Abstract
Objective
Post-traumatic osteoarthritis (PTOA) occurs after anterior cruciate ligament (ACL) injury. PTOA may be initiated by early expression of proteolytic enzymes capable of causing degradation of the articular cartilage at time of injury. This study investigated the production of three of these key proteases in multiple joint tissues after ACL injury and subsequent markers of cartilage turnover.
Design
ACL transection was performed in adolescent minipigs. Collagenase (MMP-1 and MMP-13) and aggrecanase (ADAMTS-4) gene expression changes were quantified in the articular cartilage, synovium, injured ligament, and the provisional scaffold at days 1, 5, 9, and 14 post-injury. Markers of collagen degradation (C2C), synthesis (CPII) and aggrecan synthesis (CS846) were quantified in the serum and synovial fluid. Histologic assessment of the cartilage integrity (OARSI scoring) was also performed.
Results
MMP-1 gene expression was upregulated in the articular cartilage, synovium and ligament after ACL injury. MMP-13 expression was suppressed in the articular cartilage, but upregulated 100fold in the synovium and ligament. ADAMTS-4 was upregulated in the synovium and ligament but not in the articular cartilage. The concentration of collagen degradation fragments (C2C) in the synovial joint fluid nearly doubled in the first five days after injury.
Conclusion
We conclude that upregulation of genes coding for proteins capable of degrading cartilage ECM is seen within the first few days after ACL injury, and this response is seen not only in chondrocytes, but also in cells in the synovium, ligament and provisional scaffold.
Keywords: Cartilage, metalloproteinases, aggrecanase, ACL, joint tissues
Introduction
Post-traumatic osteoarthritis (PTOA) develops in more than 50% of individuals who tear their anterior cruciate ligament (ACL) within 10 to 20 years (1). Recent work has shown that patients begin to lose the proteoglycan and collagen molecules from cartilage in the first few weeks after injury, losses that are typically irrecoverable (2, 3). In this study, we employed a large animal model to study changes in gene and protein expression within the cartilage and surrounding tissues as well as cartilage degradation within the first two weeks following an induced ACL injury. There are multiple tissues which are in contact with the articular cartilage via the synovial fluid and thus could potentially influence the response of the cartilage to injury. These tissues include the synovium, the ACL, and the provisional scaffold.
Organized connective tissues which have a net anabolic response to injury and go on to heal do so in an orderly and predictable fashion. The first major step in that process is the formation of a provisional scaffold within the wound site that fills the gap between the two damaged ends of the ligament. The trigger for scaffold formation is thought to be the contact of blood with the exposed collagen of the wound edges. This contact starts a cascade of processes which result in the release of anabolic cytokines, including fibroblast growth factor 2 (FGF-2), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF)-A and -B and others, and wound healing progresses in an orderly and reliable fashion (4). However, after the ACL tears, it does not heal the way other ligaments do, even with suture repair. Recently, a mechanism has been proposed for this failure, namely that when the ACL tears inside the knee joint, the contact of blood with the newly exposed collagen results in formation of a provisional scaffold only over the torn edges of the tissue, while the joint fluid prevents formation of a bridging scaffold (4, 5). In this study, we wished to determine if this dysfunctional provisional scaffold had any catabolic effect on the surrounding cartilage of the knee joint after an ACL transection.
After injury, not only does the ACL fail to heal, but the injury leads to the development of post-traumatic osteoarthritis of the knee joint in many patients. MMP-1 and MMP-13 have been found to play a role in the development of osteoarthritis (6, 7). MMP-1 is responsible for breaking down interstitial collagens, including types I, II, and III. MMP-13 is five to ten times more active than MMP-1 in degrading collagen type II and is capable of contributing to aggrecan degradation (7, 8). Therefore, it has been suggested that MMP-13 may play an important role in articular cartilage turnover and the pathophysiology associated with PTOA (8). The characteristic fragments generated from this collagenase activity on type II collagen are quantifiable through ELISA (enzyme-linked immunosorbent assay). The C2C marker identifies fragments following type II collagen cleavage by collagenases, including MMP-1 and MMP-13 (9). The CPII marker, in contrast, is a marker of type II procollagen synthesis (10). Cartilage turnover is thus largely dependent on the balance between collagen and aggrecan synthesis and degradation.
In addition, aggrecanases are also thought to play a role in the development of PTOA. ADAMTS-4 efficiently degrades aggrecan (11). This aggrecanase activity is balanced by aggrecan synthesis, which also increases during the course of osteoarthritis (OA) (12). The chondroitin sulfate 846 epitope (CS 846) has been examined as a marker of aggrecan synthesis, with the greatest concentrations observed in the synovial fluid of osteoarthritis patients with the longest disease duration and greatest cartilage loss (12, 13).
Kraus et al. suggest that the early phase of acute injury provides a window of opportunity to decrease the destructive joint processes, which may ultimately lead to the development of PTOA (14). In this study, we hypothesized that an isolated ACL injury prompts a response in multiple tissues as early as one day after injury which results in changes in cytokine and protein expression over time. We hypothesized that even in the absence of impact trauma to the cartilage, ACL injury would produce a catabolic response in several joint tissues (i.e., ligament, synovium, cartilage, provisional scaffold). We further hypothesized that this upregulation of catabolic genes would result in aggrecan and collagen destruction within the cartilage within the first two weeks of injury.
Materials and Methods
Thirty adolescent Yucatan minipigs (Coyote CCI, Douglas, MA), aged 12-15 months, were obtained for use in this study. All minipigs were handled according to approved Institutional Animal Care and Use Committee (IACUC) protocols at Animal Resources at Children's Hospital (ARCH, Boston, MA). Minipigs were acclimated to the ARCH environment for a minimum of 3 days prior to experimental handling. Minipigs were assigned to euthanasia at one of four time points (day 1, 5, 9 or 14; n=6 per group). Minipigs in four of the groups were subjected to unilateral ACL transection, followed by tissue harvest at the designated time point. A fifth group of six non-operated Yucatans served as ACL-intact controls.
Surgical Procedure
Twenty-four minipigs underwent an ACL transection procedure on one knee as previously described (5, 15). Briefly, the ACL of anesthetized animals were exposed by performing a medial arthrotomy and partial resection of the fat pad. The ACL was then cut using a scalpel blade at the junction of the proximal and middle thirds. Functional loss of the ACL was verified using the Lachman maneuver, a clinical exam used to assess the integrity of the ACL (16). The knee was closed in layers. Animals were allowed normal nutrition and ad lib activity following surgery throughout the experimental period.
Tissue Collection
At time of euthanasia, samples of cartilage, synovium, ACL and the provisional scaffold (i.e., ACL scar tissue located between the torn ends of the ligament) were harvested. Cartilage was harvested from the weight bearing surface of the femoral condyle and a synovium sample was taken from the medial aspect of the joint at a location remote from the arthrotomy site. Each tissue specimen was submerged in a cryovial containing RNAlater ® stabilization solution (Ambion, Austin, TX, USA), then flash frozen in liquid nitrogen and stored at -80°C until gene expression analysis. A second portion of the cartilage tissue was embedded within Optimal Cutting Temperature (OCT) medium (Sakura Finetek, CA, USA), frozen, and stored at -80°C for histological analysis. Systemic blood of control animals was clotted to serve as a provisional scaffold control for the intact group. Cartilage, intact ACL tissue, and synovium samples were also harvested from the six unoperated animals.
The six minipigs euthanized at day 14 were also subjected to serial serum and synovial fluid draws. Serum and synovial fluid were sampled pre-transection, then at 5 and 14 days post-injury while the subjects were under anesthesia. Blood was collected in serum separator tubes, allowed to clot at room temperature, centrifuged at 1000×g for 10 min, and the serum aliquoted in 500 μL aliquots and stored at -80°C. Synovial fluid was centrifuged at 3000×g for 10 min to remove any cells. The supernatant was removed and stored in 120 μL aliquots in cryovials at -80°C, with approximately 240-500 μL of synovial fluid recovered at each time point.
Quantitative Real-Time PCR
The cartilage, ligament, synovium, and provisional scaffold samples were examined for mRNA expression of several genes using real-time reverse transcriptase polymerase chain reaction (qPCR) run in duplicate. Briefly, total RNA was extracted from the frozen tissue using the PureLink RNA Mini Kit (Ambion, Austin, TX, USA), treated with DNAse I (PureLink DNase, Invitrogen, Life Technologies, NY, USA) according to the manufacturer's protocol and quantified. Total RNA was reverse transcribed to generate cDNA using the RETROscript kit (Ambion, Austin, TX, USA). For use in qPCR, previously reported primers were validated by sequencing the PCR product and performing a BLAST search with these results. Primers are summarized in Table 1. Sybr Green PCR Mastermix (Applied Biosystems, Foster City, CA, USA) (10 μL), nuclease-free water, forward and reverse primer (2 μl each), and 3 μl (cartilage) or 0.5 μl (ligament, synovium, provisional scaffold) of the 1 μg cDNA were mixed and quantified in a reaction volume of 10μl. Non-template controls (NTC) were included to indicate contaminants or non-specific amplification. An Applied Biosystems 7900HT (Applied Biosystems, Foster City, CA, USA) was used for amplification and detection. The PCR profile conditions were 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, 50°C for 45 seconds, and 60°C for 45 seconds. Levels of gene expression were normalized to the housekeeping gene, GAPDH. Relative changes in gene expression were calculated using the 2-ΔΔCt method (17).
Table 1.
Sequences of porcine-specific qPCR primers.
Collagen and Aggrecan Fragment Detection by ELISA
Synovial fluid and serum C2C, CPII, CS 846 levels were determined using commercially-available ELISA kits (60-1001-001, 60-1004, and 60-1003-01; IBEX Pharmaceuticals Inc, Montreal, Canada) as previously described for porcine tissue (18-20). The IBEX C2C sequence and porcine proteome have a 100% maximum identity between the assay and porcine collagen alpha-1 (II) sequence. Spiking studies were performed to document adequate antigen retrieval for the porcine synovial fluid. Synovial fluid samples were digested using 75 units/ml recombinant Streptomyces hyaluronidase (Sigma Aldrich, USA) for 10 min at 37°C prior to ELISA testing.
Safranin-O and Active Caspase 3 Immunostaining in Porcine Cartilage Specimens
Frozen section slides of porcine cartilage specimens from control (non-injured) joints (n=2) and day14 post-ACL transection (n=5) knees were stained with Safranin-O and Fast Green. All sections were evaluated for histologic signs of cartilage degeneration using a modified Osteoarthritis Research Society International (OARSI) scoring system in which two investigators blinded to the treatment independently graded the best stained sections and arrived at a consensus score as previously described (21, 22). Active caspase 3 staining was performed using rabbit polyclonal primary antibody (Cat#ab13847, Abcam, Cambridge, MA) at 1:50 dilution overnight at 4°C and a goat anti-rabbit secondary antibody (Life Technologies, Molecular Probes) at 1:50 dilution. Vectashield mounting medium with DAPI (Vector Laboratories Inc., Burlingame, CA) was used.
Statistical Analyses
Gene expression was summarized as median (25th percentile, 75th percentile). Overall differences in gene expression levels were assessed using Kruskal-Wallis H Test followed by post-hoc Mann-Whitney U Tests. Statistical analysis on gene expression was performed using SAS (version 9.2, SAS Institute Inc., Cary, NC). Synovial fluid and serum levels of cartilage biomarkers were reported as median (25th percentile, 75th percentile). Comparisons were determined using a Wilcoxon signed-rank test through the R statistical package (provide company and its location) (23). P< 0.05 was considered statistically significant for both gene expression and cartilage biomarker analyses.
Results
MMP-1 gene expression changes in cartilage, ligament, provisional scaffold and synovium over 14 days following ACL injury
MMP-1 gene expression in the articular cartilage increased significantly by 16-fold compared to baseline levels by day 1 (P= 0.0162) and remained moderately elevated at day 14, although this difference was not significant (P= 0.0751) (Figure 1A). MMP-1 gene expression was significantly upregulated by at least 9-fold at day 14 in the synovium (P= 0.0123) and 3-fold in the ligament (P= 0.0162), compared to baseline levels within each tissue. MMP-1 gene expression was at least 9-fold higher than baseline at all time points in the provisional scaffold; however, this change was not statistically significant (P> 0.5798).
Figure 1.
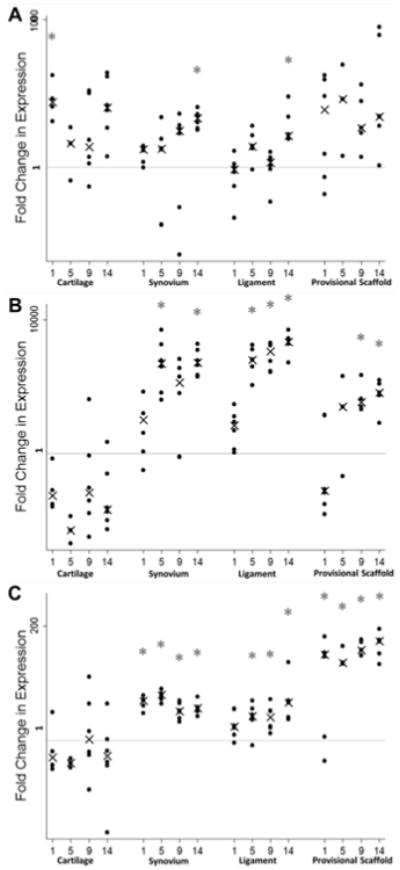
Median fold change in MMP-1 (A), MMP-13 (B), and ADAMTS-4 (C) gene expression levels in cartilage, synovium, ligament, and provisional scaffold in the initial 2 weeks following ACL injuries compared to uninjured controls (Day 0). Note the log 10 scale on the y-axis. Error bars represent the 25th and 75th percentile and asterisks indicate significant differences at p<0.05 from baseline (tissue from uninjured controls) within each tissue type for the six minipigs.
MMP-13 gene expression changes in cartilage, ligament, provisional scaffold and synovium over 14 days following ACL injury
MMP-13 gene expression in the articular cartilage was found to be suppressed by 16-fold at day 1, increasing to over 200-fold by day 5, a change that was statistically significant (P= 0.0312; Figure 1B). In contrast, in the synovium, a 350-fold increase in MMP-13 gene expression was seen by day 5, and remained that elevated at day 14 (P= 0.0123 for comparison to baseline for both time points). Changes in the ligament were similar to those seen in the synovium, with a 500-fold increase in MMP-13 gene expression relative to baseline by five days after injury (P= 0.0123), and remained upregulated by at least 1000-fold at day 9 and 14 (P< 0.0162). In the provisional scaffold, a significant 80-fold increase in MMP-13 expression was seen at both days 9 and 14 (P= 0.0342 and 0.0255, respectively).
ADAMTS-4 gene expression changes in the cartilage, ligament, synovium, and provisional scaffold over 14 days following injury
ADAMTS-4 gene expression in the articular cartilage did not change significantly over the two week experiment (Figure 1C). In the synovium, a 3-fold increase in ADAMTS-4 gene expression was from day 1 thru 14 (P= 0.0162, 0.0123, 0.0123, and 0.0123, respectively). In the ligament, a significant 2-fold upregulation of gene expression was seen by day 5, and persisted throughout the remainder of the experiment (P= 0.0123, 0.0123, 0.0162, respectively). In the provisional scaffold, a significant upregulation of 40-fold was seen on the first day after injury, and persisted throughout the entire experiment (P= 0.0255, 0.0481, 0.0342, 0.0255, respectively).
Quantification of aggrecan and collagen degradation fragments in synovial fluid and serum
The synovial fluid C2C levels increased by a factor of 1.5 in the first five days after injury and remained elevated at 14 days after injury, changes that were significant at both time points (P= 0.041 and 0.024, respectively; Figure 2). CPII levels in the synovial fluid did not change over time (P > 0.32); nor did the C2C:CPII ratios (P> 0.25). The synovial fluid CS 846 levels were 3600 ng/ml at baseline and over 10,000 ng/ml at day 14; however, this change was not statistically significant (P= 0.10). Spiking studies for C2C, CPII and CS846 resulted in a 97%, 93% and 86% recovery of the protein respectively from the synovial fluid. This recovery rate is consistent with prior reports of the use of the IBEX ELISA kit for analysis of synovial fluid (24-26). Dilution studies were not performed due to the relatively small volumes of synovial fluid obtained from the knees; however, these assays have been previously used in other reports of analysis of non-human synovial fluid (27-29).
Figure 2.
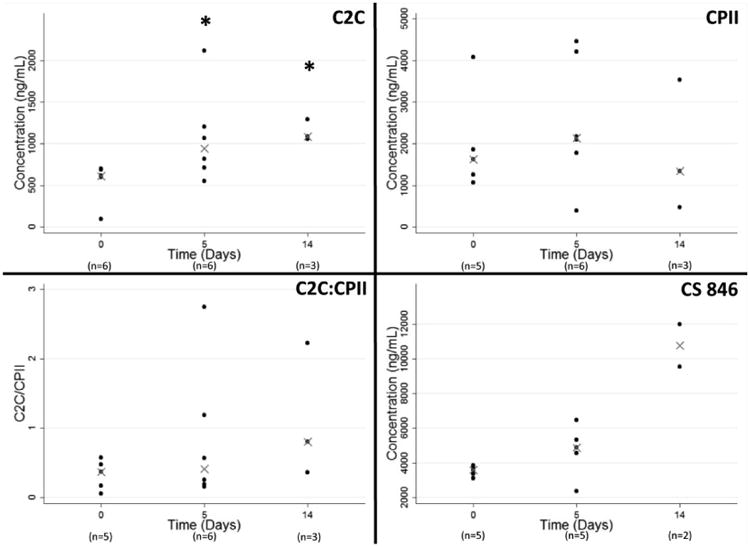
Synovial fluid concentrations of C2C, CPII, C2C:CPII, and CS 846. The synovial fluid aspirated from unoperated knees at Day 0 serves as the baseline control. Data is reported as median values, with error bars representing 25th and 75th percentiles. Asterisks indicate significant differences from control at P<0.05 (n=3 per analysis).
The C2C, CPII and CS 846 levels in porcine sera are presented in Figure 3. There was no significant increase in any of the serum biomarkers in the first two weeks following ACL injury.
Figure 3.
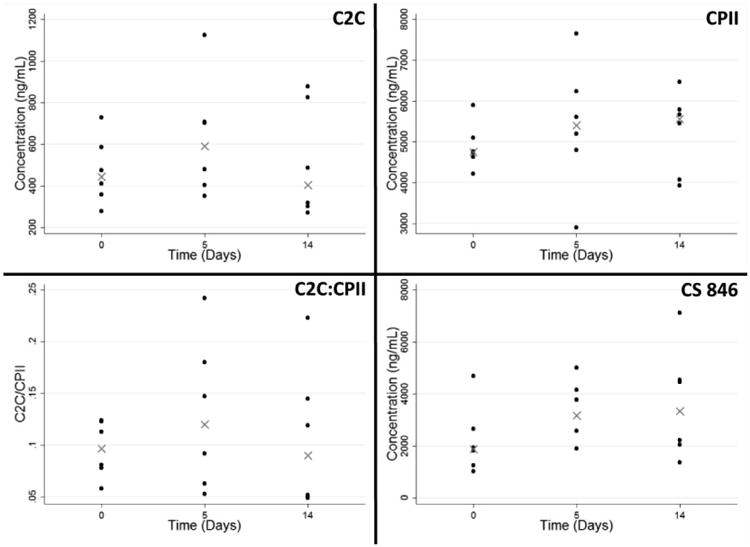
Serum concentrations of C2C, CPII, C2C:CPII, and CS 846. The synovial fluid aspirated from unoperated knees at at Day 0 serves as the baseline control. Data is reported as median values, with error bars representing 25th and 75th percentiles. No statistical differences were found across days (n=6 per analysis).
Histological assessment of Safranin-O and apoptosis
The articular cartilage in the uninjured control knees appeared smooth (OARSI score of 1.0, Figure 4A), increasing to an average OARSI score of 1.8 (Figure 4B), primarily due to an increase in surface roughness of the articular cartilage rather than observation of cellular changes or glycosaminoglycan loss. There was no observed change in C-6-S, a marker for chondroitin sulfate, seen with immunohistochemistry at the two week time point. While there was no caspase 3 detected in the cartilage from intact knees (Figure 4E), active caspase 3 was detected in the superficial zone chondrocytes at day 14 (Figure 4F).
Figure 4.
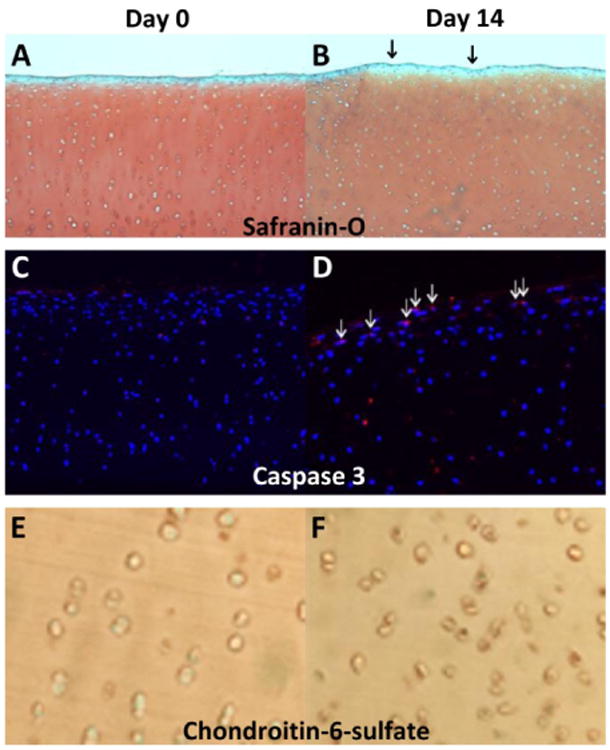
Representative Safranin-O/Fast Green (A, B) and active caspase-3 (E,F) immunostaining in porcine cartilage from control (day 0- A, C, E) and from day 14 (B, D, F) post-ACL transection. Day 14 cartilage specimens exhibited slightly rougher cartilage surface (shown by arrows). Active caspase-3 staining (red, shown by arrows) was detected in cartilage specimens at days 0 and 14.
Discussion
Results from this study convey the potential importance of an early intervention strategy following ACL injury to minimize cartilage damage. MMP-1, MMP-13 and ADAMTS-4 gene expression were upregulated in the joint as early as one day following isolated ACL injury, and the amount of type II collagen fragments in the synovial fluid nearly doubled within five days. While MMP-13 and ADAMTS-4 were not significantly upregulated in the articular cartilage, these genes were upregulated in the adjacent synovium, transected ACL, and provisional scaffold formed at the injury site; tissues which had the potential to influence cartilage degradation by releasing these degradative enzymes into the synovial fluid. The significant increase in collagen degradation fragment concentrations in the synovial fluid within the first two weeks after injury suggest that this acute phase may be suited for interventions to prevent cartilage loss after ACL injury.
Our results indicate that all of the joint tissues in this study (articular cartilage, synovium, ligament and provisional scaffold) are capable of upregulating MMP -1 expression following ACL injury. This observed increased expression of MMP-1 in the articular cartilage is consistent with prior reports of MMP-1 gene expression in cartilage of white rabbits subjected to ACL transection (30) and in humans with OA (31). In addition, the synovium and ACL were found to upregulate their gene expression of MMP-13. This is consistent with prior reports of a 28-fold increase in MMP-13 gene expression in the injured ligament after 1 week in a rabbit ACL transection model and an upregulation in both the ACL and synovium in a rat ACL injury model after 1, 2, and 3 days (32, 33). In addition, the lack of upregulation of MMP-13 in the articular cartilage is also consistent with prior reports of a lack of MMP-13 upregulation in the articular cartilage in the first 7 days following destabilization of the medial meniscus in a murine model (34).
The upregulation of both MMP-1 and MMP-13 expression by the intra-articular tissues may be responsible for the increasing C2C concentration in the synovial fluid seen in the first few days after ACL injury. The increases in C2C concentration seen in our porcine model are similar to the increased levels previously reported at day 5 after injury to the human knee (3). Increased C2C degradation fragments have also been found in the serum of patients with osteoarthritis (35). While MMP-1 regulated cartilage degradation may be due to the actions of chondrocytes as well as synoviocytes and fibroblasts, the MMP-13 regulated cartilage degradation appears to be mediated by the synovium and injured ligament, rather than the articular cartilage. MMP-1 was upregulated 3-fold in all joint tissues, where MMP-13 was upregulated more than 500-fold in the synovium and ligament. As MMP-13 is thought to be five to ten times more active in cleaving type II collagen than MMP-1, it is likely that the synovium and ligament MMP-13 production plays a key role in the early cartilage degradation seen after ACL injury.
Aggrecan has been noted to be one of the first cartilage extracellular matrix proteins to undergo measurable loss after joint injury, and thus is thought to be one of the early measures of PTOA (11, 36). In this study, while we did not see increases in the gene expression for ADAMTS-4 in the articular cartilage, we did see a 10- and 3-fold increase in gene expression in the synovium and ACL, respectively. These findings are consistent with those observed after destabilization of the medial meniscus in the murine knee joint, where ADAMTS-4 gene expression was noted to be elevated at time points as early as 6 hours after injury in whole joint preparations, following removal of the skin and muscle (34). In addition to the upregulation of gene expression for ADAMTS-4 in the synovium and ligament, we also found a relative increase in aggrecan synthesis fragments (CS 846) within the synovial fluid at day 14 post-injury, though this increase was not statistically significant (P= 0.15 and 0.10 for day 5 and 14, respectively). An increase in aggrecan synthesis fragments would be consistent with prior reports of a similar increase found in human patients within days after injury, peaking at 1 week (36, 37). The increased gene expression for ADAMTS-4 observed in the synovium and transected ACL and the associated production of aggrecanase synthesis fragments suggests that the initiation of proteoglycan loss in the articular cartilage after injury may be due to actions within tissues other than the articular cartilage.
The provisional scaffold is thought to lead the repair of extra-synovial ligaments, including the medial collateral ligament (MCL) (39). However, in this study, the provisional scaffold that forms after an intra-synovial ACL injury was found to serve a catabolic function as well, with significant upregulation of MMP-13 and ADAMTS-4. This would suggest it is a catabolic mediator of similar or greater significance than the synovium and ligament. This is the first report of the catabolic components of the response of the native provisional scaffold within the joint. This finding is consistent with what is noted clinically, where patients with recurrent hemarthroses are noted to prematurely develop cartilage loss (40, 41). Increased expression of MMPs have been observed in patients diagnosed with pigmented villonodular synovitis (PVNS), which has been suggested to play a critical role in the cartilage destruction observed clinically (41). Osteochondral damage has also been detected via magnetic resonance imaging in hemophiliac patients experiencing bleeding within the joint (40). This study suggests the provisional scaffold formed after ACL injury may play a similar role in joint destruction, ultimately leading to cartilage degradation.
In prior studies of post-traumatic osteoarthritis after ACL injury, there has been much debate over the root cause of this disease. While the impact damage is typically in the lateral compartment, the resulting osteoarthritis typically occurs in the medial compartment, suggesting it is a “whole joint” response, rather than a site-specific response to a local cartilage injury (42). Cellular apoptosis has previously been observed at both the impaction site as well as adjacent, non-impacted areas (38). Apoptosis was accompanied by progressive cartilage degeneration, even at these non-impacted sites. Additionally, delivery of a surfactant has been shown to delay the expansion of cellular apoptosis, thereby protecting cartilage integrity to a higher extent than inhibition of caspase-3 alone (38). Results of this study suggest a post-traumatic hemarthrosis (which routinely occurs after ACL injury) may thus be one of the factors that initiates the catabolic reaction of the joint to injury and the journey to PTOA. Further studies to examine the role of the coagulation proteins and blood degradation products on cartilage metabolism may shed new light on the mechanism of PTOA development.
There are several limitations for this study. First, an animal model was required in order for us to harvest the tissue and to evaluate the changes over time. It is possible that the timing of cytokine release and protease activity would be different in humans. The porcine model was chosen in part due to its large size, which is larger and easier to access surgically (i.e., more typical to that seen for humans) in comparison to smaller animal models. The porcine model was also chosen because of similarities in gait biomechanics and dependence on the ACL (43-46). Secondly, this study was performed with only a two week follow-up period. While this was long enough to accomplish the initial goals of the study - namely to determine the acute changes in the joint tissues after ACL injury - it was not long enough to see macroscopic cartilage breakdown. Future studies of longer-term time points are planned. Finally, the joint is a complex structure comprised of multiple tissue types. Though we have performed qPCR analysis on several of these tissues, it is likely that each secretes proteins and other signaling molecules into the synovial fluid, which subsequently interact with the other tissues.
Patients who have an ACL tear are at increased risk of post-traumatic osteoarthritis, whether or not they have an ACL reconstruction (47). In this study, we determined that gene expression of a key matrix metalloproteinase and aggrecanase were upregulated in the adjacent joint synovium, transected ACL, and provisional scaffold, but not in the articular cartilage. These finding suggest that cartilage changes seen after ACL injury, even without impact trauma, may be mediated not just by chondrocytes, but also by other tissues of the joint, and thus the joint should be considered a complex organ with an orchestrated response to injury, rather than a set of individual tissues. This paper suggests that multiple tissues within the articular joint, including the provisional scaffold, produce catabolic cytokines, including MMP-1 and MMP-13. The joint is a complex organ with multiple different tissue types sharing the same synovial fluid environment, which makes determining which tissue is the key player in cartilage degradation challenging. However, the upregulation of catabolic factors seen in the torn ligament, synovium, provisional scaffold and articular cartilage after ACL injury suggests the response of these tissues should be considered when studying mechanisms of post-traumatic osteoarthritis development after an ACL injury.
Acknowledgments
The authors thank the ARCH staff, Dr. Arthur Nedder, Kathryn Mullen, Dana Bolgen, and Courtney White, for their assistance and care in handling the minipigs. We would also like to thank Drs. Adele Hill, Sebastian Kalamajski, and Justin Allen for their assistance in qPCR design and Elise Magarian, Ryu Yoshida, and Dr. Patrick Vavken for their assistance in surgery and tissue collection.
Funding Sources: This investigation was supported by National Institutes of Health under NIAMS AR054099 and AR056834 (MMM and BCF) and the Ruth L. Kirschstein National Research Service Award (F32 AR061186) (CMH). Dr. Haslauer's salary was also supported by Dr. Matthew Warman and the Children's Orthopaedic Surgery Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author contributions: Conception and design of the study: MMM, BCF, KAE, and CMH
Sample and data collection: BLP, CMH, and KAE
Statistical analysis: VMJ
Drafting of manuscript: MMM, BCF, KAE, VMJ, BLP, and CMH
Critical revision of manuscript: MMM, BCF, KAE, VMJ, BLP, and CMH
Approval of final manuscript version: MMM, BCF, KAE, VMJ, BLP, and CMH
Competing interests: The authors do not have any conflicts of interest to report.
References
- 1.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. The American journal of sports medicine. 2007;35(10):1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 2.Fleming BC, Oksendahl HL, Mehan WA, Portnoy R, Fadale PD, Hulstyn MJ, et al. Delayed Gadolinium-Enhanced MR Imaging of Cartilage (dGEMRIC) following ACL injury. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2010;18(5):662–7. doi: 10.1016/j.joca.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury ( NCT00332254) Arthritis research & therapy. 2010;12(6):R229. doi: 10.1186/ar3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray MM, Spindler KP, Devin C, Snyder BS, Muller J, Takahashi M, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24(4):820–30. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 5.Murray MM, Magarian E, Harrison SL, Mastrangelo A, Zurakowski D, Fleming BC. The Effect of Skeletal Maturity on Functional Healing of the Anterior Cruciate Ligament. J Bone Joint Surg. 2010;92(11):2039–49. doi: 10.2106/JBJS.I.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tchetverikov I, Lohmander LS, Verzijl N, Huizinga TW, TeKoppele JM, Hanemaaijer R, et al. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Annals of the rheumatic diseases. 2005;64(5):694–8. doi: 10.1136/ard.2004.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. The Journal of clinical investigation. 1997;99(7):1534–45. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Frontiers in bioscience: a journal and virtual library. 2006;11:529–43. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 9.Poole AR, Ionescu M, Fitzcharles MA, Billinghurst RC. The assessment of cartilage degradation in vivo: development of an immunoassay for the measurement in body fluids of type II collagen cleaved by collagenases. Journal of immunological methods. 2004;294(1-2):145–53. doi: 10.1016/j.jim.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Nelson F, Dahlberg L, Laverty S, Reiner A, Pidoux I, Ionescu M, et al. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. The Journal of clinical investigation. 1998;102(12):2115–25. doi: 10.1172/JCI4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karsdal MA, Madsen SH, Christiansen C, Henriksen K, Fosang AJ, Sondergaard BC. Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis research & therapy. 2008;10(3):R63. doi: 10.1186/ar2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poole AR, Ionescu M, Swan A, Dieppe PA. Changes in cartilage metabolism in arthritis are reflected by altered serum and synovial fluid levels of the cartilage proteoglycan aggrecan. Implications for pathogenesis. The Journal of clinical investigation. 1994;94(1):25–33. doi: 10.1172/JCI117314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohmander LS, Ionescu M, Jugessur H, Poole AR. Changes in joint cartilage aggrecan after knee injury and in osteoarthritis. Arthritis Rheum. 1999;42(3):534–44. doi: 10.1002/1529-0131(199904)42:3<534::AID-ANR19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Kraus VB, Birmingham J, Stabler TV, Feng S, Taylor DC, Moorman CT, 3rd, et al. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: a randomized controlled pilot trial ( NCT00332254) Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2012;20(4):271–8. doi: 10.1016/j.joca.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, Kelly M, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25(1):81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 16.Magarian EM, Fleming BC, Harrison SL, Mastrangelo AN, Badger GJ, Murray MM. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. The American journal of sports medicine. 2010;38(12):2528–34. doi: 10.1177/0363546510377416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 18.Frantz NZ, Friesen KG, Andrews GA, Tokach MD, Yamka RM, Loughin TL, et al. Use of serum biomarkers to predict the development and severity of osteochondrosis lesions in the distal portion of the femur in pigs. American journal of veterinary research. 2010;71(8):946–52. doi: 10.2460/ajvr.71.8.946. [DOI] [PubMed] [Google Scholar]
- 19.Hembry RM, Dyce J, Driesang I, Hunziker EB, Fosang AJ, Tyler JA, et al. Immunolocalization of matrix metalloproteinases in partial-thickness defects in pig articular cartilage. A preliminary report. The Journal of bone and joint surgery American volume. 2001;83-A(6):826–38. doi: 10.2106/00004623-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Fosang AJ, Last K, Stanton H, Weeks DB, Campbell IK, Hardingham TE, et al. Generation and novel distribution of matrix metalloproteinase-derived aggrecan fragments in porcine cartilage explants. The Journal of biological chemistry. 2000;275(42):33027–37. doi: 10.1074/jbc.M910207199. [DOI] [PubMed] [Google Scholar]
- 21.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Jay GD, Fleming BC, Watkins BA, McHugh KA, Anderson SC, Zhang LX, et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2010;62(8):2382–91. doi: 10.1002/art.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornik K. The R FAQ. 2012. http://CRANR-projectorg/doc/FAQ/R-FAQhtml.
- 24.Matyas JR, Atley L, Ionescu M, Eyre DR, Poole AR. Analysis of cartilage biomarkers in the early phases of canine experimental osteoarthritis. Arthritis Rheum. 2004;50(2):543–52. doi: 10.1002/art.20027. [DOI] [PubMed] [Google Scholar]
- 25.Chu Q, Lopez M, Hayashi K, Ionescu M, Billinghurst RC, Johnson KA, et al. Elevation of a collagenase generated type II collagen neoepitope and proteoglycan epitopes in synovial fluid following induction of joint instability in the dog. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2002;10(8):662–9. doi: 10.1053/joca.2002.0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima T, Mwale F, Yasuda T, Girard C, Poole AR, Laverty S. Early degradation of type IX and type II collagen with the onset of experimental inflammatory arthritis. Arthritis Rheum. 2001;44(1):120–7. doi: 10.1002/1529-0131(200101)44:1<120::AID-ANR16>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 27.de Grauw JC, Donabedian M, van de Lest CH, Perona G, Robert C, Lepage O, et al. Assessment of synovial fluid biomarkers in healthy foals and in foals with tarsocrural osteochondrosis. Vet J. 2011;190(3):390–5. doi: 10.1016/j.tvjl.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson AM, Trumble TN, Merritt KA, Brown MP. Associations of horse age, joint type, and osteochondral injury with serum and synovial fluid concentrations of type II collagen biomarkers in Thoroughbreds. American journal of veterinary research. 2010;71(7):741–9. doi: 10.2460/ajvr.71.7.741. [DOI] [PubMed] [Google Scholar]
- 29.Wei L, Fleming BC, Sun X, Teeple E, Wu W, Jay GD, et al. Comparison of differential biomarkers of osteoarthritis with and without posttraumatic injury in the Hartley guinea pig model. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2010;28(7):900–6. doi: 10.1002/jor.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bluteau G, Conrozier T, Mathieu P, Vignon E, Herbage D, Mallein-Gerin F. Matrix metalloproteinase-1, -3, -13 and aggrecanase-1 and -2 are differentially expressed in experimental osteoarthritis. Biochimica et biophysica acta. 2001;1526(2):147–58. doi: 10.1016/s0304-4165(01)00122-2. [DOI] [PubMed] [Google Scholar]
- 31.Wu W, Billinghurst RC, Pidoux I, Antoniou J, Zukor D, Tanzer M, et al. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 2002;46(8):2087–94. doi: 10.1002/art.10428. [DOI] [PubMed] [Google Scholar]
- 32.Attia E, Brown H, Henshaw R, George S, Hannafin JA. Patterns of gene expression in a rabbit partial anterior cruciate ligament transection model: the potential role of mechanical forces. The American journal of sports medicine. 2010;38(2):348–56. doi: 10.1177/0363546509348052. [DOI] [PubMed] [Google Scholar]
- 33.Tang Z, Yang L, Zhang J, Xue R, Wang Y, Chen PC, et al. Coordinated expression of MMPs and TIMPs in rat knee intra-articular tissues after ACL injury. Connective tissue research. 2009;50(5):315–22. [PubMed] [Google Scholar]
- 34.Burleigh A, Chanalaris A, Gardiner MD, Driscoll C, Boruc O, Saklatvala J, et al. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum. 2012;64(7):2278–88. doi: 10.1002/art.34420. [DOI] [PubMed] [Google Scholar]
- 35.Cahue S, Sharma L, Dunlop D, Ionescu M, Song J, Lobanok T, et al. The ratio of type II collagen breakdown to synthesis and its relationship with the progression of knee osteoarthritis. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2007;15(7):819–23. doi: 10.1016/j.joca.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sward P, Frobell R, Englund M, Roos H, Struglics A. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis) -a cross-sectional analysis. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2012 doi: 10.1016/j.joca.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 37.Lohmander LS, Atley LM, Pietka TA, Eyre DR. The release of crosslinked peptides from type II collagen into human synovial fluid is increased soon after joint injury and in osteoarthritis. Arthritis Rheum. 2003;48(11):3130–9. doi: 10.1002/art.11326. [DOI] [PubMed] [Google Scholar]
- 38.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2011;29(6):802–9. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chamberlain CS, Crowley EM, Kobayashi H, Eliceiri KW, Vanderby R. Quantification of collagen organization and extracellular matrix factors within the healing ligament. Microsc Microanal. 2011;17(5):779–87. doi: 10.1017/S1431927611011925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraft J, Blanchette V, Babyn P, Feldman B, Cloutier S, Israels S, et al. Magnetic resonance imaging and joint outcomes in boys with severe hemophilia A treated with tailored primary prophylaxis in Canada. J Thromb Haemost. 2012;10(12):2494–502. doi: 10.1111/jth.12025. [DOI] [PubMed] [Google Scholar]
- 41.Uchibori M, Nishida Y, Tabata I, Sugiura H, Nakashima H, Yamada Y, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in pigmented villonodular synovitis suggests their potential role for joint destruction. The Journal of rheumatology. 2004;31(1):110–9. [PubMed] [Google Scholar]
- 42.Potter HG, Jain SK, Ma Y, Black BR, Fung S, Lyman S. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. The American journal of sports medicine. 2012;40(2):276–85. doi: 10.1177/0363546511423380. [DOI] [PubMed] [Google Scholar]
- 43.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39–44. doi: 10.1097/01.blo.0000197079.26600.09. [DOI] [PubMed] [Google Scholar]
- 44.Levy AS, Meier SW. Approach to cartilage injury in the anterior cruciate ligament-deficient knee. Orthop Clin North Am. 2003;34(1):149–67. doi: 10.1016/s0030-5898(02)00065-2. [DOI] [PubMed] [Google Scholar]
- 45.Sharma L, Lou C, Felson DT, Dunlop DD, Kirwan-Mellis G, Hayes KW, et al. Laxity in healthy and osteoarthritic knees. Arthritis Rheum. 1999;42(5):861–70. doi: 10.1002/1529-0131(199905)42:5<861::AID-ANR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 46.Seitz H, Hausner T, Schlenz I, Lang S, Eschberger J. Vascular anatomy of the ovine anterior cruciate ligament. A macroscopic, histological and radiographic study. Arch Orthop Trauma Surg. 1997;116(1-2):19–21. doi: 10.1007/BF00434094. [DOI] [PubMed] [Google Scholar]
- 47.Oiestad BE, Holm I, Engebretsen L, Aune AK, Gunderson R, Risberg MA. The prevalence of patellofemoral osteoarthritis 12 years after anterior cruciate ligament reconstruction. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2012 doi: 10.1007/s00167-012-2161-9. [DOI] [PubMed] [Google Scholar]


