Abstract
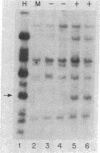
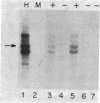
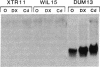
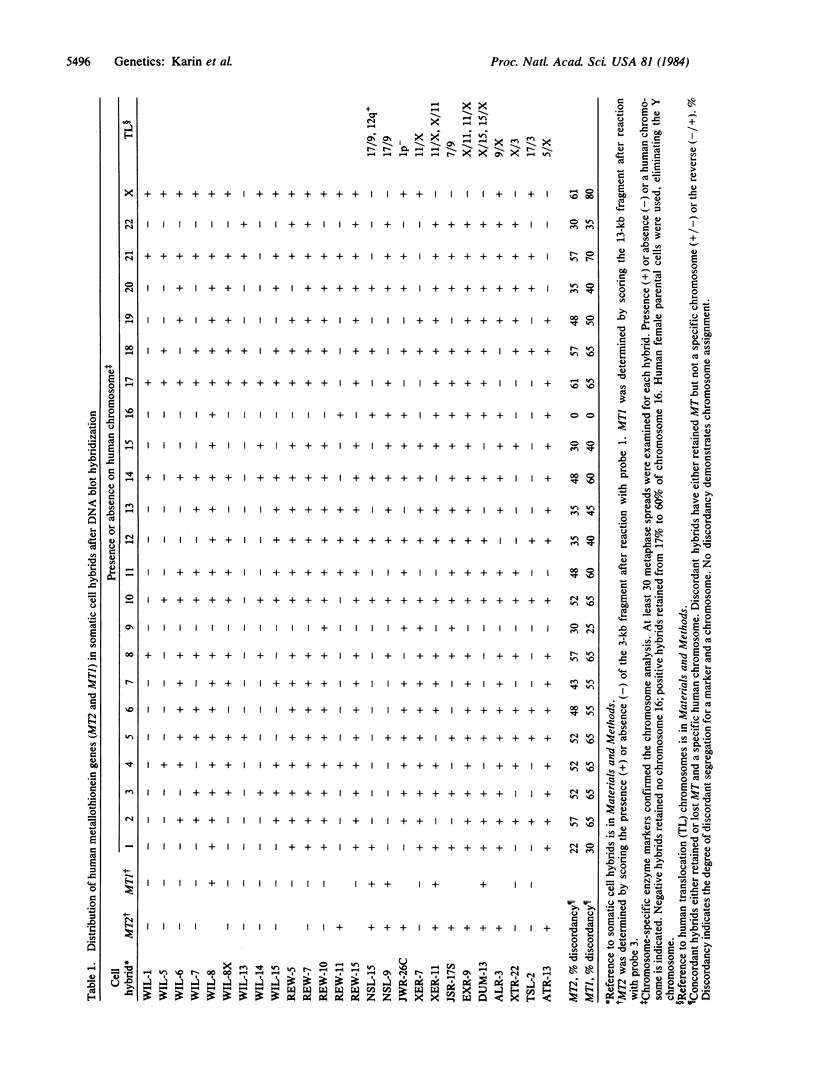
The metallothioneins are a family of heavy-metal binding proteins of low molecular weight. They function in the regulation of trace metal metabolism and in the protection against toxic heavy metal ions. In man, the metallothioneins are encoded by at least 10-12 genes separated into two groups, MT-I and MT-II. To understand the genomic organization of these genes and their involvement in hereditary disorders of trace metal metabolism, we have determined their chromosomal location. Using human-mouse cell hybrids and hybridization probes derived from cloned and functional human MT1 and MT2 genes, we show that the functional human genes are clustered on human chromosome 16. Analysis of RNA from somatic cell hybrids indicated that hybrids that contained human chromosome 16 expressed both human MT1 and MT2 mRNA, and this expression is regulated by both heavy metal ions and glucocorticoid hormones.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cousins R. J. Letter: Zinc-thionein and heritable disorders associated with aberrant zinc metabolism. Lancet. 1976 Sep 25;2(7987):686–687. doi: 10.1016/s0140-6736(76)92493-4. [DOI] [PubMed] [Google Scholar]
- Cox D. R., Palmiter R. D. The metallothionein-I gene maps to mouse chromosome 8: implications for human Menkes' disease. Hum Genet. 1983;64(1):61–64. doi: 10.1007/BF00289481. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981 Jun 10;256(11):5712–5716. [PubMed] [Google Scholar]
- Evans G. W., Bubois R. S., Hambidge K. M. Wilson's disease: identification of an abnormal copper-binding protein. Science. 1973 Sep 21;181(4105):1175–1176. doi: 10.1126/science.181.4105.1175. [DOI] [PubMed] [Google Scholar]
- Hata A., Tsunoo H., Nakajima H., Shintaku K., Kimura M. Acute cadmium intoxication in inbred mice: a study on strain differences. Chem Biol Interact. 1980 Oct;32(1-2):29–39. doi: 10.1016/0009-2797(80)90066-6. [DOI] [PubMed] [Google Scholar]
- Karin M., Andersen R. D., Slater E., Smith K., Herschman H. R. Metallothionein mRNA induction in HeLa cells in response to zinc or dexamethasone is a primary induction response. Nature. 1980 Jul 17;286(5770):295–297. doi: 10.1038/286295a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Cathala G., Nguyen-Huu M. C. Expression and regulation of a human metallothionein gene carried on an autonomously replicating shuttle vector. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4040–4044. doi: 10.1073/pnas.80.13.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Cathala G., Slater E., Baxter J. D. Activation of a heterologous promoter in response to dexamethasone and cadmium by metallothionein gene 5'-flanking DNA. Cell. 1984 Feb;36(2):371–379. doi: 10.1016/0092-8674(84)90230-7. [DOI] [PubMed] [Google Scholar]
- Karin M., Herschman H. R. Dexamethasone stimulation of metallothionein synthesis in HeLa cell cultures. Science. 1979 Apr 13;204(4389):176–177. doi: 10.1126/science.432639. [DOI] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes: molecular cloning and sequence analysis of the mRNA. Nucleic Acids Res. 1982 May 25;10(10):3165–3173. doi: 10.1093/nar/10.10.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labadie G. U., Hirschhorn K., Katz S., Beratis N. G. Increased copper metallothionein in Menkes cultured skin fibroblasts. Pediatr Res. 1981 Mar;15(3):257–261. doi: 10.1203/00006450-198103000-00012. [DOI] [PubMed] [Google Scholar]
- Lane P. W., Eicher E. M. Gene order in linkage group XVI of the house mouse. J Hered. 1979 Jul-Aug;70(4):239–244. doi: 10.1093/oxfordjournals.jhered.a109246. [DOI] [PubMed] [Google Scholar]
- Naylor S. L., Sakaguchi A. Y., Shows T. B., Law M. L., Goeddel D. V., Gray P. W. Human immune interferon gene is located on chromosome 12. J Exp Med. 1983 Mar 1;157(3):1020–1027. doi: 10.1084/jem.157.3.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian E. V., Iwai J., Leitl G., Tuthill R. Genetic influence on cadmium-induced hypertension. Am J Physiol. 1978 Oct;235(4):H385–H391. doi: 10.1152/ajpheart.1978.235.4.H385. [DOI] [PubMed] [Google Scholar]
- Prins H. W., Van den Hamer J. A. Abnormal copper-thionein synthesis and impaired copper utilization in mutated brindled mice: model for Menkes' disease. J Nutr. 1980 Jan;110(1):151–157. doi: 10.1093/jn/110.1.151. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Heguy A., Karin M. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell. 1984 May;37(1):263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roderick T. H., Lalley P. A., Davisson M. T., O'Brien S. J., Womack J. E., Créau-Goldberg N., Echard G., Moore K. L. Report of the Committee on Comparative Mapping. Cytogenet Cell Genet. 1984;37(1-4):312–339. doi: 10.1159/000132013. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Alper C. A., Bootsma D., Dorf M., Douglas T., Huisman T., Kit S., Klinger H. P., Kozak C., Lalley P. A. International system for human gene nomenclature (1979) ISGN (1979). Cytogenet Cell Genet. 1979;25(1-4):96–116. doi: 10.1159/000131404. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Brown J. A., Haley L. L., Byers M. G., Eddy R. L., Cooper E. S., Goggin A. P. Assignment of the beta-glucuronidase structural gene to the pter leads to q22 region of chromosome 7 in man. Cytogenet Cell Genet. 1978;21(1-2):99–104. doi: 10.1159/000130882. [DOI] [PubMed] [Google Scholar]
- Shows T., Eddy R., Haley L., Byers M., Henry M., Fujita T., Matsui H., Taniguchi T. Interleukin 2 (IL2) is assigned to human chromosome 4. Somat Cell Mol Genet. 1984 May;10(3):315–318. doi: 10.1007/BF01535253. [DOI] [PubMed] [Google Scholar]
- Sobocinski P. Z., Canterbury W. J., Jr, Mapes C. A., Dinterman R. E. Involvement of hepatic metallothioneins in hypozincemia associated with bacterial infection. Am J Physiol. 1978 Apr;234(4):E399–E406. doi: 10.1152/ajpendo.1978.234.4.E399. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]