair enzymes that monitor substrate dynamics
Acknowledgments
This work was supported by NIH grant 2R01AI066025, the Howard Hughes Medical Institute, and the David and Lucile Packard Foundation. Part of this research was conducted using instruments at the Malaria Research Institute (CD spectrometer), and CHESS (beamline supported by NSF grant DMR-0936384 and NIH grant GM-103485). SU designed the study. RB and SU developed the inducible co-reconstitution system, SWD developed the real-time assay, performed all experiments except those in Figure 1A, 1B, 1D, and S1B, and prepared the figures. SC solved the structure of GlpG at neutral and low pH. SU wrote the paper, and all authors approved the final manuscript.
Footnotes
The authors declare that no financial conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adrain C, Freeman M. New lives for old: evolution of pseudoenzyme function illustrated by iRhoms. Nature reviews Molecular cell biology. 2012;13:489–498. doi: 10.1038/nrm3392. [DOI] [PubMed] [Google Scholar]
- Baker RP, Urban S. Architectural and thermodynamic principles underlying intramembrane protease function. Nature chemical biology. 2012;8:759–768. doi: 10.1038/nchembio.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RP, Young K, Feng L, Shi Y, Urban S. Enzymatic analysis of a rhomboid intramembrane protease implicates transmembrane helix 5 as the lateral substrate gate. Proc Natl Acad Sci U S A. 2007;104:8257–8262. doi: 10.1073/pnas.0700814104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casci T, Freeman M. Control of EGF receptor signalling: Lessons from fruitflies. Cancer and Metastasis Rev. 1999;18:181–201. doi: 10.1023/a:1006313122373. [DOI] [PubMed] [Google Scholar]
- Chavez-Gutierrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M, Lismont S, Zhou L, Van Cleynenbreugel S, Esselmann H, et al. The mechanism of gamma-Secretase dysfunction in familial Alzheimer disease. EMBO J. 2012;31:2261–2274. doi: 10.1038/emboj.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Annaert W. Novel research horizons for presenilins and gamma-secretases in cell biology and disease. Annu Rev Cell Dev Biol. 2010;26:235–260. doi: 10.1146/annurev-cellbio-100109-104117. [DOI] [PubMed] [Google Scholar]
- Deckert G, Warren PV, Gaasterland T, Young WG, Lenox AL, Graham DE, Overbeek R, Snead MA, Keller M, Aujay M, et al. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature. 1998;392:353–358. doi: 10.1038/32831. [DOI] [PubMed] [Google Scholar]
- Doucet A, Butler GS, Rodriguez D, Prudova A, Overall CM. Metadegradomics: toward in vivo quantitative degradomics of proteolytic post-translational modifications of the cancer proteome. Mol Cell Proteomics. 2008;7:1925–1951. doi: 10.1074/mcp.R800012-MCP200. [DOI] [PubMed] [Google Scholar]
- Drag M, Salvesen GS. Emerging principles in protease-based drug discovery. Nature reviews. 2010;9:690–701. doi: 10.1038/nrd3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod JP, Hogg JL, Quinn DM, Venkatasubban KS, Schowen RL. Protonic Reorganization and Substrate Structure in Catalysis by Serine Proteases. J Am Chem Soc. 1980;102:3917–3922. [Google Scholar]
- Fersht A. Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding. New York: W. H. Freeman and Company; 1999. [Google Scholar]
- Fleig L, Bergbold N, Sahasrabudhe P, Geiger B, Kaltak L, Lemberg MK. Ubiquitin-Dependent Intramembrane Rhomboid Protease Promotes ERAD of Membrane Proteins. Mol Cell. 2012;47:558–569. doi: 10.1016/j.molcel.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Fluhrer R, Steiner H, Haass C. Intramembrane proteolysis by signal peptide peptidases: a comparative discussion of GXGD-type aspartyl proteases. J Biol Chem. 2009;284:13975–13979. doi: 10.1074/jbc.R800040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JI, Stivers JT. Detection of damaged DNA bases by DNA glycosylase enzymes. Biochemistry (Mosc) 2010;49:4957–4967. doi: 10.1021/bi100593a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallio M, Sturgill G, Rather P, Kylsten P. A conserved mechanism for extracellular signaling in eukaryotes and prokaryotes. Proc Natl Acad Sci U S A. 2002;99:12208–12213. doi: 10.1073/pnas.192138799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel JP, Dreyer WJ. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Huntington JA. Thrombin plasticity. Biochim Biophys Acta. 2012;1824:246–252. doi: 10.1016/j.bbapap.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Kuznetsov NA, Koval VV, Nevinsky GA, Douglas KT, Zharkov DO, Fedorova OS. Kinetic conformational analysis of human 8-oxoguanine-DNA glycosylase. J Biol Chem. 2007;282:1029–1038. doi: 10.1074/jbc.M605788200. [DOI] [PubMed] [Google Scholar]
- Lazareno-Saez C, Arutyunova E, Coquelle N, Lemieux MJ. Domain swapping in the cytoplasmic domain of the Escherichia coli rhomboid protease. J Mol Biol. 2013;425:1127–1142. doi: 10.1016/j.jmb.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Lemberg MK, Freeman M. Functional and evolutionary implications of enhanced genomic analysis of rhomboid intramembrane proteases. Genome Res. 2007;17:1634–1646. doi: 10.1101/gr.6425307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Dang S, Yan C, Gong X, Wang J, Shi Y. Structure of a presenilin family intramembrane aspartate protease. Nature. 2013;493:56–61. doi: 10.1038/nature11801. [DOI] [PubMed] [Google Scholar]
- Li X, Wang B, Feng L, Kang H, Qi Y, Wang J, Shi Y. Cleavage of RseA by RseP requires a carboxyl-terminal hydrophobic amino acid following DegS cleavage. Proc Natl Acad Sci U S A. 2009;106:14837–14842. doi: 10.1073/pnas.0903289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Bond JS. Proteases: multifunctional enzymes in life and disease. J Biol Chem. 2008;283:30433–30437. doi: 10.1074/jbc.R800035200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinoshima H, Glickman MS. Site-2 proteases in prokaryotes: regulated intramembrane proteolysis expands to microbial pathogenesis. Microbes Infect. 2006;8:1882–1888. doi: 10.1016/j.micinf.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Moin SM, Urban S. Membrane immersion allows rhomboid proteases to achieve specificity by reading transmembrane segment dynamics. eLife. 2012a;1:e00173. doi: 10.7554/eLife.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osenkowski P, Ye W, Wang R, Wolfe MS, Selkoe DJ. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J Biol Chem. 2008;283:22529–22540. doi: 10.1074/jbc.M801925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona JJ, Craik CS. Evolutionary divergence of substrate specificity within the chymotrypsin-like serine protease fold. J Biol Chem. 1997;272:29987–29990. doi: 10.1074/jbc.272.48.29987. [DOI] [PubMed] [Google Scholar]
- Rather PN, Ding X, Baca-DeLancey RR, Siddiqui S. Providencia stuartii genes activated by cell-to-cell signaling and identification of a gene required for production or activity of an extracellular factor. J Bacteriol. 1999;181:7185–7191. doi: 10.1128/jb.181.23.7185-7191.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman CA. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. J Bacteriol. 1970a;104:882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman CA. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970b;104:890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE, 3rd, Sudhof T, Yu G. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122:435–447. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Renatus M, Meldal M, Salvesen GS. Internally quenched fluorescent peptide substrates disclose the subsite preferences of human caspases 1, 3, 6, 7 and 8. Biochem J. 2000;350(Pt 2):563–568. [PMC free article] [PubMed] [Google Scholar]
- Stevenson LG, Strisovsky K, Clemmer KM, Bhatt S, Freeman M, Rather PN. Rhomboid protease AarA mediates quorum-sensing in Providencia stuartii by activating TatA of the twin-arginine translocase. Proc Natl Acad Sci U S A. 2007;104:1003–1008. doi: 10.1073/pnas.0608140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strisovsky K, Sharpe HJ, Freeman M. Sequence-specific intramembrane proteolysis: identification of a recognition motif in rhomboid substrates. Mol Cell. 2009;36:1048–1059. doi: 10.1016/j.molcel.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer JC, Zhu W, Pop C, Regan T, Snipas SJ, Eroshkin AM, Riedl SJ, Salvesen GS. Structural and kinetic determinants of protease substrates. Nature structural & molecular biology. 2009;16:1101–1108. doi: 10.1038/nsmb.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S. Making the cut: central roles of intramembrane proteolysis in pathogenic microorganisms. Nature Reviews Microbiology. 2009;7:411–423. doi: 10.1038/nrmicro2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S. Taking the plunge: integrating structural, enzymatic and computational insights into a unified model for membrane-immersed rhomboid proteolysis. Biochem J. 2010;425:501–512. doi: 10.1042/BJ20090861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S, Baker RP. In vivo analysis reveals substrate-gating mutants of a rhomboid intramembrane protease display increased activity in living cells. Biol Chem. 2008;389:1107–1115. doi: 10.1515/BC.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S, Dickey SW. The rhomboid protease family: a decade of progress on function and mechanism. Genome biology. 2011;12:231. doi: 10.1186/gb-2011-12-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S, Wolfe MS. Reconstitution of intramembrane proteolysis in vitro reveals that pure rhomboid is sufficient for catalysis and specificity. Proc Natl Acad Sci U S A. 2005;102:1883–1888. doi: 10.1073/pnas.0408306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinothkumar KR, Strisovsky K, Andreeva A, Christova Y, Verhelst S, Freeman M. The structural basis for catalysis and substrate specificity of a rhomboid protease. EMBO J. 2010;29:3797–3809. doi: 10.1038/emboj.2010.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilderman PJ, Vasil AI, Martin WE, Murphy RC, Vasil ML. Pseudomonas aeruginosa synthesizes phosphatidylcholine by use of the phosphatidylcholine synthase pathway. J Bacteriol. 2002;184:4792–4799. doi: 10.1128/JB.184.17.4792-4799.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS. Intramembrane proteolysis. Chem Rev. 2009;109:1599–1612. doi: 10.1021/cr8004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka M, Lesner A, Legowska A, Jaskiewicz A, Miecznikowska H, Rolka K. Designing of substrates and inhibitors of bovine alpha-chymotrypsin with synthetic phenylalanine analogues in position P(1) Protein and peptide letters. 2008;15:260–264. doi: 10.2174/092986608783744180. [DOI] [PubMed] [Google Scholar]
- Xue Y, Chowdhury S, Liu X, Akiyama Y, Ellman J, Ha Y. Conformational change in rhomboid protease GlpG induced by inhibitor binding to its S′ subsites. Biochemistry (Mosc) 2012;51:3723–3731. doi: 10.1021/bi300368b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Ha Y. Catalytic mechanism of rhomboid protease GlpG probed by 3,4-dichloroisocoumarin and diisopropyl fluorophosphonate. J Biol Chem. 2012;287:3099–3107. doi: 10.1074/jbc.M111.310482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Kovach IM. Deuterium solvent isotope effect and proton-inventory studies of factor Xa-catalyzed reactions. Biochemistry (Mosc) 2006;45:14175–14182. doi: 10.1021/bi061218m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Moin SM, Urban S, Zhang Y. An internal water-retention site in the rhomboid intramembrane protease GlpG ensures catalytic efficiency. Structure. 2012;20:1255–1263. doi: 10.1016/j.str.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.